Abstract
BACKGROUND
Symptoms of gastro-esophageal reflux disease (GERD) affect health-related quality of life (HRQOL). When a questionnaire is translated into a new language, linguistic validation is necessary, yet insufficient, unless the psychometric characteristics have been verified. The aim of this study is to document the translation and psychometric validation of the Persian translation of the Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire.
METHODS
After translation and cultural adaptation of QOLRAD to Persian, fifty patients with clinical GERD from the Prospective Acid Reflux Study of Iran (PARSI) database who had at least one of the symptoms of acid regurgitation, heartburn, non-cardiac chest pain, or dysphagia for at least four weeks over the past three months completed the QOLRAD and Short Form Health Survey-36 (SF-36). After two weeks, QOLRAD was again completed by the patients. Cronbach alpha and Intra-class Correlation Coefficient (ICC) were used to test reliability and Pearson correlation was used to compare the dimensions of SF-36 and QOLRAD.
RESULTS
The translation was approved by MAPI Research Institute. Fifty patients completed the SF-36 and QOLRAD questionnaires at the first visit. Mean (SD) age of the participants was 38.4 (14.6) years and 68% were females. The internal consistency and reliability of QOLRAD ranged from 0.78–0.92. The test-retest reliability of QOLRAD was from 0.87–0.93. Relevant QOLRAD domains significantly correlated with the majority of SF-36 domains, with the exception of sleep disturbance.
CONCLUSION
The psychometric characteristics of the Persian translation of QOLRAD were found to be good, with satisfactory reliability and validity.
Keywords: QOLRAD, Questionnaire, Validation, Iran
INTRODUCTION
Gastroesophageal reflux disease (GERD) is one of the most common causes for referral to general practitioners and specialists. Evaluation of reflux with heart burn and acid regurgitation, the most relevant symptoms to GERD, are considered the best way to suspect and diagnose the disease.1 In addition to being a symptom of GERD, heartburn affects other aspects of patients’ lives such as their daily activities, personal relations, ability to have a good night sleep and unrestricted eating and drinking.2 Symptom assessment, management and resolution remain the goals of medical intervention for both patients and physicians. Symptoms affect health-related quality of life (HRQOL) and treatment satisfaction. HRQOL instruments [e.g., Quality of Life in Reflux and Dyspepsia (QOLRAD)3, Reflux-Qual Short Form4, and GERDyzer 5] measure the influence of disease on the patient’s physical, psychological and social function, and can be considered as one of the endpoints in treatment of diseases such as GERD. Both HRQOL and treatment satisfaction tools aim to provide additional dimensions of treatment response which may influence a patient’s choice of medication or compliance with therapy, but are not usually captured by assessing only symptom frequency or severity. However, both improved quality of life and greater treatment satisfaction correlate with reduced GERD symptom severity and/or frequency.3,6
QOLRAD is one of the most popular questionnaires for this purpose.1 Patient reported questionnaires must be documented to meet scientific standards and linguistic validation as well as cultural adaptation in order to be useful in a new cultural population. QOLRAD has been translated and validated in different languages and used to assess the impact of GERD on patients’ HRQOL. Its factor structure was also replicated in several translations.1, 2, 7-13, In this study, we translated and assessed the reliability and validity of the Persian version of the QOLRAD (P-QOLRAD) in Iranian patients with GERD.
MATERIALS AND METHODS
The Prospective Acid Reflux Study of Iran (PARSI) database for selection of patients was used. Briefly, the PARSI is designed to follow 1,250 volunteer GERD patients for five years in its initial phase. All participants undergo a structured interview covering detailed demographic, habitual and clinical data as well as their past medical history.14, 15 Frequency and severity of heartburn symptoms were also recorded and a composite symptom score calculated. Short Form Health Survey-36 (SF-36) was used as a means for comparing the validity of P-QOLRAD. We used test-retest to assess reliability of P-QOLRAD. The heartburn version of the QOLRAD is a disease specific instrument and contains 25 questions addressing concerns associated with gastrointestinal symptoms.1 The questions are rated on a seven- grade Likert scale; the lower the value, the more severe the impact on daily functions.11-13 The questions are categorized into five areas: emotional distress (six questions), sleep disturbance (five questions), vitality (three questions), food/drink problems (six questions) and physical/social functioning (five questions).
The study protocol and consent form were approved by the Digestive Disease Research Center Ethics Committee in accordance with the revised Declaration of Helsinki. The patients were free to discontinue participation in the study at any time without affecting their medical care.
Short Form Health Survey-36 (SF-36)
The SF-36 is an extensively used generic questionnaire containing 36 items clustered in eight dimensions.16 Item scores for each dimension are coded, summed and transformed to a scale from 0 (worst possible health state) to 100 (best possible health state). The SF-36 is well documented in terms of reliability and validity in other languages17-19as well as in Persian.15 This study used the acute version of the SF-36, e.g., a one week recall period.
Cultural adaptation
The linguistic validation of a patient reported outcomes (PRO) instrument is the first step in the process called cultural adaptation which has two validation phases: linguistic and psychometric.20, 21 The aim of the linguistic validation of a PRO questionnaire is the production of a conceptually equivalent version in a language other than the original language. Linguistic validation was done according to MAPI Research Institute guidelines (Table 1).22 The MAPI Research Institute is an organization which assists research worldwide to produce target language versions of questionnaire instruments that are conceptually equivalent to the original source instruments. Such documents are pivotal in obtaining accurate information, data pooling and/or comparisons across countries (http://www.mapi-research. fr/i-02-meth.htm).
Table 1 . Steps of linguistic validation (the MAPI Protocol) .
| Stages | Questionnaire |
| Forward translation by two independent translators | Forward translation A1 (Persian) and forward translation A2 (Persian) |
| Merging session (analysis and reconciliation) with presence of translators and the executive manager | Forward translation B |
| Backward translation by another independent translator | Backward translation (English) |
| Comparing the main questionnaire with the backward translation by the MAPI Research Institute | Forward translation (Persian) C |
| Review by physician interested in GERD | Forward translation (Persian) D |
| Final checking and amendment | Forward translation (Persian) E |
Psychometric validation
Reliability
Internal consistency refers to the extent to which the items are interrelated. Cronbach’s coefficient is the method most widely used to assess internal consistency. Cronbach’s alpha was calculated in each dimension of the instruments to assess the internal consistency reliability. A Cronbach-alpha coefficient ≥0.70 is considered as excellent.15 Test-retest reliability refers to the stability of a score derived from serial administrations of a measure by the same rater. Repeated measurements are made in the same individuals, presumably with a time interval long enough to ensure independence. Here, patients in the stable phase (between visits one and two) and in whom the treatment remained unchanged were assessed. A reliability coefficient above 0.7015 was considered acceptable.
Construct validity:
Construct validity is the relationship between the new questionnaire and accepted references (SF-36). Good correlation is considered to be present if Pearson Correlation is ≥0.60. Values between 0.30 and 0.60 point to a moderate correlation and values less than 0.3 as unacceptable correlation.2 To assess the construct validity of P-QOLRAD (e.g., comparability of QOLRAD performance to measure the desired domains effectively with an existing tool), we compared the P-QOLRAD with the PARSI questionnaire.
Discriminative validity of the instruments
Correlating QOLRAD with the severity and frequency of GERD symptoms also tested the discriminant validity of this instrument. Physician-assessed overall severity of symptoms and its relation to the QOLRAD dimensions were also evaluated. The PARSI database information was used to assess severity of GERD symptoms and the SF-36 summary scores were calculated for the physical component summary scale (PCS) and the mental component summary scale (MCS) based on Iranian data.16
According to previous studies, a picture which shows the site of sensing “heartburn” was added to the first page to make the questionnaire more user-friendly and provide face validity.20 Content validity of the questionnaire was assessed in its original language.1 Test-retest was used to check reliability.20
After validation of the translated QOLRAD, we selected patients with gastro-esophageal reflux who were enrolled in the PARSI database. Patients had to be able to complete the Patient-Reported Outcomes, PRO instruments themselves and be able to read and write in the Persian language. Patients completed two PRO instruments: QOLRAD and SF-36. QOLRAD was completed at the first and second visits (two weeks apart) and SF-36 was filled out only once at the first visit.
Statistical methods
In case of missing data in the QOLRAD, the mean of the completed items in one dimension was imputed to substitute for the missing item, provided that more than 50% of the items in one dimension were completed.23 Cronbach’s alpha and intra-class correlation coefficient (ICC) were used to test reliability and Pearson correlation to compare dimensions of SF-36 and QOLRAD.24 A p value of <0.05 was considered significant. The relationship of QOLRAD scores to various domains of SF-36 was calculated as a means of determining concurrent validity. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Fifty GERD patients completed both the QOLRAD and SF-36. Of these,22 returned for the retest [response rate: 44%, mean age (SD): 38.4 (14.6) years, range: 15-77 years]. Demographics are shown in Table 2. Most recruited patients had mild or moderate severity of clinical GERD according to their symptom scores (mild: 48%, moderate: 48%, severe: 4%). Cronbach’s alpha measure for QOLRAD dimensions ranged from 0.781 (vitality) to 0.926 (emotional distress). In QOLRAD, the ICC ranged from 0.83 (food/drink problems) to 0.94 (physical/social functioning), respectively (Table 3).
Table 2 . Demographic details of patients at the baseline visit (N=50) .
| Variables | Number | Percent | |
| Female/Male | 34/16 | 68/32 | |
| Education | |||
| High school or less | 10 | 20 | |
| High school diploma | 20 | 40 | |
| University degree | 19 | 38 | |
| PhD or doctor | 1 | 2 |
Table 3 . Cronbach’s alpha at visit 1 and test-retest reliability (ICC) for QOLRAD .
| QOLRAD domains | Cronbach’s alpha | ICC (95 % confidence interval) |
| Emotional distress | 0.926 | 0.927 (0.820-0.970) |
| Food/drink problems | 0.864 | 0.837 (0.598-0.934) |
| Physical/social functioning | 0.838 | 0.935 (0.844-0.973) |
| Sleep disturbance | 0.856 | 0.889 (0.733-0.954) |
| Vitality | 0.781 | 0.871 (0.682-0.948) |
Convergent and discriminant validity of SF-36 and QOLRAD.
Pearson correlation coefficients used to assess the convergent and discriminant validity are shown in Table 4.
Table 4 . Pearson’s correlation coefficient between SF-36 and QOLRAD.
| QOLRAD | ||||||
| Instruments | Emotional distress | Sleep disturbance | Food/drink problems | Physical/social functioning | Vitality | |
| SF-36 | Bodily pain | 0.51* | 0.40* | 0.44* | 0.59* | 0.52* |
| General health | 0.53* | 0.19 | 0.45* | 0.43* | 0.28 | |
| Mental health | 0.51* | 0.11 | 0.28 | 0.43* | 0.26 | |
| Physical | 0.68* | 0.45* | 0.59* | 1.00* | 0.74* | |
| functioning | ||||||
| Role-emotional | 0.38* | -0.04 | 0.27 | 0.29* | 019 | |
| Role-physical | 0.38* | 0.05 | 0.35* | 0.58* | 0.34* | |
| Social | 0.49* | 0.12 | 0.33* | 0.61* | 0.39* | |
| functioning | ||||||
| Vitality | 0.68* | 0.11 | 0.49* | 0.58* | 0.39* | |
| PCS | 0.43* | 0.15 | 0.40* | 0.68* | 0.38* | |
| MCS | 0.58* | 0.08 | 0.42* | 0.53* | 0.31* | |
| General symptom score (GSS) | 0.04 | -0.36 | -0.19 | -0.18 | -0.30 | |
*Coefficients are significant at p<0.03.
There was a positive correlation between the QOLRAD and SF-36 in nearly all domains checked (QOLRAD domains “emotional distress”, “food/drink functioning” and “physical/social functioning” with all domains of SF-36 “physical functioning”, “vitality” and “bodily pain”). The QOLRAD domain “sleep disturbance” did not correlate with the respective SF-36 domains. The SF-36 domains “role emotional” correlated negatively with QOLRAD domains “sleep disturbance”. All domains of QOLRAD differentiated between mild and moderate groups of symptoms in GERD patients (Figure1) except for emotional disturbance.
Figure 1 .
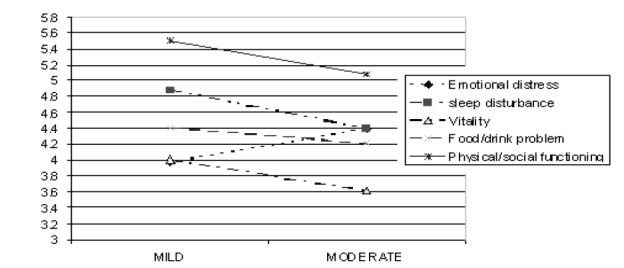
Severity of GE RD symptoms in PARSI and QOL RAD domain scores.
DISCUSSION
Cross-cultural validation studies are worthy for using an instrument in a setting other than its original milieu. PRO instruments make possible health status comparison between countries. They also provide validated instruments to monitor health of the population.16 One of the most established, validated, reliable and responsive instruments available in this area is the QOLRAD,1,2 which has been proven to have excellent psychometric characteristics when tested in clinical trials.7-13,25
The aims of the present study were to translate, and assess the reliability and validity of the Persian version of the QOLRAD in patients with GERD. According to our data, the internal consistency and reliability of the QOLRAD was quite high for this sample, suggesting that all items and subscales held together as a single conceptual unit. These results are comparable to those obtained from other studies.1, 2, 7-13 We were able to include 22 cases for test-retest; had this been done with more cases, the data would have been more reliable. Our data also support a good correlation for convergent validity between the domains of QOLRAD and SF36.
Clear and consistent associations were found between the SF-36 and QOLRAD domains,2, 7-13 in the original language as well as in studies done by Wiklund (0.44 to 0.71)1 and in German translation studies (0.37-0.71).2 Our data are also consistent with these findings. General symptom score (GSS) of patients, as assessed by PARSI data, shows a negative correlation with QOLRAD domains. This is because increased severity and frequency of GERD symptoms (e.g., increased GSS) can reduce quality of life. Our data, although not meant to assess this, revealed that GERD patients with moderate reflux symptoms had reduced QOL (Figure 1). The relevance of the sample of patients was confirmed since we have recruited patients suffering from heartburn and/or acid regurgitation from PARSI data base.
The primary goal of this study of documenting the psychometric characteristics of the Persian translation of the QOLRAD was achieved. regurgitation from PARSI data base. The primary goal of this study of documenting the psychometric characteristics of the Persian translation of the QOLRAD was achieved. The reliability of the most relevant QOLRAD domain was satisfactory, but the “sleep disturbance” domain was not optimal. The low reliability of this domain in our study may be due to a lower prevalence of night time GERD episodes in our patients than reported in other studies (10%7). The SF-36 does not have questions pertaining to sleep disturbance.26-28 More research is needed to explore this issue.
In conclusion, our data showed that all domains of the QOLRAD had excellent internal consistency and test-retest reliability. We should not forget that both long- and short term evaluations as well as an evaluation of the responsiveness of a questionnaire to treatment are important, of which all have been covered in the QOLRAD’s original language.29-32
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
REFERENCES
- 1.Wiklund IK, Junghard O, Grace E, Talley NJ, Kamm M, Veldhuyzen van zanten S. et al. Quality of life in reflux and dyspepsia patientsPsychometric documentation of a new disease-specific questionnaire (QOLRAD)Eur J Surg. suppl. 1998;583:41–9. [PubMed] [Google Scholar]
- 2.Kulich KR, Malfertheiner P, Madisch A, Labenz J, Bayerdörffer E, Miehlke S. et al. Psychometric validation of the German translation of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in patients with reflux disease. Health Qual Life Outcomes. 2003;1:62. doi: 10.1186/1477-7525-1-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degl’ Innocenti A, Guyatt GH, Wiklund I, Heels-Ansdell D, Armstrong D, Fallone CA. et al. The influence of demographic factors and health-related quality of life on treatment satisfaction in patients with gastroesophageal reflux disease treated with esomeprazole. Health Qual Life Outcomes. 2005;3:4. doi: 10.1186/1477-7525-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amouretti M, Nalet B, Robaszkiewicz M, Wainsten JP, de la Loge C, Benmedjahed K. et al. Validation of the short-form REFLUX-QUAL (RQS), a gastro-esophageal reflux disease (GERD) specific quality of life questionnaire. Gastroenterol Clin Biol. 2005;29:793–801. doi: 10.1016/s0399-8320(05)86350-x. [DOI] [PubMed] [Google Scholar]
- 5.Holtmann G, Chassany O, DeVault K. Validation of a new scale for the assessment of health related quality of life in gastroesophageal reflux disease (GERD) Gut. 2005;54:52. [Google Scholar]
- 6.Vakil N. Treatment of gastroesophageal reflux diseasedefining endpoints that are important to patients. Rev Gastroenterol Disord. 2004;4:S3–7. [PubMed] [Google Scholar]
- 7.Shaker R, Brunton S, Elfant A, Golopol L, Ruoff G, Stanghellini V. Review article: impact of night-time reflux on lifestyle - unrecognized issues in reflux disease. Aliment Pharmacol Ther. 2004;20:3–13. doi: 10.1111/j.1365-2036.2004.02237.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulich KR, Wiklund I, Junghard O. Factor structure of the Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire evaluated in patients with heartburn predominant reflux disease. Qual Life Res. 2003;12:699–708. doi: 10.1023/a:1025192100450. [DOI] [PubMed] [Google Scholar]
- 9.Kulich RK, Ujszászy L, Tóth GT, Bárány L, Carlsson J, Wiklund I. Psychometric validation of the Hungarian translation of the gastrointestinal symptom rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) questionnaire in patients with reflux disease. Orv Hetil. 2004;145:723–9, 739. [PubMed] [Google Scholar]
- 10.Kulich KR, Piqué JM, Vegazo O, Jiménez J, Zapardiel J, Carlsson J. et al. [Psychometric validation of translation to Spanish of the gastrointestinal symptoms rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) in patients with gastroesophageal reflux disease] Rev Clin Esp. 2005;205:588–94. doi: 10.1016/s0014-2565(05)72651-5. [DOI] [PubMed] [Google Scholar]
- 11.Talley NJ, Fullerton S, Junghard O, Wiklund I. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001;96:1998–2004. doi: 10.1111/j.1572-0241.2001.03932.x. [DOI] [PubMed] [Google Scholar]
- 12. Wiklund I. Fullerton S, Junghard O, Talley NJ. Interpretability and meaningfulness of quality of life changes in patients with heartburn. Annual meeting at d.d.week,aga sandiego,california2000.
- 13.Kulich KR, Wiklund I, Junghard O. Factor structure of the Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire evaluated in patients with heartburn predominant reflux disease. Qual Life Res. 2003;12:699–708. doi: 10.1023/a:1025192100450. [DOI] [PubMed] [Google Scholar]
- 14.Nasseri-Moghaddam S, Razjouyan H, Alimohamadi SM, Mamarabadi M, Ghotbi MH, Mostajabi P. et al. Prospective Acid Reflux Study of Iran (PARSI): methodology and study design. BMC Gastroenterol. 2007;7:42. doi: 10.1186/1471-230X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasseri-Moghaddam S, Rafat-zand KH, Habibi R, Razjouyan H, Ahrari B, Nouraie M. et al. Reliability, Validity, and Feasibility of the Mayo Gastro-Esophageal Reflux Questionnaire (GERQ) in an Iranian Population. Iranian J Publ Health. 2008;37:64–74. [Google Scholar]
- 16.Montazeri A, Goshtasebi A, Vahdaninia M, Gandek B. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res. 2005;14:875–82. doi: 10.1007/s11136-004-1014-5. [DOI] [PubMed] [Google Scholar]
- 17.Rothman M, Farup C, Stewart W, Helbers L, Zeldis J. Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci. 2001;46:1540–9. doi: 10.1023/a:1010660425522. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE Jr, Kosinski M, Gandek B, Aaronson NK, Apolone G, Bech P. et al. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA ProjectInternational Quality of Life Assessment. J Clin Epidemiol. 1998;51:1159–65. doi: 10.1016/s0895-4356(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE Jr, Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J. et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA ProjectInternational Quality of Life Assessment. J Clin Epidemiol. 1998;51:1167–70. doi: 10.1016/s0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
- 20.Borgaonkar MR, Irvine EJ. Quality of life measurement in gastrointestinal and liver disorders. Gut. 2000;47:444–54. doi: 10.1136/gut.47.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technalogy Assesment. 1998;2:1–74. [PubMed] [Google Scholar]
- 22. MAPI Research Institute, Linguistic validation. Available at:http://www.mapi-research.fr/i_02_meth.htmAccessed April 2, 2005.
- 23. Halling K, Långström G, Wiklund I . Handling missing data in quality of life questionnaires: experience from clinical trials. (paper presented at the 6th annual conference of the international society for quality of life research, barcelona, spain) 1999.
- 24.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 25.Wahlqvist P, Carlsson J, Stålhammar NO, Wiklund I. Validity of a Work Productivity and Activity Impairment questionnaire for patients with symptoms of gastro-esophageal reflux disease (WPAI-GERD)--results from a cross-sectional study. Value Health. 2002;5:106–13. doi: 10.1046/j.1524-4733.2002.52101.x. [DOI] [PubMed] [Google Scholar]
- 26.Chassany O, Sagnier P, Marquis P, Fullerton S, Aaronson NK. Patient-reported outcomes: the example of health-related quality of life. Drug Inf J. 2002:36209–38. [Google Scholar]
- 27.Chassany O, Marquis P, Scherrer B, Read NW, Finger T, Bergmann JF. et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527–33. doi: 10.1136/gut.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farup C, Kleinman L, Sloan S, Ganoczy D, Chee E, Lee C. et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161:45–52. doi: 10.1001/archinte.161.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Irvine E. Quality of life assessment in gasteroesophageal reflux disease. GUT. 2004;53:iv35–9. doi: 10.1136/gut.2003.034314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JY, Woloshin S, Laycock WS, Rothstein RI, Finlayson SR, Schwartz LM. Symptoms and treatment burden of gastroesophageal reflux disease: validating the GERD assessment scales. Arch Intern Med. 2004;164:2058–64. doi: 10.1001/archinte.164.18.2058. [DOI] [PubMed] [Google Scholar]
- 31.Kitapcioglu G, Mandiracioglu A, Bor S. Psychometric and methodological characteristics of a culturally adjusted gastroesophageal reflux disease questionnaire. Dis Esophagus. 2004;17:228–34. doi: 10.1111/j.1442-2050.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 32.Crawley J, Frank L, Joshua-Gotlib S, Flynn J, Frank S, Wiklund I. Measuring change in quality of life in response to helicobacter pylori eradication in peptic ulcer disease. Dig Dis Sci. 2001;46:571–80. doi: 10.1023/a:1005655317121. [DOI] [PubMed] [Google Scholar]


