Abstract
BACKGROUND
Epidemiology of diseases changes over time with changes in socio-economic status, culture and health care systems. Gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD) are among the diseases whose epidemiology has changed over the past few decades in the west. Studies addressing the trend of GERD and PUD occurrence in Iran are lacking. We aimed to look at the time trends of GERD and PUD in a referral endoscopy clinic in Tehran, Iran.
METHODS
All patients with dyspeptic symptoms who underwent upper GI endoscopy from 1993 to 2005 (inclusive) in a tertiary outpatient GI referral center in Tehran were enrolled. Erosive esophagitis (EE, used as a proxy for GERD as a whole), PUD, rapid urease test (RUT) status and demographic characteristics were recorded from the endoscopy reports according to the year the endoscopy was performed.
RESULTS
Over a period of 13 years, 8,029 endoscopic examinations were performed. The most common endoscopic diagnosis was EE that occurred in 4,808 patients (59.8%) followed by duodenal ulcer in 2,188 (27.3%) and gastric ulcer in 88 (1.1%). Over 13 years (1995-2005), the proportion of EE increased from 14.1% in 1993 to 75.1% in 2005 among dyspeptic patients in this referral clinic. The proportion of each grade of GERD according to the Los Angeles classification was as follows: GERD-A 76.0%, GERD-B 20.9%, GERD-C 2.8% and GERD-D 0.3%. RUT positivity decreased from 71.4% to 9.5% during the study period.
CONCLUSION
This study shows a remarkable increase in EE with a concomitant decrease in PUD and RUT positivity among dyspeptic patients in Tehran over a decade. This change in trend is important for future health care planning.
Keywords: Gastroesophageal reflux, Peptic ulcer disease, Endoscopy, Time Trend, Iran
INTRODUCTION
Gastro-esophageal reflux disease (GERD) is one of the most prevalent diseases worldwide.1-4 Clinical manifestations of GERD are diverse and include heartburn, acid regurgitation, dysphagia, non-cardiac chest pain and a variety of the so-called minor symptoms such as water brash, nausea, epigastric pain centered below the xiphoid process (subxiphoid pain) and other less well-established ones.5 Incompetence and/ or transient relaxation of the lower esophageal sphincter (LES) are important pathophysiological factors of GERD.6,7 Unhealthy lifestyle, including high-energy diets and fast foods, inactivity and obesity are among plausible risk factors for GERD.4, 8 Recent animal studies as well as ecological studies on humans have implied that dietary nitrates may be important in the pathogenesis of GERD.9-11
The cost of GERD, including direct medical costs as well as absenteeism from work is substantial.12 The annual direct and indirect costs of GERD in the United States are estimated to be more than 10 billion US dollars as compared
to that of peptic ulcer disease (PUD), which is estimated to be about 5 billion US dollars.12 Long-term consequences of GERD are also notable. Adenocarcinoma of the distal esophagus arising in Barrett’s epithelium is a related complication, the prevalence of which is rising.13-15 Despite these epidemiological links, the majority of patients with GERD will never suffer from a malignant transformation; therefore, this should be addressed when taking care of GERD patients to avoid unnecessary anxiety.
Diagnosis of GERD mostly relies on clinical symptoms. However, endoscopy is commonly performed to look for erosive esophagitis (EE), other concomitant upper GI disorders (e.g., PUD), and columnar metaplasia in the distal esophagus. Western studies have shown a decline in the prevalence and incidence of PUD in the past three decades, 16, 17 whereas GERD has increased. This may be partly due to decreasing prevalence of Helicobacter pylori in developed countries.18-21Surveys in Asian countries have also shown a similar trend, albeit at a lower pace,22-27 but large scale studies in our region are scarce. Hence, long-term studies to monitor longitudinal changes in the pattern of GERD and PUD among different generations are needed.
In the current study we have analyzed time trends of gastric and duodenal ulcers (GU and DU) and have compared them with concomitant time trends of EE as a proxy for GERD as a whole in a large dyspeptic population who referred to a tertiary outpatient GI referral center in Tehran.
MATERIALS AND METHODS
All patients who underwent upper gastrointestinal endoscopy in a referral GI center in Tehran, Iran from 1993 to the end of the year 2005 were enrolled in the present study. Patients came from both rural and urban areas.
All endoscopy reports were reviewed and their final diagnoses were recorded using the Los Angeles classification.28 The endoscopies were performed by a single gastroenterologist. Thus, there was no inter-observer variability in the diagnosis of EE.29, 30
Additional data recorded included age, sex, final diagnosis and rapid urease test results.
The prevalence of endoscopic findings was expressed as percentages. Between group differences were assessed using chi-square or student t-test. Binary logistic regression was used to determine the independent predictors of EE detected in endoscopy. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated, and p-values <0.05 were considered as significant. All variables with a p -value <0.05 in univariate analysis were included in the multivariate model. All data was analyzed using the Statistical Package for the Social Sciences for Windows (SPSS, Chicago, IL) version 16.00.
RESULTS
A total of 8,029 patients fulfilled the inclusion criteria. Among them,3, 942 (49.1%) were female. Mean age at presentation was 41.1 ± 14.9 years (range of 15 to 97 years). A total of 4,934 patients (61.5%) were found to have EE, 2,188 (27.3%) had DU and 88 (1.1%) had GU. Table 1 demonstrates demographic characteristics of the study population and the endoscopic findings according to the year of endoscopy. Figure 1 shows the time trends of EE, GU, DU, normal findings and positive urease test from 1993 to 2005.
Table 1 . The demographic characteristics of the study population, prevalence of EE, GU, DU and positive urease test, the proportion of males among EE and PUD patients, according to the year of endoscopy.
| year | N | Age (Mean ± SD) in years | Male N (%) |
EE
N (%) |
GU
N (%) |
DU
N (%) |
Normal
N (%) |
Positive Urease test |
EE
Males (%) |
PUD Males (%) |
| 1993 | 106 | 39.6 ± 14.5 | 56 (52.8%) | 15 (14.1%) | 2 (1.8%) | 44 (41.5%) | 90 (84.9%) | 71.4% | 69.2% | 65.9% |
| 1994 | 453 | 39.8 ± 14.9 | 237 (52.3%) | 116 (25.6%) | 9 (2.0%) | 151 (33.3%) | 252 (55.6%) | 79.9% | 60.3% | 62.3% |
| 1995 | 629 | 39.7 ± 13.5 | 340 (54.1%) | 241 (38.3%) | 10 (1.6%) | 223 (35.5%) | 337 (53.6%) | 75.4% | 57.7% | 66.8% |
| 1996 | 832 | 39.9 ± 14.4 | 459 (55.2%) | 338 (40.6%) | 16 (1.9%) | 318 (38.2%) | 327 (39.3%) | 74.9% | 58.9% | 65.7% |
| 1997 | 971 | 40.2 ± 14.4 | 471 (48.5%) | 345 (35.5%) | 12 (1.2%) | 303 (31.2%) | 377 (38.8%) | 70.0% | 54.5% | 60.7% |
| 1998 | 905 | 41.5 ± 15.9 | 446 (49.3%) | 582 (64.3%) | 9 (1.0%) | 303 (33.5%) | 236 (26.1%) | 63.9% | 49.7% | 57.8% |
| 1999 | 800 | 40.1 ± 15.1 | 426 (53.2%) | 641 (80.1%) | 2 (0.3%) | 242 (30.3%) | 124 (15.5%) | 64.3% | 54.5% | 64.5% |
| 2000 | 660 | 41.0 ± 14.9 | 341 (51.7%) | 494 (74.8%) | 1 (0.2%) | 185 (28.0%) | 129 (19.5%) | 62.7% | 53.6% | 68.0% |
| 2001 | 521 | 41.9 ± 14.8 | 262 (50.3%) | 397 (76.2%) | 2 (0.4%) | 134 (25.7%) | 126 (24.2%) | 65.1% | 51.3% | 69.8% |
| 2002 | 380 | 42.8 ± 14.6 | 186 (48.9%) | 296 (77.9%) | 1 (0.3%) | 70 (18.4%) | 99 (26.1%) | 68.4% | 51.0% | 67.2% |
| 2003 | 634 | 41.9 ± 14.8 | 296 (46.7%) | 507 (80.0%) | 10 (1.6%) | 70 (11.1%) | 109 (17.2%) | 62.0% | 47.4% | 71.4% |
| 2004 | 668 | 42.4 ± 15.5 | 341 (51.0%) | 498 (74.6%) | 10 (1.5%) | 87 (13.0%) | 146 (21.9%) | 44.0% | 55.8% | 69.1% |
| 2005 | 470 | 44.2 ± 14.4 | 226 (48.1%) | 353 (75.1%) | 4 (0.9%) | 58 (12.3%) | 98 (20.8%) | 49.5% | 49.3% | 70.1% |
| Total | 8029 | 41.1 ± 14.9 | 4078 (50.9%) | 4808 (59.8%) | 88 (1.1%) | 2188 (27.3%) | 2450 (30.5%) | 65.3% | 53.1% | 63.0% |
Figure 1 .
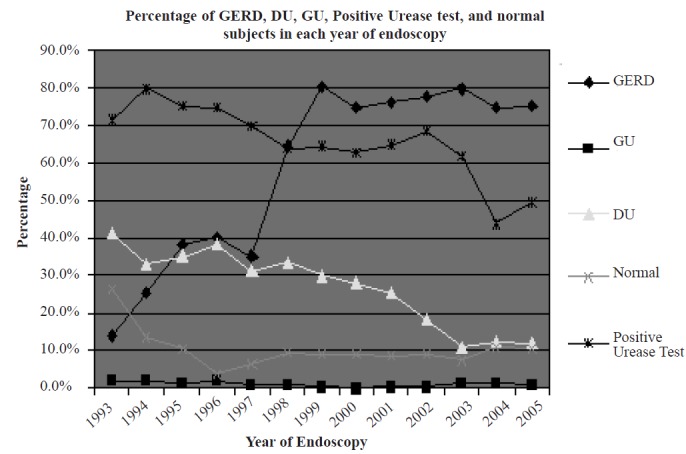
Prevalence rates of endoscopic findings and positive urease test results by year of endoscopy
EE shows a significant rise during 13 years of follow-up (p<0.001), while both types of PUD (GU and DU) show a significant decline (p <0.001). It seems that the decline in DU prevalence is greater than GU, but this could not be shown statistically, because of the low prevalence of GU. The proportion of positive urease test results also shows a significant decline (p<0.001).
We also found that positive rapid urease test results were more prevalent among patients suffering from PUD (82.4% versus 58.5%, p<0.001). On the other hand, patients having EE were slightly less likely to have a positive rapid urease test (62.7% versus 69.5%, p<0.001).
There was no significant difference in mean age between the three categories of EE, GU, and DU. Figure 2 shows the distribution of EE severity according to the LA classification over different years. The trend has been depicted from 2000 to 2005, e.g., the era when the LA classification was introduced and gained popularity. As shown in Figure 2, there is no difference in the percentage of the three EE categories from 2000 to 2005.
Fig. 2 .
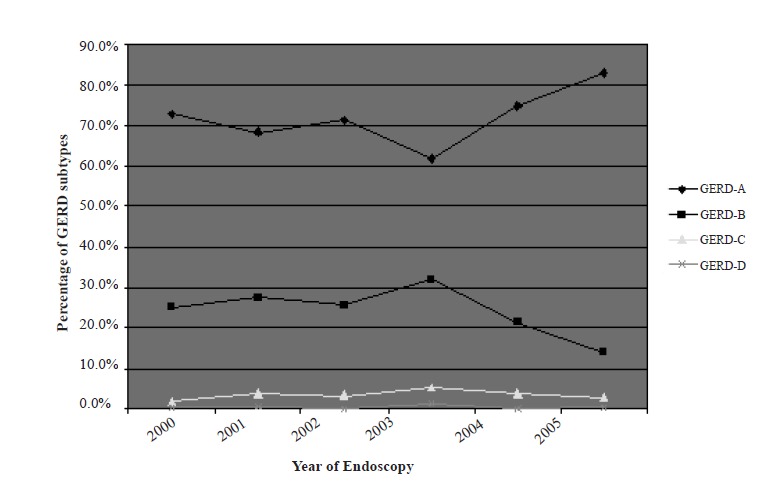
Percentages of GERD subtypes by year of endoscopy.
Table 2 shows the relation between EE prevalence and year of endoscopy, age, gender and results of the rapid urease test.
Table 2 . Univariate logistic regression of GERD on year of endoscopy, age, gender and results of the urease test.
| Variables | OR | 95% Confidence interval | p -value |
| Year of endoscopy | 1.25 | 1.23-1.27 | <0.001 |
| Age(for each 10 years) | 1. 02 | 0.99-1.006 | 0.159 |
| Gender | |||
| Female | 1 | ||
| Male | 1.22 | 1.11-1.35 | <0.001 |
| Urease test | |||
| Negative | 1 | ||
| Positive | 0.72 | 0.65-0.81 | <0.001 |
As noticed in the table, the OR is significant for year of endoscopy, male gender and positive urease test. However, in the final multivariate logistic regression demonstrated in Table 3, only the year of endoscopy and gender remain in the model. A similar analysis was done for PUD and as shown in Tables 4 and 5, the OR for the year of the endoscopy, age, male gender and positive urease test was significant in both univariate and multivariate regression.
Table 3 . Multivariate logistic regression of GERD on independent variables using backward Wald method.
| Variables in the equation OR | 95% Confidence interval | p -value | |
| Year of endoscopy | 1.26 | 1.24-1.28 | <0.001 |
| Gender | |||
| Female | 1 | ||
| Male | 1.45 | 1.29-1.62 | <0.001 |
Table 4 . Univariate logistic regression of PUD on year of endoscopy, age, gender and results of the urease test.
| Variables tested | OR | 95% Confidence interval | p -value |
| Year of endoscopy | 0.86 | 0.85-0.88 | <0.001 |
| Age(for each 10 years) | 1.06 | 1.02-1.09 | 0.002 |
| Gender | |||
| Female | 1 | ||
| Male | 1.99 | 1.79-2.22 | <0.001 |
| Urease test | |||
| Negative | 1 | ||
| Positive | 3.33 | 2.90-3.82 | <0.001 |
Table 5 . Multivariate logistic regression of PUD on independent variables using backward Wald method.
| Variables in the equation | OR | 95% Confidence interval | p -value |
| Year of endoscopy | 0.86 | 0.85-0.88 | <0.001 |
| Age(for each 10 years) | 1. 07 | 1.03-1.12 | 0.002 |
| Gender | |||
| Female | 1 | ||
| Male | 1.99 | 1.79-2.25 | <0.001 |
| Urease test | |||
| Negative | 1 | ||
| Positive | 2.81 | 2.44-3.24 | <0.001 |
DISCUSSION
This study shows two distinct and opposing patterns for common diseases that affect the gastro-duodenum and esophagus. PUD prevalence has decreased continuously during recent years; on the other hand, EE has increased over the same period of time. Both types of PUD are strongly correlated with gastric infection by H. pylori.31H. pylori seems to play an essential role in the pathogenesis of PUD and the general decline in its prevalence rate provides the most likely explanation for the time trends of these three diseases. Although the prevalence of H. pylori is very high in Iran32, its prevalence is declining. Resolution of H. pylori induced atrophic change in the gastric corpus results in restoration of acid secretion. This in turn may be responsible for development or worsening EE.33, 34
Due to the limitation of this study regarding its retrospective nature that reflects the experience of a single center, the results cannot be extrapolated to the general population. But we believe that the merits of this study lie in the large number of patients involved and relatively high diagnostic accuracy.
We found that EE is becoming more prevalent in the Iranian population, which supports previous studies in Iran.35, 36 GERD-A is the most common type encountered and this is also similarly reported by others.37-39 GERD-B and GERD-C also show a rising pattern, especially in male patients. There was a slight male preponderance in the study patients which is in accordance with previous studies.26, 38
Based on logistic regression, year of the endoscopy and male gender are independent predictors of EE, which is congruent with previous studies.40 Year of the endoscopy is inversely associated with PUD, while age, male gender and positive urease test are all predictors of PUD. Also, we have observed that time trends of PUD and EE are in opposing directions which may be due to the decline in H. pylori infection. We believe that careful population- based studies should be carried out to elucidate the true prevalence rates of different forms of GERD in the general population.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
REFERENCES
- 1.Spechler SJ. Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion. 1992;51:24–9. doi: 10.1159/000200911. [DOI] [PubMed] [Google Scholar]
- 2. Johansson KE , Tibbling L . Esophageal body motor disturbances in gastroesophageal reflux and the effects of fundoplication. Scand J Gastroenterol Suppl. 1988;155:82–8. doi: 10.3109/00365528809096289. [DOI] [PubMed] [Google Scholar]
- 3.Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol. 2009;44:518–34. doi: 10.1007/s00535-009-0047-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti H, Achem SR, Hinder RA. Impact of changing epidemiology of gastroesophageal reflux disease on its diagnosis and treatment. J Gastrointest Surg. 2008;12:373–81. doi: 10.1007/s11605-007-0294-9. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenberg A, Townsend WF, Muller AD. Evaluation of dyspepsia and functional gastrointestinal disorders: a cost-benefit analysis of different approaches. Eur J Gastroenterol Hepatol. 1995;7:655–9. [PubMed] [Google Scholar]
- 6.Dent J, Dodds WJ, Friedman RH, Sekiguchi T, Hogan WJ, Arndorfer RC. et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–67. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castell DO. The lower esophageal sphincterPhysiologic and clinical aspects. Ann Intern Med. 1975;83:390–401. doi: 10.7326/0003-4819-83-3-390. [DOI] [PubMed] [Google Scholar]
- 8.Festi D, Scaioli E, Baldi F, Vestito A, Pasqui F, Di Biase AR. et al. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J Gastroenterol. 2009;15:1690–1701. doi: 10.3748/wjg.15.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo H, Iijima K, Asanuma K, Ara N, Ito H, Asano N. et al. Exogenous luminal nitric oxide exposure accelerates columnar transformation of rat esophagus. Int J Cancer. 2010;127:2009–19. doi: 10.1002/ijc.25227. [DOI] [PubMed] [Google Scholar]
- 10.Ishiyama F, Iijima K, Asanuma K, Ara N, Yoshitake J, Abe Y. et al. Exogenous luminal nitric oxide exacerbates esophagus tissue damage in a reflux esophagitis model of rats. Scand J Gastroenterol. 2009;44:527–37. doi: 10.1080/00365520802699260. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Iijima K, Moriya A, McElroy K, Scobie G, Fyfe V. et al. Conditions for acid catalysed luminal nitrosation are maximal at the gastric cardia. Gut. 2003;52:1095–101. doi: 10.1136/gut.52.8.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veldhuyzen van Zanten SJ, Sherman PM. Indications for treatment of Helicobacter pylori infection: a systematic overview. CMAJ. 1994;150:189–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12:576–3. [PubMed] [Google Scholar]
- 14.Crane SJ, Locke GR, 3rd 3rd, Harmsen WS, Diehl NN, Zinsmeister AR, Melton LJ, 3rd 3rd. et al. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol. 2007;102:1596–602. doi: 10.1111/j.1572-0241.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 15.Haghdoost AA, Hosseini H, Chamani G, Zarei MR, Rad M, Hashemipoor M. et al. Rising incidence of adenocarcinoma of the esophagus in Kerman, Iran. Arch Iran Med. 2008;11:364–70. [PubMed] [Google Scholar]
- 16.Sonnenberg A. Temporal trends and geographical variations of peptic ulcer disease. Aliment Pharmacol Ther. 1995;9:3–12. [PubMed] [Google Scholar]
- 17.Boring CC, Squires TS, and Tong T. Cancer statistics 1992. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Schutze K, Hentschel E, Dragosics B, and Hirschl AM. Helicobacter pylori reinfection with identical organisms: transmission by the patients’ spouses. Gut. 1995;36:831–3. doi: 10.1136/gut.36.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labenz J, Blum AL, Bayerdorffer E, Meining A, Stolte M, Borsch G. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442–7. doi: 10.1016/s0016-5085(97)70024-6. [DOI] [PubMed] [Google Scholar]
- 20.Holtmann G. Reflux disease: the disorder of the third millennium. Eur J Gastroenterol Hepatol. 2001;13:S5–11. [PubMed] [Google Scholar]
- 21.Gisbert JP, Pajares JM, Losa C. Helicobacter pylori and gastroesophageal reflux disease: friends or foes? Hepatogastroenterology. 1999;46:1023–9. [PubMed] [Google Scholar]
- 22.Yeom JS, Park HJ, Cho JS, Lee SI, Park IS. Reflux esophagitis and its relationship to hiatal hernia. J Korean Med Sci. 1999;14:253–6. doi: 10.3346/jkms.1999.14.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee PL. Association between Helicobacter pylori and gastro-esophageal reflux disease. Korean J Gastroenterol. 2003;42:179–82. [PubMed] [Google Scholar]
- 24.Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett’s esophagus: the long and short of it all. Dig Dis Sci. 2004;49:237–42. doi: 10.1023/b:ddas.0000017444.30792.94. [DOI] [PubMed] [Google Scholar]
- 25.Khoshbaten M. Gastro-esophageal reflux disease in northwestern Tabriz, Iran. Indian J Gastroenterol. 2003;22:138–9. [PubMed] [Google Scholar]
- 26.Heading RC. Epidemiology of oesophageal reflux disease. Scand J Gastroenterol Suppl. 1989;24:33–7. [PubMed] [Google Scholar]
- 27.Li YM, Du J, Zhang H, Yu CH. Epidemiological investigation in outpatients with symptomatic gastroesophageal reflux from the Department of Medicine in Zhejiang Province, east China. J Gastroenterol Hepatol. 2008;23:283–9. doi: 10.1111/j.1440-1746.2007.05045.x. [DOI] [PubMed] [Google Scholar]
- 28.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP. et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasseri-Moghaddam S, Razjouyan H, Nouraei M, Alimohammadi M, Mamarabadi M, Vahedi H. et al. Inter- and intra-observer variability of the Los Angeles classification: a reassessment. Arch Iran Med. 2007;10:48–53. [PubMed] [Google Scholar]
- 30.Dent J. Endoscopic grading of reflux oesophagitis: the past, present and future. Best Pract Res Clin Gastroenterol. 2008;22:585–99. doi: 10.1016/j.bpg.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–81. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S. et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sipponen P, Seppala K, Aarynen M, Helske T, Kettunen P. Chronic gastritis and gastroduodenal ulcer: a case control study on risk of coexisting duodenal or gastric ulcer in patients with gastritis. Gut. 1989;30:922–9. doi: 10.1136/gut.30.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 35.Nouraie M, Razjouyan H, Assady M, Malekzadeh R, Nasseri-Moghaddam S. Epidemiology of gastroesophageal reflux symptoms in Tehran, Iran: a population-based telephone survey. Arch Iran Med. 2007;10:289–94. [PubMed] [Google Scholar]
- 36.Ehsani MJ, Maleki I, Mohammadzadeh F, Mashayekh A. Epidemiology of gastroesophageal reflux disease in Tehran, Iran. J Gastroenterol Hepatol. 2007;22:1419–22. doi: 10.1111/j.1440-1746.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto K, Iwakiri R, Okamoto K, Oda K, Tanaka A, Tsunada S. et al. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol. 2003;38:3–6. [PubMed] [Google Scholar]
- 38.el-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut. 1997;41:594–9. doi: 10.1136/gut.41.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borsch G, Gude C. Diagnostic yield of upper gastrointestinal endoscopy in relation to age: retrospective analysis of 8,043 patients. Hepatogastroenterology. 1989;36:113–9. [PubMed] [Google Scholar]
- 40.Ho KY, Chan YH, Kang JY. Increasing trend of reflux esophagitis and decreasing trend of Helicobacter pylori infection in patients from a multiethnic Asian country. Am J Gastroenterol. 2005;100:1923–8. doi: 10.1111/j.1572-0241.2005.50138.x. [DOI] [PubMed] [Google Scholar]


