Abstract
Purpose
Protein phosphorylation mediated by protein kinases controls numerous cellular processes. A genetically encoded, generalizable split firefly luciferase (FL)-assisted complementation system was developed for noninvasive monitoring phosphorylation events and efficacies of kinase inhibitors in cell culture and in small living subjects by optical bioluminescence imaging.
Procedures
An Akt sensor (AST) was constructed to monitor Akt phosphorylation and the effect of different PI-3K and Akt inhibitors. Specificity of AST was determined using a non-phosphorylable mutant sensor containing an alanine substitution (ASA).
Results
The PI-3K inhibitor LY294002 and Akt kinase inhibitor perifosine led to temporal- and dose-dependent increases in complemented FL activities in 293T human kidney cancer cells stably expressing AST (293T/AST) but not in 293T/ASA cells. Inhibition of endogenous Akt phosphorylation and kinase activities by perifosine also correlated with increase in complemented FL activities in 293T/AST cells but not in 293T/ASA cells. Treatment of nude mice bearing 293T/AST xenografts with perifosine led to a 2-fold increase in complemented FL activities compared to that of 293T/ASA xenografts. Our system was used to screen a small chemical library for novel modulators of Akt kinase activity.
Conclusion
This generalizable approach for noninvasive monitoring of phosphorylation events will accelerate the discovery and validation of novel kinase inhibitors and modulators of phosphorylation events.
Keywords: Phosphorylation, Kinases, Noninvasive, Repetitive imaging in living subjects, Optical bioluminescence imaging in living subjects, Drug development, Akt
Introduction
The investigation of different biological processes involves examination of pathways at the molecular level, in part by monitoring promoter activity, gene expression [1], and protein–protein interactions [2, 3] through different chemical, biochemical, and cell-based assays as well as direct and indirect imaging [4, 5]. However, examination of posttranslational modification events in living subjects poses a much greater challenge due to the variety, kinetics, and localization of the enzymes involved. One of the most important posttranslation modifications is protein phosphorylation, which is mediated by kinases and reversed by protein phosphatases [6]. Protein kinases can be activated through different ligand and kinase/phosphatase equilibria along the signal transduction cascades that play crucial roles in normal cellular processes and cancer initiation, progression, and metastasis [7].
One prime example is Akt, which is a 57-kDa serine/threonine kinase. Akt is activated by the phosphatidylinosi-tol-3-kinase (PI-3K) pathway and becomes fully activated upon phosphorylation at T308 and S473 [8, 9]. Activated Akt phosphorylates multiple substrates that are crucial in cell cycle progression, protein synthesis, survival, glucose metabolisms, and inhibition of apoptosis [10, 11] at their Akt kinase motif (AKM): RxRxxS/T. Akt is highly activated in multiple human cancers, and this correlates with tumor metastasis and resistance to chemotherapy [12, 13] and radiation therapy [14, 15]. Investigation of Akt kinase activity is usually performed by kinase assays and Western blotting using phosphorylation-specific antibodies [16], which are invasive in nature and may not reflect the nature of Akt kinase activity in intact cells. Genetically encoded fluorescence reporters including the B kinase activity reporter (BKAR) [17] and Aktus [18] were used to monitor Akt kinase activity in intact cells based on changes in fluorescence resonance energy transfer (FRET) ratios mediated by the interactions between the phosphorylated AKM and the respective phospho-binding domain [18]. Although these reporters provide single-cell resolution of Akt kinase activity [19], the small changes in FRET ratios cannot be easily monitored in living-animal imaging due to the high autofluorescence and low signal-to-background ratios. Other approaches have also been developed to monitor different events pertaining to protein–protein interactions, including beta-lactamase complementation [20–22] for monitoring translocation of the pleckstrin homology (PH) domain of Akt to the plasma membrane [23], split Gaussia luciferase complementation [24, 25], and split yellow fluorescence protein complementation [26, 27].
Different Akt kinase inhibitors have been developed to target its adenosine triphosphate-binding site, PH-binding domain, and substrate-binding motifs [28]. PI-3K inhibitors have also been developed to inhibit Akt activation [29]. The Akt inhibitors that are in advanced development are perifosine and RX-0201, which are now in phase I/II clinical trials for patients with advanced malignancies [30–32]. Although some of these Akt inhibitors have shown efficacy for growth inhibition in cell culture and xenograft models in living mice [28, 33, 34], longitudinal studies for monitoring their efficacies cannot be achieved noninvasively without sacrificing the mice at each time point prior to excision of tumors for in vitro analyses. To construct a genetically encoded reporter (AS) for indirect monitoring of Akt kinase activity and discovery of novel Akt kinase inhibitors in living subjects, we adapted the firefly luciferase (FL) protein-fragment-assisted complementation (SFL-PFAC) technology developed by our laboratory [1, 2, 5, 35, 36] and others [37, 38] for noninvasive monitoring of protein–protein interactions by optical bioluminescence imaging. SFL-PFAC is based on the complementation of two inactive halves on the full-length FL mediated by the interaction between two proteins and was used for monitoring the interactions between 14-3-3ε/Cdc25, Stat1 homodimerization [37, 38], Id/MyoD, Hif1-α/VHL, Rapamycin-mediated mTOR/FKBP12 interactions, and Herpes simplex virus type I thymidine kinase homodimerization in cell culture studies and living mice [2].
We hypothesized that: (1) the AS serves as a substrate for activated Akt (Fig. 1a); (2) interactions between the phosphorylated AKM and the phoshothereonine binding domain (FHA2) lead to decrease in complementation of split FL activity; whereas (3) inhibition of Akt kinase activity leads to reduced AKM/FHA2 interactions and increases split FL complementation that leads to signal amplification and light production in the presence of its substrate D-luciferin. In the current work, we first established the sensitivity and specificity of AS to indirectly examine the kinetics of Akt kinase activity and monitor the efficacy of known PI-3K and Akt inhibitors in cell culture. This was followed by noninvasive, repetitive bioluminescence imaging in living mice bearing tumor xenografts for dynamic indirect monitoring of Akt kinase activity in response to perifosine. We also extended our system for drug screening using a chemical library for novel Akt kinase inhibitors. This is our first attempt to monitor phosphorylation events in intact cells in cell culture and noninvasively in living mice using the same genetically encoded reporters that can be extended for discovery and validation of novel kinase inhibitors.
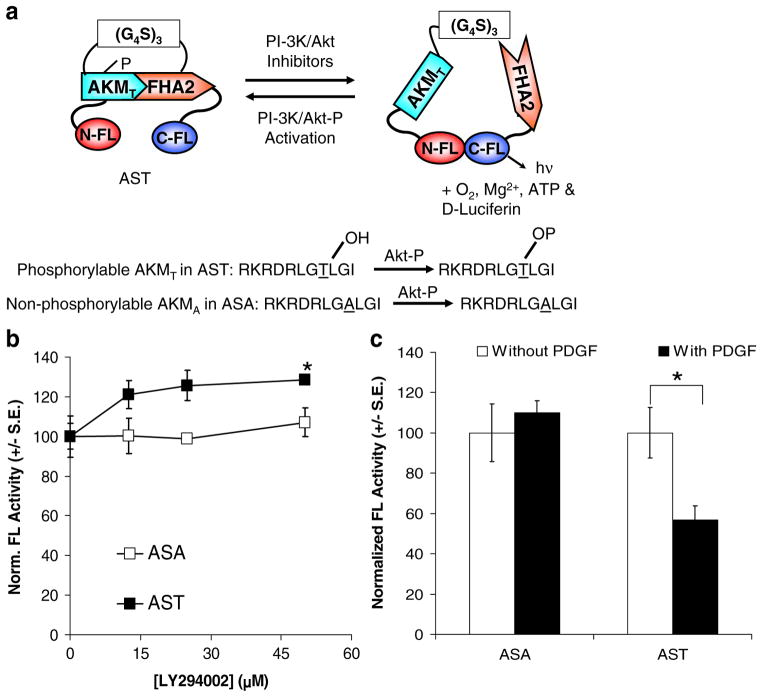
Fig. 1.
a Schematic diagram for Akt kinase sensor (AST). Inhibition of Akt kinase activity by PI-3K/Akt inhibitors leads to decreased phosphorylation of AST at the Akt kinase motif (AKMT) and interaction with the phosphothreonine binding domain (FHA2). This leads to increased complementation of split FL fragments (NFL and CFL) and light production in the presence of the FL substrate D-luciferin. On the other hand, activation of Akt kinase activity (by PI-3K/AKT-P) leads to phosphorylation of AST at the AKMT and increased interaction with FHA2, thus hinders complementation between NFL/CFL. The ASA sensor with a non-phosphorylable AKMA motif served as a negative control. b Inhibition of Akt kinase activity led to increase in complemented FL activity in BT474 cells transiently transfected with AST. BT474 cells were transiently transfected with ASA or AST for 24 h in the presence of the PI-3K inhibitor LY294002 or carrier control prior to bioluminescence imaging of intact cells upon addition of D-luciferin. Total flux (complemented FL activities) was normalized for transfection efficiency using RL activities, protein content, and to carrier control treated cells (100%). LY led to a dose-dependent increase in complemented FL activities in BT474 cells transiently transfected with AST compared to that of carrier control treated cells. *p<0.05 relative to carrier control treated cells. c Activation of Akt kinase activity by platelet-derived growth factor led to decrease in complemented FL activity. 293T cells transiently transfected with ASA or AST for 24 h were treated with PDGF or carrier control for 30 min prior to analysis of complemented FL activity as described in 1B. In 293T cells transiently transfected with AST, PDGF led to decrease in complemented FL activity. On the other hand, in 293T cells transiently transfected with ASA, PDGF did not lead to significant decrease in complemented FL activity. *p<0.05 relative to carrier control treated cells.
Materials and Methods
Chemicals, Enzymes, and Reagents
The plasmids pCMV-hRL encoding the full-length synthetic Renilla luciferase (RL) and the pCMV-FL encoding the full-length firefly luciferase, LARII substrate for FL assay and 5× passive lysis buffer were purchased from Promega (Madison, WI, USA). Restriction enzymes, modification enzymes, and ligase were purchased from New England Biolabs (Beverly, MA, USA). TripleMaster TaqDNA polymerase for polymerase chain reaction (PCR) amplification was purchased from Brinkmann Eppendorf (Hamburg, Germany). Ampicillin and 5′-guanidinonaltrindole di (trifluoroacetate) salt hydrate were purchased from Sigma (St. Louis, MO, USA). Platelet-derived growth factor (PDGF) was purchased from Calbiochem (San Diego, CA, USA). Bacterial culture media and growth factor-reduced Matrigel™ were purchased from BD Biosciences (Sparks, MD, USA). Plasmid and DNA gel extraction kits were purchased from Qiagen (Valencia, CA, USA). D-Luciferin was purchased from Biosynth (Switzerland). Animal cell culture media, fetal bovine serum (FBS), the antibiotics streptomycin and penicillin, plastic wares for cell culture, lipofectamine2000 transfection reagent, and 4–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels were purchased from Invitrogen (Carlsbad, CA, USA). Akt inhibitor perifosine and PI-3K inhibitor LY294002 (LY) were purchased from Cayman Chemicals (Ann Arbor, MI, USA) and Cell Signaling (Beverly, MA, USA) respectively.
Construction of Plasmid Vectors
Plasmid vectors ASA and AST were constructed by using split FL fragments (NFL, amino acids 1–416 and CFL, amino acids 394–550), Akt kinase motif peptide sequence (RKRDRLGTLGI), and the yeast phosphothreonine FHA2 binding domain. The PCR amplified fragment of NFL with overhang primers coding for peptides for AKT (RKRDRLGTLGI) and AKA (RKRDRLGALGI) with HindIII restriction enzymes and the forward primer flanking the NFL with start codon and the NheI restriction enzyme site were cloned into corresponding enzyme digested pcDNA 3.1 (+) vector backbone previously cloned with (G4S)3 linker in HindIII/EcoRI restriction enzyme site (pcDNA 3.1 (+)-NFL-AKM/AKT-(G4S)3. The PCR amplified fragment of FHA2 (cloned in EcoRI/BamHI) and CFL (cloned in BamHI/XhoI) in pcDNA 3.1 (−) were digested with BamHI and XbaI restriction enzymes cloned to the corresponding enzyme-digested pcDNA 3.1 (+)-NFLUC-AKM/AKT-(G4S)3 site to construct pcDNA 3.1 (+)-NFL-AKT-(G4S)3-FHA2-CFL and pcDNA 3.1 (+)-NFLUC-AKA-(G4S)3-FHA2-CFL.
Cell Culture
All cell lines used were purchased from American Type Culture Collection (Manassas, VA, USA). Human 293T embryonic kidney cancer cells were grown in minimal essential medium (MEM) supplemented with 10% FBS and 1% penicillin/streptomycin (P/S) solutions. Human BT474 invasive breast ductal carcinoma cells were grown in Dubelcco modified MEM supplemented with 10% FBS and 1% P/S solutions.
Transient Cell Transfection, Firefly, and Renilla Luciferase Assays
Transfections were performed in 80% confluent 24 h-old cultures of 293T and BT474 cells. For transient transfection, 250 ng/well of pcDNA molar equivalent of each ASA or AST were used in 12-well tissue culture plates, and 1.5 μl of lipofectamine2000 was used per transfection. Five nanograms of pCMV-hRL was co-transfected per well to normalize for transfection efficiency. The cells were assayed after 24 h incubation at 37°C at 5% CO2. To determine the effect of LY and PDGF on complemented FL activities, cells were treated with LY or PDGF at the time of transfection or 23 h posttransfection, respectively. Bioluminescence imaging of complemented FL activities in intact cells were performed using the IVIS-50 cooled charge-coupled device (CCD) camera (Caliper Life Sciences, Mountain View, CA, USA) by addition of 0.225 mg of D-luciferin in 500 μl of phosphate-buffered saline (PBS), with an acquisition time of 1 min. To quantify the measured light, regions of interest were drawn over the area of each well, and the total flux (photons s−1 cm−2) were obtained as validated previously [1–3, 5, 35, 36]. RL activities were determined by luminometer assay as previously described [1–3, 5]. Protein concentrations in cell lysates were determined by Bradford Assay (Bio-Rad). FL activities in each well were normalized for protein content and for transfection efficiency using RL activity. Average normalized FL activities in triplicate wells were expressed as relative light units per microgram protein per minute of counting (RLU μg−1 protein min−1).
Selection of 293T Cells Stably Expressing AST and ASA and Evaluation of the Efficacies of Different PI-3K and Akt Inhibitors in Cell Culture
To generate 293T cells stably expressing ASA (293T/ASA) or AST (293T/AST), 5×106 293T cells were plated in each 10-cm tissue culture plate and allowed to attach for 24 h. Cells were transfected with 5 μg each of pcDNA3.1+ expressing ASA or AST for 24 h using 20 μl of lipofectamine2000, prior to trypsinizing and replating in 1.5 μg/ml of puromycin hydrochloride for 2 weeks at low density (~1,000 cells) to allow formation of individual colonies. To select for 293T/ASA or 293T/AST stable clones, cell culture medium was aspirated, 0.5 mg of D-luciferin diluted in 3 ml of PBS was added, and bioluminescence imaging of FL activities was performed as described above. Positive colonies with the highest FL activities were trypsinized, transferred to individual wells in a 24-well plate, and expanded in medium containing 1.5 μg/ml puromycin hydrochloride for subsequent experiments.
To determine the effect of PI-3K and Akt inhibitors on complementation of FL activity, 2.5×105 293T/ASA and 293T/AST cells were plated in each 12-well plate and allowed to attach for 24 h prior to treatment with different concentrations of LY, perifosine, or their respective carrier controls for 6, 12, and 24 h. FL activities in intact cells were determined by bioluminescence imaging of intact cells using the above IVIS imaging parameters. Cells were subsequently lysed in 1% NP40 buffer (Cell Signaling) for 15 min on ice followed by centrifugation at 10,000×g to remove cell debris. Protein concentrations were determined by Bio-Rad protein Dc assay (Bio-Rad). FL activities were normalized for protein content and to that of carrier control treated cells and expressed as normalized FL activity±standard error of means (SEM) for the triplicate wells.
Akt Western Blotting and Kinase Assay
To determine the expression of total and phosphorylated Akt in 293T/ASA and 293T/AST cells treated with perifosine, cell lysates from the triplicate wells upon imaging were combined, and the protein concentrations were re-determined. Western blotting for phosphorylated (S473)/total Akt and Akt kinase activities were performed as previously described [16]. Akt kinase activities upon perifosine treatment were determined using an in vitro Akt kinase assay kit (Cell Signaling) with 200 μg total protein from cell lysates. Briefly, Akt was immunoprecipitated using the immobilized Akt antibody, followed by an in vitro kinase assay at 30°C for 1 h using 1 μg of the purified GSK-3β fusion protein as the substrate. The kinase reaction was terminated with 3× loading buffer and heating at 95°C for 3 min. One third of the kinase reaction (25 μl) was resolved by 4–16% SDS-PAGE gel for Western analysis of phosphorylated and total Akt (S473) and phospho-GSK-3β. In all cases, band intensities were quantified by Image J (National Institute of Health, Bethesda, MD, USA).
Optical Cooled CCD Imaging in Living Mice
All of the animal handling was performed in accordance with Stanford University Animal Research Committee guidelines. Mice were anesthetized using isofluorane (2% isofluorane in 100% oxygen) during all injection and imaging procedures. Mice were imaged using a cooled CCD camera (IVIS100; Caliper Life Sciences). 293T cells (3×106) stably co-transfected with AST or 3×105 293T cells stably transfected with ASA mixed with 2.7×106 293T cells were implanted subcutaneously in the bottom left and right flanks of each female nude mouse of 7 weeks old (nu/nu, Charles River), respectively, for 7 days for tumor establishment. The animals were placed prone in a light-tight chamber, and a gray scale reference image was obtained under low-level illumination. To determine baseline complemented FL activity in the implanted tumors in living mice, 3 mg of D-luciferin in 100 μl of PBS was injected intraperitoneally (i.p.) prior to serial imaging with the IVIS100 system an acquisition time of 10 s until signals peaked. Images were obtained using Living Image Software (Xenogen Corp., Alameda, CA, USA) and Igor Image Analysis Software (Wavemetrics, Seattle, WA, USA). To quantify the measured light, regions of interest were drawn over the area of the implanted cells, and the maximum photons per second per square centimeter per steradian (sr) were obtained as validated previously [1–3, 5, 35, 36]. Mice were then given 30 mg/kg of perifosine dissolved in 200 μl saline or 200 μl saline as carrier control by oral gavage and re-imaged at 6, 15, 27, and 39 h upon i.p. injection of 3 mg D-luciferin. Max photons (photons s−1 cm−2 per sr) analysis was performed as previously described [39] to avoid inaccuracies in determining tumor boundaries for total flux analysis. Max photons were normalized to that of time 0 h for each individual mouse and expressed as normalized average max photons±SEM.
High-Throughput Screening of Novel Akt Kinase Inhibitors
To identify novel Akt kinase inhibitors, the library of pharmacologically active compounds (LOPAC) from Sigma was utilized. High-throughput screening was performed at Stanford High-throughput Bioscience Center. 293T/ASA or AST cells (9×103) were plated in each well of the 384-white bottom plate from columns 1–22 (E & K Scientific, Santa Clara, CA, USA) in 60 μl of medium using the Matrix Wellmate (Thermo Scientific, Hudson, NH, USA) and allowed to attach for 24 h. Sixty microliters of medium (no cells) was added to column 23–24 to control for background signals due to substrate alone. Approximately 100 nl of each LOPAC compound (10 mM stock in dimethylsulfoxide (DMSO)) was then added to each well in columns 3–22 (final concentration ~17 μM) using a PinTool (V&P Scientific, San Diego, CA, USA) on a Sciclone ALH3000 (Caliper Sciences, Hopkinton, MA, USA). Cells in columns 1–2 that were not treated with the compounds were used to determine the baseline Akt activities. A total of four compound plates were used for 293T/ASA and 293T/AST cells. Cells were incubated for another 24 h at 5% CO2 at 37°C. Complemented FL activity in each well was determined 10 min after addition of 10 μl of Steady-Glo® Luciferase system reagents (Promega) using the Analyst GT (Molecular Device, Sunnyvale, CA, USA), with an acquisition time of 0.2 s per well. Complemented FL activities in each LOPAC-library-treated well were normalized to the mean FL activities of untreated cells.
To validate the efficacy of the lead compound 5′GU from the LOPAC library screen, 3×104 293T/ASA and 293T/AST cells were plated in each well of the 96 black-well clear bottom plates (Corning, Corning, NY, USA) and allowed to attach for 24 h. Cells were then treated with 0–50 μM of 5′GU for 24 h. Complemented FL activities in intact cells were determined, and total flux in each well was quantitated as described above. Total flux was normalized for cell number using the Sulfohodamine B assay as previously described [40] and to carrier control treated cells (100%).
Data Analysis
Each experiment was repeated at least three times, and results were expressed as mean±SEM. Statistical differences were determined by Student’s t test using p<0.05 as the cutoff point.
Results
Akt Kinase Sensor (AST) is a Genetically Encoded Reporter Based on Complementation of Split FL Fragments upon Akt Dephosphorylation
AST was constructed using the overlapping split sites of N terminus and C terminus of FL [2]. The AKM (RKRDRLGTLGI) and FHA2 binding domain previously used for the fluorescence-based BKAR [17] were cloned in between the NFL and CFL. A corresponding AS with the non-phosphorylable AKM (RKRDRLGALGI) containing an alanine substitution (ASA) was used as a negative control. The AKM and FHA2 domains were connected by a flexible (G4S)3 peptide linker. Figure 1a shows the configuration of the AST and ASA fusion constructs. We hypothesized that activation of endogenous Akt kinase activity results in phosphorylation of AST at the AKM, while subsequent interactions with FHA2 hinder complementation between the NFL/CFL and decrease complemented FL activities. Inhibition of Akt kinase activities by PI-3K/Akt inhibitors reduces phosphorylation of AKM in AST and AKM/FHA2 interactions, increases complementation of NFL/CFL, and leads to higher light production in the presence of the FL substrate D-luciferin. On the other hand, complementation of NFL/CFL in ASA with the non-phosphorylable AKM should not be affected by changes in endogenous Akt kinase activity.
AS was Sensitive and Specific for Monitoring Akt Kinase Activity
The sensitivity and specificity of AST was first examined using BT474 human breast ductal carcinoma cells, which exhibit constitutively active Akt kinase activity [16]. To test the hypothesis that inhibition of Akt kinase activity leads to decreased AKM/FHA2 interactions and increase in split FL complementation, BT474 cells were transiently transfected with AST or ASA constructs in the presence of different concentrations of LY294002 (a PI-3K inhibitor that inhibits Akt activation) or carrier control (0.1% DMSO) for 24 h. FL activity was determined by bioluminescence imaging of intact cells using a cooled CCD camera upon addition of D-luciferin. Total flux was measured by drawing regions of interest (ROI) over each well optimally using a 5% threshold for border detection prior to normalization for protein content and transfection efficiency. LY treatment of BT474 cells transiently transfected with AST led to a dose-dependent increase in FL activity in (1.3-fold maximum increase, p<0.05 at 50 μM, relative to carrier control treated cells). On the other hand, in BT474 cells transiently transfected with the non-phosphorylable ASA, there was no significant increase in complemented FL activity (p>0.05; Fig. 1b).
We next tested the hypothesis that activation of Akt activity would led to increased phosphorylation of AKM and interaction with FHA2 and subsequent inhibition of split FL complementation. 293T cells transiently transfected with AST and ASA were treated with platelet-derived growth factor for 30 min prior to analysis of FL activity. A 60% decrease in FL activity was seen in 293T cells transiently transfected with AST and treated with PDGF, compared to that of carrier control treated cells (p<0.05; Fig. 1c). On the other hand, PDGF treatment did not significantly decrease FL activity in 293T cells transiently transfected with ASA (p>0.05); thus, AST can be used to monitor both the activation and inhibition of Akt kinase activity.
AST Revealed Differential Kinetics of PI-3K Inhibitor LY294002 and Akt Kinase Inhibitor Perifosine on Inhibition of Akt Kinase Activity
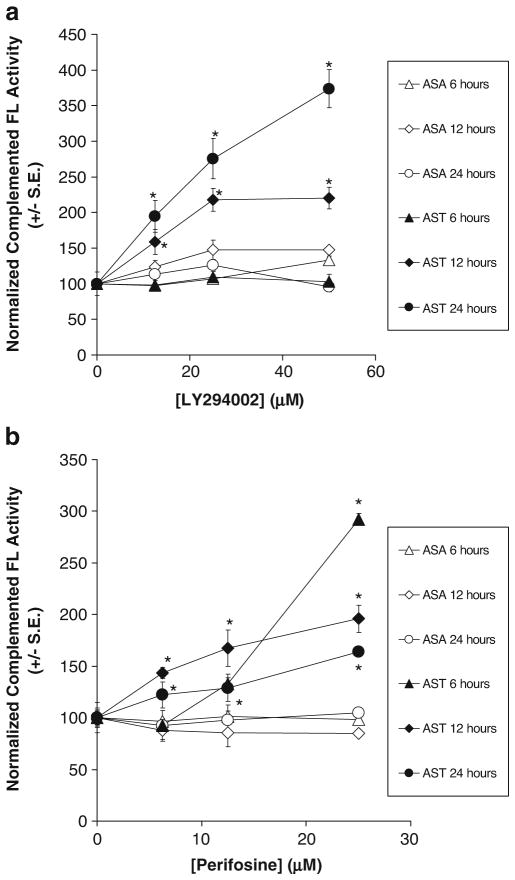
To circumvent the changes in expression of ASA and AST in transient transfection studies, 293T cells stably transfected with AST (293T/AST) or ASA (293T/ASA) were used for subsequent studies. To determine the kinetics and dynamic range of using AST to indirectly monitor the effect of LY on inhibition of Akt kinase activity, 293T/AST and 293T/ASA cells were treated with different concentrations of LY or carrier control (1% DMSO) for 6, 12, and 24 h. Complemented FL activity at each time point was determined by bioluminescence imaging of intact cells upon addition of D-luciferin (Fig. 2a). LY treatment led to a dose-dependent increase in complemented FL activity for 293T/AST at 12 and 24 h (p<0.05 at all LY concentrations) but not at 6 h (p>0.05), compared to that of 293T/ASA cells treated with same concentrations of LY.
Fig. 2.
Differential temporal- and dose-dependent increase in complemented FL activity by the PI-3K inhibitor LY294002 and Akt kinase inhibitor perifosine in 293T cells stably transfected with ASA (293T/ASA) or AST (293T/AST). a 293T/AST cells were treated with different concentrations of LY or carrier control for 6, 12, and 24 h prior to analysis of complemented FL activity by bioluminescence imaging of intact cells. Quantitation of bioluminescence signals in 293T/ASA and 293T/AST stable cells treated with LY was performed as follow: Total flux was determined by drawing ROI over each well with 5% border detection limit. Protein content in each well was determined upon cell lysis. Complemented FL activity in each well was normalized for protein content, averaged first and then normalized to carrier control treated cells. Each data point represents the average normalized complemented FL activity±SEM. *p<0.05 relative to ASA cells treated with same concentrations of LY for the same duration. b Increase complemented FL activities in 293T/ASA and 293T/AST cells upon treatment with Akt kinase inhibitor perifosine were determined as in a, except 0.1% ethanol was used as the carrier control. Quantitation of bioluminescence signals was performed as described (a). *p<0.05 relative to ASA cells treated with same concentrations of perifosine for the same duration.
To establish the kinetics of using AS to monitor the efficacy of the Akt kinase inhibitor perifosine, stable 293T/AST and 293T/ASA cells were treated with different concentrations of perifosine or carrier control (0.08% ethanol) for 6, 12, and 24 h prior to imaging of complemented FL activity and normalization of protein content, as described above for LY. Figure 2b shows that perifosine treatment led to a dose-dependent increase in complemented FL activity for 293T/AST at all time points and concentrations tested, compared to that of 293T/ASA cells (p<0.05). Furthermore, the maximum increase in complemented FL activity in 293T/AST cells occurred at 6 h, compared to that of 24 h for LY-treated 293T/AST cells. All together, these data show AST was sensitive and specific for indirect monitoring of Akt kinase activity in intact cells in response to both PI-3K and Akt kinase inhibitors.
AST was Reversible and Indirectly Monitored Changes in Endogenous Akt Kinase Activity
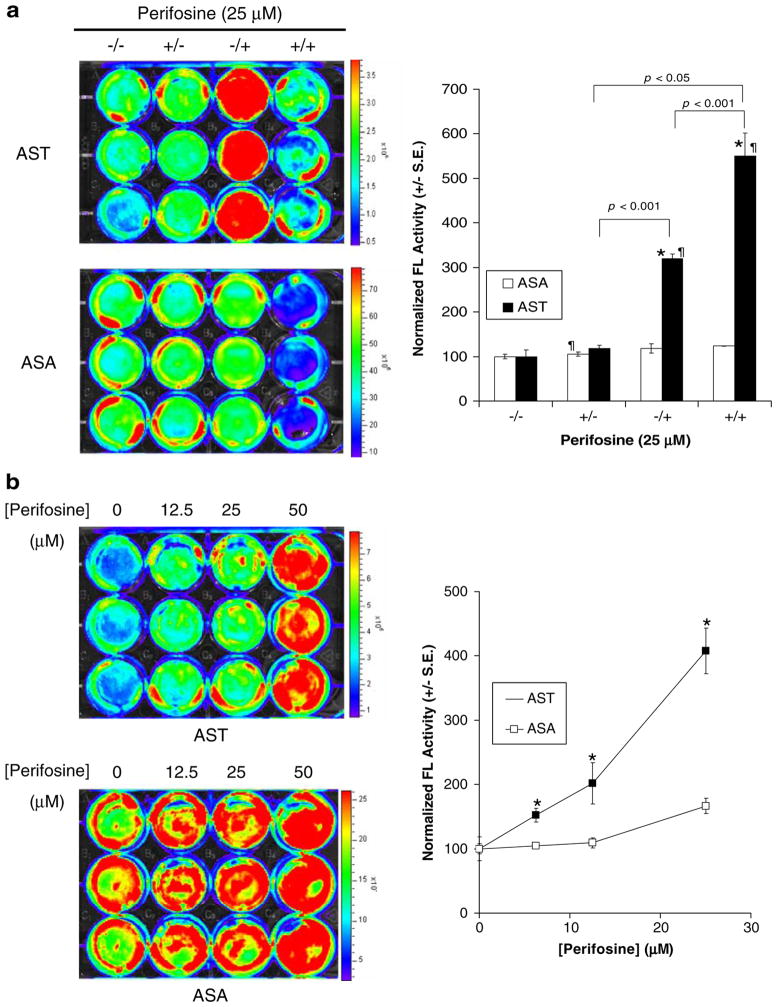
To determine the reversibility of AST to inhibition of Akt kinase activity by perifosine, 293T/AST and 293T/ASA cells were first treated with 25 μM of perifosine (6 h). This was followed by washout and replacement of medium with of 25 μM perifosine (+/+) or carrier control (+/−) for 6 h prior to determination of complemented FL activity and normalization for protein content as described above. 293T/AST stable cells that were first treated with carrier controls then with perifosine (−/+) served as positive controls, while 293T/AST and 293T/ASA cells treated with medium for the entire 12 h (−/−) served as negative controls. Figure 3a shows that normalized complemented FL activity in 293T/AST cells that was treated with perifosine followed by incubation with carrier control (+/−) was substantially lower than that of 293T/AST cells that were first treated with carrier control and then with perifosine (−/+, p<0.05) or with perifosine for the entire 12 h (+/+, p<0.05). The normalized complemented FL activity in 293T/AST cells treated with carrier control first followed by perifosine (−/+) or with perifosine for the entire 12 h (+/+) was also higher than that of corresponding 293T/ASA cells (p<0.05). Furthermore, there was no significant difference in complemented FL activities between all perifosine and carrier control treated 293T/ASA cells (p>0.05).
Fig. 3.
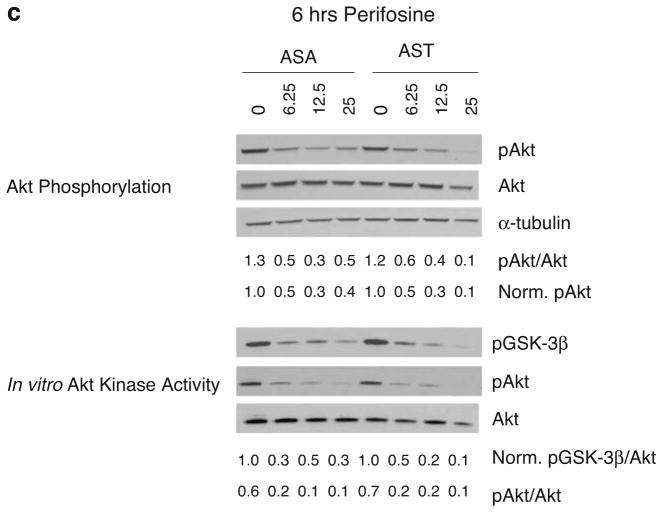
Validation of AST for indirect imaging of Akt kinase activity in cell culture studies. a Reversibility of AST for indirect monitoring of Akt kinase activity. 293T/AST and 293T/ASA cells were treated with 25 μM of perifosine for 6 h, prior to medium change with 25 μM perifosine (+/+) or carrier control (+/−) for another 6 h. Likewise, cells were treated with carrier control for 6 h, prior to medium change with 25 μM perifosine (−/+) or carrier control (−/−) for another 6 h. Complemented FL activities in 293T/AST cells was determined as in Fig. 2b (left) and normalized to for protein content and then to carrier control treated cells (right). *p<0.05 relative to ASA; ¶p<0.05 relative to carrier control treated cells. b Increase in complemented FL activities upon inhibition of Akt kinase activity by perifosine. 293T/ASA and 293T/AST cells were treated with 25 μM of perifosine or carrier control for 6 h. Complemented FL activity was determined by bioluminescence imaging (left), followed by normalization of protein content (right). *p<0.05 relative to ASA treated with the same concentrations of perifosine or carrier control for 6 h. c Increase in complemented FL activities corresponded to decrease in endogenous Akt phosphorylation and kinase activities in 293T/AST cells but not in 293T/ASA cells Akt phosphorylation and kinase activity in perifosine treated cells from b. (Top) The amount of phosphorylated Akt at Ser473 (pAkt) was determined by phosphorylation-specific Western blotting, followed by stripping and reprobing for total Akt. α-Tubulin was used as a loading control. The ratio of phosphorylated/total Akt, and normalized pAkt (to α-tubulin and to carrier control treated cells) were indicated. (Bottom) in vitro Akt kinase activity in cell lysates was determined using purified GSK-3β as a substrate. The amount of phosphorylated GSK-3β was normalized for amount of total Akt immunoprecipitated for each sample and to carrier control treated cells. The ratio of phosphorylated to total Akt for each sample was also indicated on the bottom.
To validate AST as a genetically encoded reporter for indirect imaging of endogenous Akt kinase activity, we examined the correlation between inhibition of endogenous Akt phosphorylation and kinase activities with increase in complemented FL activities in perifosine-treated 293T/AST cells. 293T/AST and 293T/ASA cells were treated with 25 μM of perifosine or carrier control for 6 h and complemented FL activities were determined as described in Fig. 2b. Akt phosphorylation and kinase activities in cell lysates were determined by phosphorylation-specific Western blotting and in vitro kinase assay using purified glycogen synthase kinase 3β (GSK-3β) as the substrate, respectively. Figure 3b shows the complemented FL activities 293T stably expressing AST was higher than that of ASA at all perifosine concentrations tested (p<0.05). At the same time, Fig. 3c shows that perifosine led to similar dose-dependent decrease in phosphorylated endogenous Akt (as determined by Western blotting using phospho-Akt (Ser473)-specific antibodies) and Akt kinase activity (as reflected by the decrease in the amount of phosphorylated GSK-3β by in vitro kinase assay/phospho-GSK3β Western blotting) for both 293T/ASA and 293T/AST cells. Thus, the increase in complemented FL activities correlates with decrease in endogenous Akt phosphorylation and kinase activity only for 293T/AST cells expressing the Akt sensor but not for 293T/ASA cells expressing the control sensor. Thus, our data demonstrated that AST was sensitive, specific, and reversible for indirect monitoring of endogenous Akt kinase activity in intact cells.
The Akt Inhibitor Perifosine is Shown to be Efficacious Using AS in Living Mice
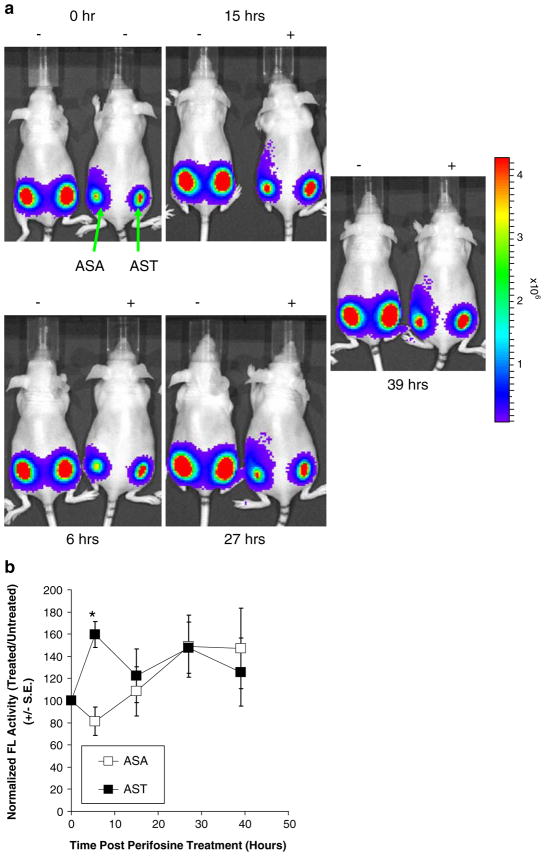
To noninvasively monitor the efficacy of perifosine in inhibition of Akt kinase activity in living subjects, 293T/AST and 293T/ASA cells were implanted at the lower left and right flank of nude mice for xenograft establishment. We chose perifosine over LY for our animal experiments since it is water soluble and currently being evaluated in clinical trials [30, 31]. Since the bioluminescence signals from untreated 293T/ASA cells was about 10-fold higher than that of 293T/AST cells in cell culture studies (Fig. 3), we mixed 10% 293T/ASA cells with 90% 293T cells (no FL activity) prior to implantation to normalize the signals to that of 293T/AST cells. Complemented FL activity in living mice was determined by bioluminescence imaging using a cooled CCD camera upon i.p. injection of D-luciferin at time 0 h. Mice were then treated with 30 mg/kg of perifosine or carrier control and re-imaged at 6, 15, 27, and 39 h thereafter. Figure 4a shows the bioluminescence images of complemented FL activity in 293T/AST (left) and 293T/ASA (right) xenografts at different time points post perifosine treatment. At 6 h, perifosine led to a 20% decrease in complemented FL activity in 293T/ASA but a 60% increase in complemented FL activity in 293T/AST relative to carrier control treated mice, corresponding to the 2-fold difference between AST and ASA (p<0.05; Fig. 4b). At later time points, there was no significant difference in complemented FL activity in 293T/AST and 293T/ASA relative to carrier control treated mice (p> 0.05). Thus, we have indirectly monitored the efficacy and specificity of perifosine in living mice by repetitive, noninvasive imaging of Akt kinase activity.
Fig. 4.
Inhibition of Akt kinase activity by perifosine in living mice. a The efficacy of perifosine in inhibition of Akt kinase activity in living mice was determined by bioluminescence imaging of nude mice bearing 293T/ASA (left) and 293T/AST (right) xenografts. Upon determination of base-line signals at time 0 h, mice were treated with 30 mg/kg of perifosine or carrier control and re-imaged at 6, 15, 27, and 39 h upon i.p. injection of D-luciferin. b Max photons at each time point was normalized to that of carrier control treated mice and expressed as average normalized max photons±SEM for each xenograft. *p<0.05 relative to mice bearing 293T/ASA xenografts.
AST can be Used for High-Throughput Screening of Novel Akt Kinase Inhibitors
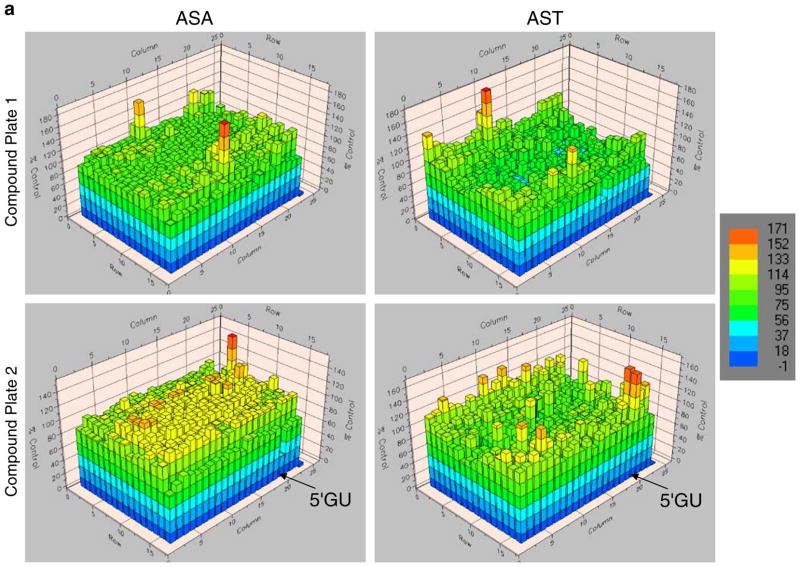
To utilize the AST system for identification of novel inhibitors of Akt kinase activity, a high-throughput drug-screening strategy was initially implemented using the 1280 LOPAC compound library. 293T/AST cells in 384-well format were treated with 17 μM of each compound (one compound per well for columns 3–22) for 24 h prior to determination of complemented FL activities and normalization to carrier control treated cells (columns 1–2). Inhibition of Akt kinase activities would lead to increase in complemented FL activities in 293T/AST cells, as in Figs. 2 and 3. 293T/ASA cells served a negative control for compounds that led to changes in bioluminescence signals independent of inhibition of Akt kinase activities. Figure 5a, b show the FL activities in 293T/AST (left columns) and 293T/ASA cells (right columns) in each well treated with compounds (plates 1–4). For each plate, cells in columns 1–2 were treated with medium, while columns 23–24 had no cells to indicate the background signals due to the FL substrate.
Fig. 5.
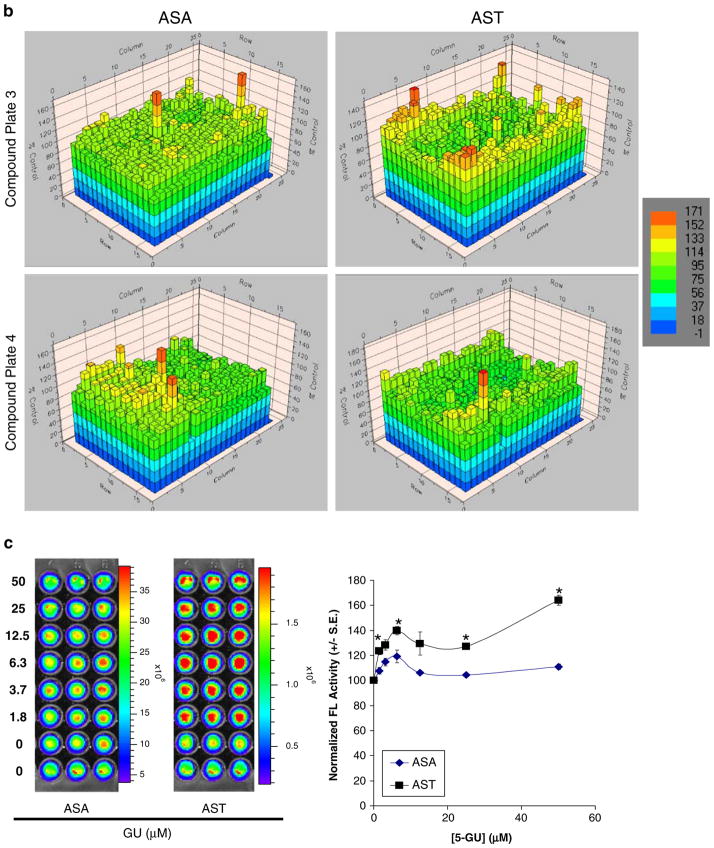
High-throughput screening of novel Akt kinase inhibitors using 293T/AST and 293T/ASA cells. a, b 293T/ASA (left columns) and 293T/AST (right columns) cells were treated with 17 μM of each of the 1280 LOPAC library compounds (plates 1–4) for 24 h prior to analysis of complemented FL activities and normalization to carrier control treated cells (100%). Cells in columns 1–2 were treated with medium alone, while columns 23–24 had no cells to indicate background signals due to substrate alone. Results of each well of the 384-well plate were shown as heat maps (color bar on the right). Location of the lead compound 5′GU was indicated with the arrow for both 293T/ASA and 293T/AST plates. c Validation of the mechanism of 5-GU as an inhibitor of Akt kinase activities. 293T/ASA and 293T/AST cells were plated in each of the 96 black-well plated and treated with different concentrations of the κ-opiate receptor antagonist 5-GU or carrier control for 24 h. Complemented FL activities were determined upon addition of D-luciferin, as described in Figs. 2 and 3 (left) followed by normalization of cell number (right). 5-GU led to selective increase in complemented FL activity in 293T/AST cells, relative to the control 293T/ASA cells. *p<0.05 relative to ASA cells treated with same concentrations of 5-GU.
Among all the compounds tested, 5′-guanidinonaltrin-dole di(trifluoroacetate) salt hydrate (5-GU; a κ-opiate receptor antagonist) led to the greatest difference in fold changes between 293T/AST and 293T/ASA cells (~5-fold). Activation of κ-opiate receptors led to activation of the PI-3K/Akt pathway [41]. To validate 5-GU as an inhibitor of Akt kinase activities, 293T/ASA and 293T/AST cells were treated with 5-GU for 24 h prior to analysis of complemented FL activities by bioluminescence imaging of intact cells, as described in Figs. 2 and 3. Quantitation of bioluminescence signals and normalization for cell number showed that 5-GU led to dose-dependent increase in complemented FL activities in 293T/AST cells to a greater extent compared to that of 293T/ASA cells (Fig. 5c). Since 293T cells express low levels of κ-opiate receptors, we are currently validating the specificity of 5′GU using MCF-7 cells, which express high levels of κ-opiate receptor and stably transfected with AST or ASA cells (unpublished data).
Discussion
One of the most important mechanisms of protein regulation and cellular function is phosphorylation/dephosphorylation, which leads to conformational changes that ultimately affect activity, localization, and stability of proteins [42]. Akt is one of the most prominent kinases due to the plethora of substrates it can act on and has also emerged as an active target for chemotherapy since it is involved in cell cycle regulation, apoptosis, and drug resistance [43]. In this study, we have indirectly monitored the Akt kinase activity in intact cells and in living mice using a previously developed novel split FL protein-fragment-assisted complementation system. There was a separate report that describes a development of split FL-based sensor for monitoring Akt kinase activity in cell culture and in xenograft models in living mice using a less optimal split sites for FL and different Akt substrate motif [44]. In this communication, our ultimate goal is to utilize and establish a platform for development of novel modulators of kinase activities [45]. Therefore, we have further extended our system for high-throughput screening of novel Akt kinase inhibitors using a small chemical library and validated the specificity of the lead compound 5-GU in inhibition of Akt kinase activities under cell culture conditions.
Sensitivity, Specificity, and Reversibility of AS
The feasibility of using split FL complementation-based sensors to monitor Akt phosphorylation was first established in BT474 cells treated with the PI-3K inhibitor LY to inhibit Akt phosphorylation and in 293T cells treated with PDGF to activate Akt phosphorylation. The genetically encoded AST and the corresponding negative control sensor ASA were subsequently stably transfected into 293T cells to evaluate their kinetics, sensitivity, and specificity for indirect monitoring of Akt kinase activity in intact cells in response to the LY and perifosine, which inhibit Akt activation [28]. The specificity of AST to indirectly monitor Akt kinase activity was further confirmed using ASA as a negative control sensor. Reversibility of AST to Akt inhibition by perifosine was established by wash-out experiments. Furthermore, the decreases in endogenous Akt phosphorylation and kinase activities by perifosine corresponded to increased complemented FL activity in 293T/AST but not for 293T/ASA cells. The efficacy of perifosine in inhibition of Akt kinase activity in living mice was also validated by noninvasive, repetitive optical bioluminescence imaging at different time points posttreatment.
Comparison of PI-3K and Akt Inhibitors in Their Kinetics of Inhibition of Akt Kinase Activity
Even though LY and perifosine both led to inhibition Akt phosphorylation and its subsequent activation, they exhibit different kinetics. Complemented FL activity in LY treated 293T/AST cells increased from 12 to 24 h (Fig. 2a, b). On the other hand, perifosine led to maximum increase in complemented FL activity at 6 h post treatment, followed by gradual decrease at 12 and 24 h (Fig. 2c, d). Since LY is an upstream inhibitor of Akt activation, it may take longer to inhibit Akt activation/kinase activity compared to perifosine which led to inhibition of Akt kinase activity [28]. Likewise, the difference kinetics between LY and perifosine may be in part due to their different solubility (LY is water insoluble, while perifosine is water soluble) and stability issues under cell culture conditions. Lastly, LY may be affecting other kinases and proteins in addition to PI-3K. In fact, Gharbi et al. [46] demonstrated that, in addition to PI-3K, LY also binds to other PI-3K related kinases, including targets that are unrelated to PI-3K family.
Comparison with Other Split Fluorescence Protein-Based Sensors
Even though both AST and FRET-based sensors (29–30) allow indirect imaging of Akt kinase activity, AST offers the advantages of (1) much greater dynamic range (4-fold maximum induction vs. 1.2-fold max induction for FRET-based sensors) in response to LY; (2) lower background for bioluminescence imaging in living mice compared to that of fluorescence imaging that requires external light excitation; and (3) the same system is currently extended for high-throughput screening of novel PI-3K/Akt inhibitors (130,000 compounds) and validation in living mice (unpublished data). The advantages of using a unified system that allows direct transition from cell culture into living mice include: (1) the immediate effects of PI-3K/Akt inhibitors on disruption of Akt kinase activity can be indirectly monitored; (2) pharmacokinetics and delivery in living mice can be directly optimized; (3) repeated monitoring of efficacy can be accomplished with molecular imaging; and (4) individual variations can be studied much better because each mouse serves as its own control.
Optimization of AST and Extension to Imaging Other Phosphorylation Events in Living Subjects
The AST sensor can be further optimized for their dynamic range and specificity using different split reporter systems that are compatible with both cell culture and animal imaging: (1) using different fragments and introducing mutations in split FL, split RL [47], and other luciferases; (2) different fusion strategies [1, 35]; (3) bioluminescence resonance energy transfer systems [48]; (4) modifying the peptide linkers that connect the split reporters with that of the AKM/FHA2 domains; and (5) novel substrates for luciferases with better pharmacokinetic and imaging properties in living subjects. The current genetically encoded reporters are now being generalized for studying different kinases/phosphorylation events and evaluating new inhibitors/modulators of signal transduction pathways in cell culture and in living subjects. This is being accomplished by replacing the AKM and FHA2 domains in AST with that of the kinases of interest and the appropriate cell lines (unpublished data).
Conclusion
Molecular imaging of phosphorylation events with pharmacological modulators in living subjects was accomplished using a novel split reporter strategy that can be utilized for studying phosphorylation events and screening the next generation of kinase inhibitors with improved pharmacodynamics and pharmacokinetic properties.
Acknowledgments
This study was supported by NIH RO1 CA082214 and NCI ICMIC P50 at Stanford University (S.S. Gambhir). C.T. Chan is supported in part by a postdoctoral fellowship award from Susan G. Komen for the Cure. We thank Dr. Tim Doyle of the Animal Imaging Facility at Stanford University for helpful discussion.
References
- 1.Paulmurugan R, Gambhir SS. An intramolecular folding sensor for imaging estrogen receptor–ligand interactions. Proc Natl Acad Sci U S A. 2006;103(43):15883–15888. doi: 10.1073/pnas.0607385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulmurugan R, Gambhir SS. Combinatorial library screening for developing an improved split-firefly luciferase fragment-assisted complementation system for studying protein–protein interactions. Anal Chem. 2007;79(6):2346–2353. doi: 10.1021/ac062053q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulmurugan R, et al. Imaging protein–protein interactions in living subjects. TrAC, Trends Anal Chem. 2005;24(5):446. [Google Scholar]
- 4.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17(5):545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 5.Massoud TF, Paulmurugan R, Gambhir SS. Molecular imaging of homodimeric protein–protein interactions in living subjects. Faseb J. 2004;18(10):1105–1107. doi: 10.1096/fj.03-1128fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen P. Protein kinases-the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1(4):309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 7.Bode AM, Dong Z, Kivie M. Progress in nucleic acid research and molecular biology. Academic Press; 2005. Signal transduction pathways in cancer development and as targets for cancer prevention; pp. 237–297. [DOI] [PubMed] [Google Scholar]
- 8.Andjelkovic M, et al. Domain swapping used to investigate the mechanism of protein kinase B regulation by 3-phosphoinositide-dependent protein kinase 1 and Ser473 kinase. Mol Cell Biol. 1999;19(7):5061–5072. doi: 10.1128/mcb.19.7.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananthanarayanan B, et al. Live-cell molecular analysis of Akt activation reveals roles for activation loop phosphorylation. J Biol Chem. 2007;282(50):36634–36641. doi: 10.1074/jbc.M706227200. [DOI] [PubMed] [Google Scholar]
- 10.Franke TF, et al. Direct regulation of the akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275(5300):665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 11.Calleja V, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5(4):e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki F, et al. Acquired resistance to Erlotinib in A-431 epidermoid cancer cells requires down-regulation of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res. 2007;67(12):5779–5788. doi: 10.1158/0008-5472.CAN-06-3020. [DOI] [PubMed] [Google Scholar]
- 13.Clark AS, et al. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Molec Cancer Ther. 2002;1(9):707–717. [PubMed] [Google Scholar]
- 14.Guo G, et al. Expression of ErbB2 enhances radiation-induced NF-kappaB activation. Oncogene. 2004;23(2):535–545. doi: 10.1038/sj.onc.1207149. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarti A, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22(10):1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 16.Chan C, Metz M, Kane S. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91:187–201. doi: 10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel MT, et al. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J Biol Chem. 2005;280(7):5581–5587. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki K, Sato M, Umezawa Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J Biol Chem. 2003;278(33):30945–30951. doi: 10.1074/jbc.M212167200. [DOI] [PubMed] [Google Scholar]
- 19.Millson SH, et al. Investigating the protein–protein interactions of the yeast Hsp90 chaperone system by two-hybrid analysis: potential uses and limitations of this approach. Cell Stress Chaperones. 2004;9(4):359–368. doi: 10.1379/CSC-29R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehrman T, et al. Protein–protein interactions monitored in mammalian cells via complementation of beta -lactamase enzyme fragments. Proc Natl Acad Sci U S A. 2002;99(6):3469–3474. doi: 10.1073/pnas.062043699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi FM, et al. Monitoring protein–protein interactions in live mammalian cells by beta-galactosidase complementation. Methods Enzymol. 2000;328:231–251. doi: 10.1016/s0076-6879(00)28400-0. [DOI] [PubMed] [Google Scholar]
- 22.Blakely BT, et al. Epidermal growth factor receptor dimerization monitored in live cells. Nat Biotechnol. 2000;18(2):218–222. doi: 10.1038/72686. [DOI] [PubMed] [Google Scholar]
- 23.Wehrman TS, et al. Enzymatic detection of protein translocation. Nat Methods. 2005;2(7):521–527. doi: 10.1038/nmeth771. [DOI] [PubMed] [Google Scholar]
- 24.Michnick SW, et al. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat Rev Drug Discov. 2007;6(7):569–82. doi: 10.1038/nrd2311. [DOI] [PubMed] [Google Scholar]
- 25.Remy I, et al. Application of protein-fragment complementation assays in cell biology. Biotechniques. 2007;42(2):137. doi: 10.2144/000112396. [DOI] [PubMed] [Google Scholar]
- 26.Kerppola TK, Kerppola TK. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc. 2006;1(3):1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerppola TK, Kerppola TK. Complementary methods for studies of protein interactions in living cells.[comment] Nat Methods. 2006;3(12):969–971. doi: 10.1038/nmeth1206-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondapaka SB, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2(11):1093–1103. [PubMed] [Google Scholar]
- 29.Raynaud FI, et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007;67(12):5840–5850. doi: 10.1158/0008-5472.CAN-06-4615. [DOI] [PubMed] [Google Scholar]
- 30.Van Ummersen L, et al. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10(22):7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 31.Bailey HH, et al. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107(10):2462–2467. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- 32.Malik SM, et al. Phase I study of RX-0201 in patients with advanced or metastatic solid tumors. J Clin Oncol. 2006;24(18_suppl):13102. [Google Scholar]
- 33.Mandal M, et al. The Akt inhibitor KP372–1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42(4):430–439. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawyers CL. Will kinase inhibitors have a dark side? N Engl J Med. 2006;355(3):313–315. doi: 10.1056/NEJMcibr062354. [DOI] [PubMed] [Google Scholar]
- 35.Paulmurugan R, Gambhir SS. Novel fusion protein approach for efficient high-throughput screening of small molecule-mediating protein–protein interactions in cells and living animals. Cancer Res. 2005;65(16):7413–7420. doi: 10.1158/0008-5472.CAN-05-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulmurugan R, et al. Molecular imaging of drug-modulated protein–protein interactions in living subjects. Cancer Res. 2004;64(6):2113–2119. doi: 10.1158/0008-5472.can-03-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luker GD, et al. Noninvasive imaging of protein–protein interactions in living animals. Proc Natl Acad Sci U S A. 2002;99(10):6961–6966. doi: 10.1073/pnas.092022399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luker KE, et al. Kinetics of regulated protein–protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101(33):12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JC, et al. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Molec Ther: J Am Soc Gene Ther. 2001;4(4):297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 40.Chan CT, et al. Molecular imaging of the efficacy of heat shock protein 90 inhibitors in living subjects. Cancer Res. 2008;68(1):216–226. doi: 10.1158/0008-5472.CAN-07-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kallergi G, et al. Distinct signaling pathways regulate differential opioid effects on actin cytoskeleton in malignant MCF7 and nonmalignant MCF12A human breast epithelial cells. Exp Cell Res. 2003;288(1):94–109. doi: 10.1016/s0014-4827(03)00210-6. [DOI] [PubMed] [Google Scholar]
- 42.Cohen P. The origins of protein phosphorylation. Nat Cell Biol. 2002;4(5):E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 43.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9(6):667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, et al. Molecular imaging of Akt kinase activity. Nat Med. 2007;13(9):1114–1119. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy BT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 46.Gharbi SI, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404(1):15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loening AM, et al. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng, Des Sel. 2006;19(9):391–400. doi: 10.1093/protein/gzl023. [DOI] [PubMed] [Google Scholar]
- 48.De A, Loening AM, Gambhir SS. An improved bioluminescence resonance energy transfer strategy for imaging intracellular events in single cells and living subjects. Cancer Res. 2007;67:7175–7183. doi: 10.1158/0008-5472.CAN-06-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]