Abstract
Gamma-aminobutyric acid (GABA) is an important neurotransmitter, but recent reports have revealed the expression of GABAergic components in peripheral, non-neural tissues. GABA administration induces natriuresis and lowers blood pressure, suggesting renal GABA targets. However, systematic evaluation of renal GABAergic components has not been reported. In this study, kidney cortices of Wistar-Kyoto rats (WKY) were used to assay for messenger RNAs of GABA-related molecules using RT-PCR. In WKY kidney cortex, GABAA receptor subunits, α1, β3, δ, ε and π, in addition to both types of GABAB receptors, R1 and R2, and GABAC receptor ρ1 and ρ2 subunit mRNAs were detected. Kidney cortex also expressed mRNAs of glutamate decarboxylase (GAD) 65, GAD67, 4-aminobutyrate aminotransferase and GABA transporter, GAT2. Western blot and/or immunohistochemistry were performed for those molecules detected by RT-PCR. By immunofluorescent observation, co-staining of α1, β3, and π subunits was observed mainly on the apical side of cortical tubules, and immunoblot of kidney protein precipitated with π subunit antibody revealed α1 and β3 subunit co-assembly. This is the first report of GABAA receptor π subunit in the kidney. In summary, unique set of GABA receptor subunits and subtypes were found in rat kidney cortex. As GABA producing enzymes, transporters and degrading enzyme were also detected, a possible existence of local renal GABAergic system with an autocrine/paracrine mechanism is suggested.
Introduction
Gamma-aminobutyric acid (GABA) acts as a major inhibitory neurotransmitter in the central nervous system. GABA is synthesized in the brain from glutamic acid via glutamic acid decarboxylase (GAD). There are two types of GAD with different molecular weights, GAD67 and GAD65. Released GABA acts on two general classes of its receptors, GABAA/GABAC and GABAB. GABAA and GABAC receptors are ligand-gated chloride channels composed of a combination of various subunits as pentamers. The unique sensitivity of GABAA receptor to its agonist such as benzodiazepines is known to depend on the combination of subunits including α, β, γ, δ, ε, π, and θ [1], [2]. Meanwhile, GABAB is a metabotropic G-protein coupled receptor coupling with Gi/o. GABAB receptor controls neuronal activity via activation of K+ channels, inhibition of Ca2+ channels, or both. Similar to the GABAA receptor-mediated increase in the conductance of Cl−, the activation of K+ channels from the GABAB signaling induces hyperpolarization of the cell membrane, which produces chronic stabilization of neuronal activity [3]. GABA secreted from neurons subsequently undergoes re-uptake via GABA transporters (GAT) and is metabolized by the GABA degrading enzyme, 4-aminobutyrate aminotransferase (ABAT) [4].
Considerable evidence suggests that GABA also mediates diverse responses in non-neuronal tissues. GABAA receptor subunits have been identified in tissues such as the lung [5], retina [6], uterus [7], [8], spermatozoa [9], adrenal medulla [10], pituitary lobes [11], and pancreatic β-cells [12]. Mizuta et al. [13] detected functional GABAB receptors in airway epithelium and airway epithelial cell lines.
The kidney is a key organ responsible for blood pressure regulation by controlling water and sodium balance. It is previously reported that GABA causes natriuresis and lowers blood pressure in human as well as rodents [14], [15]. In addition, GABAA receptor agonist muscimol and GABAB agonist baclofen increased fractional excretion of water and sodium in isolated rat kidney perfusion [16]. These suggest that GABA may play a pivotal role in the renal pathophysiology of hypertension. To date, there are some reports of the presence of GABAergic system in the kidney. GABA immunoreactivity was found in the renal medulla and cortex but not in neuronal structures [17], and depolarizing stimuli such as ouabain increased GABA release from rat kidney slice [18]. Sarang et al. examined the expression of GABAA receptor α1, α2, α4, α5, β1, γ1, γ2 and δ subunits in the kidney cortex from Sprague-Dawley rats and found the expressions of α1, α5, β1, γ1 and γ2 subunits. They also showed that GABA and a GABAA receptor agonist, muscimol, increased Cl− uptake in primary cultured renal proximal tubular cells [19]. The authors also reported the expression of β2 and β3 subunits in the renal proximal tubule [20], but some of the GABAA receptor subunits such as α6, γ3, ε, π and θ have not been examined.
GABA may have an important role in the kidney, but a systematic evaluation of GABA-related molecules has not been performed. Therefore, in this report, the expressions of GABA-related molecules, GABA producing enzyme, receptor components, transporters and degrading enzyme, were examined in rat kidney.
Materials and Methods
Animals
All animal experimental procedures were approved by the Fukushima Medical University School of Medicine Animal Committee. Seven-week old Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) were obtained from Japan SLC Inc., Sendai, Japan and used for the experiments at 8 weeks of age. Each animal was housed under controlled temperature (21°C) and light conditions (light 7∶00–19∶00, dark 19∶00–7∶00) with either 0.3% or 8% NaCl chow (Oriental Yeast Co., Tokyo, Japan) for a week with tap water ad libitum. Rats were anesthetized using pentobarbital (50 µg/kg) intraperitoneal injection. After opening the abdominal cavity and drawing blood from the abdominal aorta, kidneys and brain were harvested and stored for further analyses. The animals were euthanized by pentobarbital overdose (intraperitoneal administration of over 200 mg/kg pentobarbital sodium). Eight rats per group were used for the experiment.
Total RNA extraction and reverse transcription
Total RNA was extracted from the rat kidney cortex and brain preserved in RNAlater (Life Technologies, Carlsbad, CA, USA) using RNeasy plus mini kit (QIAGEN K.K., Tokyo, Japan) following the manufacturer’s instructions. Kidneys from 5 rats were used in the study. Universal RNA from rat normal tissues (BioChain, San Francisco, CA, USA) and brain RNA were used as positive controls. RNA concentration was measured using UV spectrometer, and 0.25 µg of total RNA was reverse transcribed in 20 µl reaction volume using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The reverse transcriptase step (5 min at 25.0°C, 30 min at 42.0°C, 5 min at 85.0°C) was carried out using My Cycler (Bio-Rad).
Human RNA samples
Human Kidney Total RNA and Human Brain Total RNA were purchased from Clontech Laboratories, Inc. (Mountain View, CA, USA). The Human Kidney Total RNA was extracted from a normal kidney of 40-year old Caucasian female with a sudden death. The Human Brain Total RNA was extracted from a normal whole brain from a 43-year-old Caucasian male with a sudden death.
Qualitative RT-PCR
Qualitative PCR was performed using the renal cortical mRNA isolated from WKY fed normal chow. Rat GABAA receptor α1-6, β1-3, γ1-3, δ, ε, π, θ subunits, GAD65, GAD67, GAT1-3, and ABAT nucleotide sequences were identified in Gen Bank database. PCR primers were designed using Primer Blast (Table 1). One µl of the reverse transcriptase products was amplified by PCR using Go Taq Green Maser Mix (Promega, Fitchburg, WI, USA) and 60 nM each of forward and reverse primers. The PCR condition was 2 min at 50.0°C. 10 min at 95.0°C, then 35 cycles of 15 s at 95.0°C and 1 min at 60.0°C, followed by 5 min at 72.0°C. Amplifications for rat GABAB R1 and R2 subtypes and GABAC ρ1-3 subunits were carried out using TaqMan Gene Expression Assay (Life Technologies) following the manufacturer’s protocol (Table 1). All PCR reactions were conducted in iCycler (Bio-Rad). Five µl of PCR products were analyzed by electrophoresis in 10% TBE Gel (Life Technologies) stained with ethidium bromide, and visualized on a UV transilluminator. Rat brain RNA or commercially available reference RNA were used as positive control.
Table 1. Primer information.
| Gene | Forward primer (3′–5′) | Reverse primer (3′–5′) | Accession No.or assay ID | Ampliconsize (bp) |
| GABAA α1 | tgacagtcattctctcccaagtc | tcagaacggtcgtcactcc | NM_183326 | 87 |
| GABAA α2 | gactgtcattctctcccaagtgt | tcattgtcaaaacagttgttactcc | NM_001135779 | 92 |
| GABAA α3 | cctacttgccatgtatcatgactg | ggtcatggtgagaacagtggt | NM_017069 | 113 |
| GABAA α4 | cttctggatcaataaggagtctgtt | tcatcgtgaggactgtggtt | NM_080587 | 68 |
| GABAA α5 | gccttggaagcagctaaaatc | gtacccacagcattcccagt | NM_017295 | 146 |
| GABAA α6 | agtcccagaaagccgaaag | ttcttcagatggtacttggagtca | NM_021841 | 124 |
| GABAA β1 | ccctctggatgagcaaaact | ccctctcctccattccagta | NM_012956 | 90 |
| GABAA β2 | catcgatatggtttctgaagtcaa | gggattacattgtaggacagtctctt | NM_012957 | 96 |
| GABAA β3 | atcgagctcccacagttctc | tcaatgagagtcgagggtagg | NM_017065 | 91 |
| GABAA γ1 | gatgcgcactggataacaac | tggagttgaaggtagcattctg | NM_080586 | 110 |
| GABAA γ2 | acagaaaatgacgctgtgga | atctgacttttggctagtgaagc | NM_183327 | 70 |
| GABAA γ3 | agcgagtggagaccaagc | ctcttccaccctcctggac | NM_024370 | 124 |
| GABAA δ | aatgacattggggactacgtg | gccacattcactggaggac | NM_017289 | 125 |
| GABAA ε | ccgctgagatgttgcctaa | tccagtttaatgtgaggtccaa | NM_023091 | 88 |
| GABAA π | gttgcagctggaaagttgg | gagtccacgtaccgaatcgt | NM_031029 | 82 |
| GABAA θ | tgatgtccgactgagaccaa | tctgagatctgttcaatactggagac | NM_031733 | 87 |
| GAD65 | gtacgccatgctcattgccc | agagaggatcaaaagccccg | NM_012563 | 299 |
| GAD67 | gctggaaggcatggaaggtttta | acgggtgcaatttcatatgtgaacata | NM_017007 | 222 |
| GAT1 | ttcaagggtgtgggcctcgc | ccacggcagagtcgtggtga | NM_024371 | 120 |
| GAT2 | gcagcgaacacaagcgcatcc | atcccacctgcagccgctact | NM_133623 | 126 |
| GAT3 | tcgtgttgagcgtagcgggag | ggaatgcccctccgccgtt | NM_024372 | 90 |
| ABAT | cctgggcatcctgcctccaga | ttgggcgccaccgacatca | NM_031003 | 70 |
| GABAB R1 | Rn00578911_m1 | 113 | ||
| GABAB R2 | Rn00582550_m1 | 87 | ||
| GABAC ρ1 | Rn00568768_m1 | 61 | ||
| GABAC ρ2 | Rn00568783_m1 | 86 | ||
| GABAC ρ3 | Rn00594210_m1 | 88 |
Abbreviations: GABA; γ-aminobutyric acid, GAD; glutamate decarboxylase, GAT; GABA transporter, ABAT; 4-aminobutyrate aminotransferase.
Quantitative PCR
Quantitative PCR was performed to compare mRNA expression levels in GABAA receptor subunits among WKY and SHR fed normal- or high-salt chow. Quantitative PCR was also performed using human kidney and brain RNA commercially available from Clontech Inc. (Mountain View, CA). One µl of reverse-transcription sample was used for real-time quantitative PCR using the iQ5 Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio Rad) or StepOnePlus Real-Time PCR System and TaqMan Fast Advanced Master Mix (Life Technologies). PCR reactions were performed in triplicate, and mRNA was quantified based on the Ct value, normalized to GAPDH, and expressed as relative amounts.
Immunoprecipitation
Protein lysate was incubated with Protein G-protein Mag Sepharose (GE Health care Life Sciences, Uppsala, Sweden) closslinked to anti-GABAA receptor π subunit antibody for 12 hours at 4°C. The immunoprecipitated protein samples eluted from the pellets were suspended in sample buffer and resolved by SDS-PAGE. Western blotting was performed with anti-GABAA receptor α1 or β2/3 subunit antibody.
Membrane protein preparation
WKY kidney membrane protein was separated using Mem-PER Eukaryotic Membrane Protein Extraction Kit (Thermo Scientific, Carlsbad, CA) according to the instruction from manufacturer. The hydrophobic fraction containing membrane proteins was diluted to decrease the detergent concentration. The membrane proteins in SDS sample buffer were resolved by SDS-PAGE and detected by immunoblot.
Western Blotting
The rat kidney cortex and brain were immediately snap-frozen in liquid nitrogen and stored at −80°C until use. The tissue was homogenized in lysis buffer (50 mM Tris-Cl pH 8.0, 150 mM NaCl. 0.02% sodium azide and 1% NP-40) containing cOmplete protease inhibitor mix (Roche, Tokyo, Japan) and centrifuged at 14000 g for 10 min at 4°C. Protein concentrations were determined by BCA protein assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Five to 30 µg/lane of protein were separated on NuPAGE Novex 4–12% Bis-Tris Gels (Life Technologies) and transferred onto PVDF membrane (MILLIPORE, Billerica, MA, USA). The membranes were blocked with Immunoblock (DS Pharma Biomedical Co., Ltd., Osaka, Japan). The membranes were probed with a 1∶1000 dilution of anti-GABAA receptor α1 subunit rabbit polyclonal antibody (MILLIPORE), a 1∶500 dilution of β2/3 subunit mouse monoclonal antibody (MILLIPORE), a 1∶200 dilution of π subunit rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, sc-25708), ρ1 antibody (Santa Cruz, sc-25707), ρ2 antibody (Santa Cruz, sc-21343) or 1∶2000 dilution of ABAT antibody (Sigma-aldrich, St. Louis, MO, USA. Immuno Shot reagents (COSMO BIO Co., LTD, Tokyo, Japan) were used to enhance the immune reaction in some assays. The membranes were washed and probed with 1∶5000 goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated IgG (Santa Cruz, Santa Cruz, Ca, USA). Proteins were detected by the ECL system (GE healthcare, Buckinghamshire, UK).
Immunohistochemistry
Three-µm tissue sections were incubated with primary antibodies including 1∶50 to 1∶100 dilution of GABAA α1, β2/3 and π subunits, GABAB receptor R1 subtype (Cell-Signaling), R2 subtype (Cell Signaling), GAD67 (Santa Cruz, GAD65 (MILLIPORE) GAT2 (MILLIPORE) or ABAT. Immuno Shot reagents (COSMO BIO) were used to enhance the immune reaction in some assays. Chromogenic immunostaining was performed with an avidin–biotin immunoperoxidase kit (Vectastain Elite kit or ABC/Peroxidase kit, Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine (Sigma Fast DAB Tablets, Sigma-Aldrich). The kidneys were lightly counterstained with hematoxylin. Fluorescent immunostaining was performed using secondary antibody of Alexa Flour 568 conjugated goat anti-rabbit antibody and Alexa Flour 488 conjugated goat anti-mouse antibody (Life Technologies). GABAA π subunit antibody (Abcam: ab26055) was used for immunofluorescence studies. Normal rabbit IgG (Santa Cruz: sc-2027) and mouse monoclonal IgG1 (Abcam: ab81032) were used as negative control. Nuclei were stained by DAPI (Life Technologies). FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan) was used for examination and image acquisition.
Results
Expressions of GABAA receptor α1, β3, δ, ε and π subunits were detected in rat kidney cortex
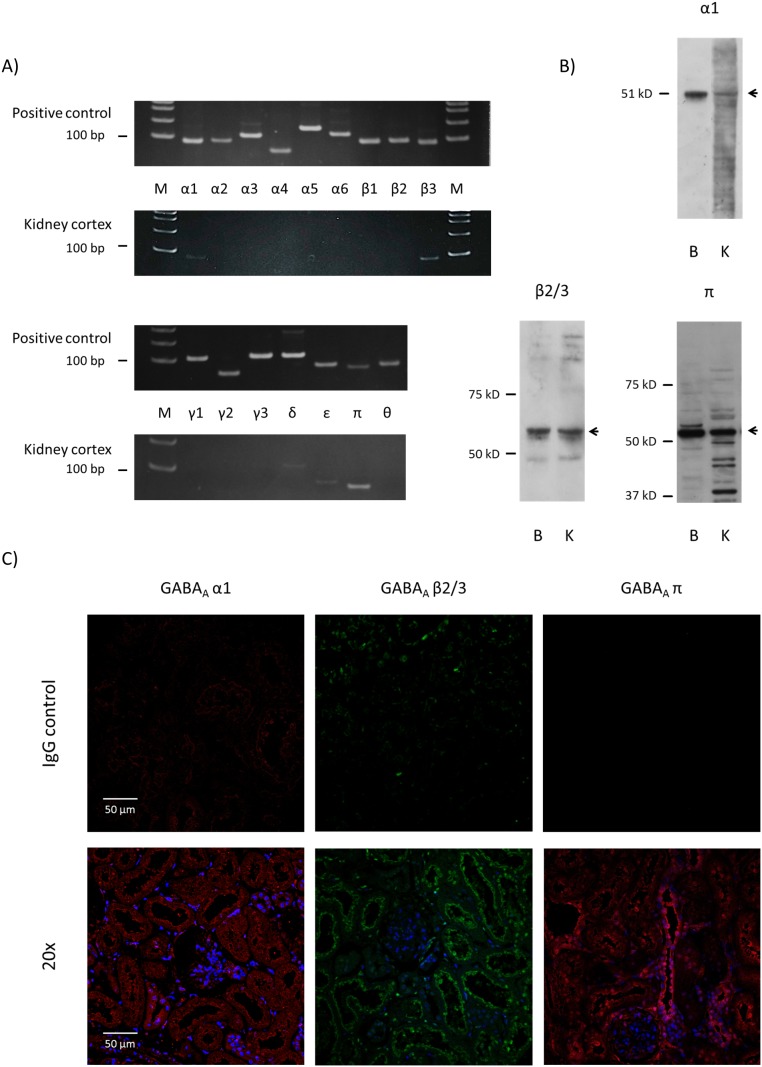
Messenger RNAs of GABAA receptor subunits, α1, β3, δ, ε and π, were detected by RT-PCR in WKY kidney cortex (Figure 1A). All PCR products showed the theoretical molecular weight and their identities were confirmed by sequencing. Clear single bands were detected for all positive controls (brain or universal RNA). However, mRNAs of GABAA receptor subunits, α2, α3, α4, α5, α6, β1, β2, γ1, γ2, γ3 and θ were not detectable in the kidney cortex. Those genes showing clear mRNA signal, namely, α1, β3 and π subunits, were tested for protein expression by immunoblotting. The major band for α1 subunit in the kidney was around 55 kDa, which was the same as a band of the brain sample on the immunoblot. Immunoblotting using an antibody against β2/3 subunit showed a similar pattern in the brain and kidney, with a major band around the theoretical 55 kDa and a secondary band around 40 kDa. The kidney may express similar or greater amounts of β3 subunit than the brain. Immunoblotting for π subunit detected a major band around 50 kDa for both brain and kidney tissue, with a weak secondary band slightly larger than the major band in the brain and smaller bands for kidney samples (Figure 1B). Then, to elucidate the protein localization, immunofluorescence study was performed for α1, β3 and π subunits (Figures 1C and 2A). Alpha 1 subunit distributed widely in the cortical tubules, especially proximal-like tubules. Specific staining of β3 subunit was observed in the apical region of the proximal-like tubules with tall cells and a brush border-like structure and also in the apical region of distal-like tubules. No staining for β3 subunit was detected in the glomeruli. Pi subunit staining was present mostly in the distal-like tubules and less in the proximal-like tubules (Figure 1C).
Figure 1. GABAA receptor subunits are expressed in rat kidney.
A) RT-PCR results for GABAA receptor subunits in rat (WKY on normal diet) kidney cortex. Appropriately-sized bands were detected for GABAA receptor α1, β3 and π subunits in at least five independent experiments. Rat brain RNA or commercial universal RNA was used as positive control. M: molecular marker. B) Immunoblot for GABAA receptor α1, β3 and π subunits in rat kidney cortex. B: brain control, K: kidney cortex. C) Immunofluorescent examination of GABAA receptor α1, β3 and π subunits in rat kidney cortex. Staining with normal IgG instead of primary antibody served as negative control.
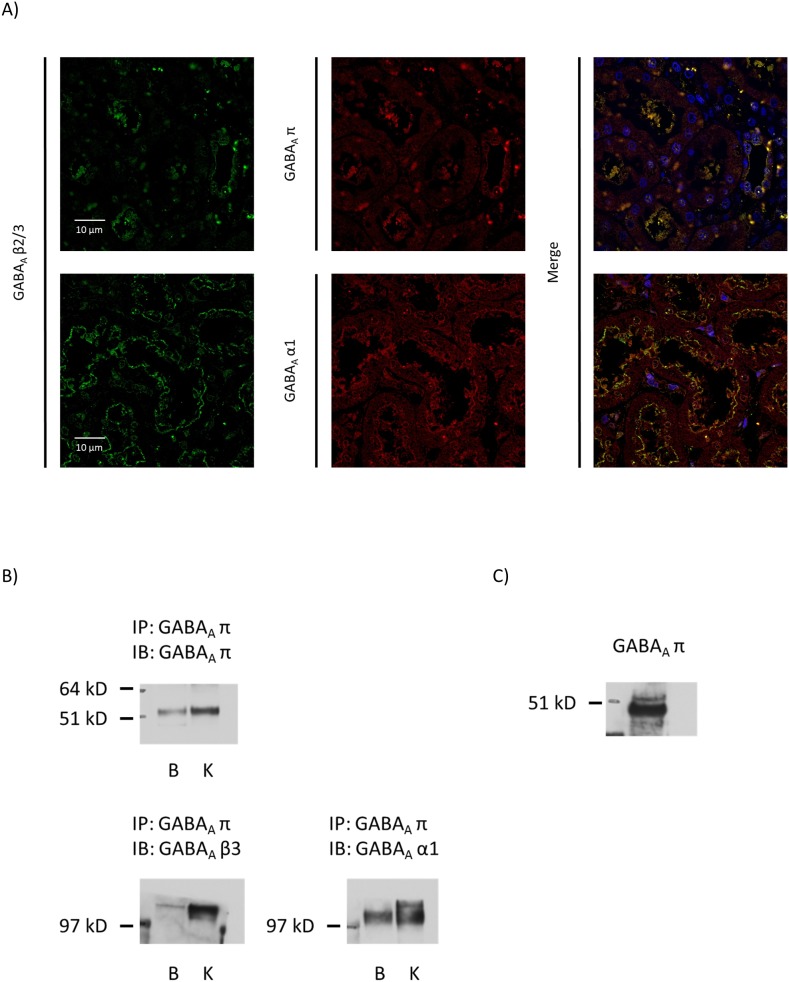
Figure 2. GABAA α1, β3 and π subunits are co-expressed in rat kidney.
A) Immunofluorescent images of GABAA α1, β3 and π subunits with DAPI counterstain. B) Immunoblot of rat kidney cortex protein immunoprecipitated with GABAA receptor π subunit antibody. Molecular sizes of α1 and β3 subunits around 110 kDa suggest dimerization. C) Immunoblot of rat kidney cortex membrane protein for GABAA receptor π subunit.
GABAA receptor α1, β3 and π subunits co-localized in the apical membranes of cortical tubules
By immunofluorescent observation, co-staining of α1, β3 and π subunits was observed mainly on the apical side of cortical tubules (Figure 2A). Kidney protein immunoprecipitated with π subunit antibody revealed α1 and β3 subunits, and the molecular weights of blotted α1 and β3 subunits were close to 110 kDa, suggesting dimerization (Figure 2B). Pi subunit may be the characteristic GABAA receptor subunit in the kidney, possibly constituting α1/α1/β3/β3/π pentamers. By immunohistochemical observation, it seemed that π subunit may be present in the cytosol. However, because membrane protein preparation of rat kidney clearly showed π subunit signal on the immunoblot (Figure 2C), some of the π subunit molecules are on the membrane and seem to associate with other subunits.
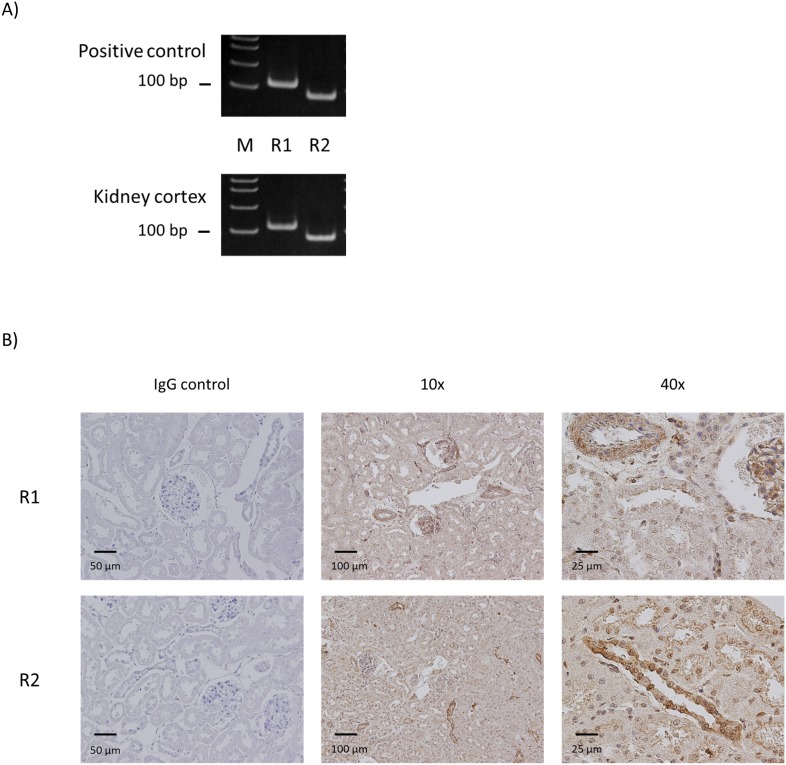
Both GABAB receptor R1 and R2 subtypes were expressed in rat kidney cortex
Both types of GABAB receptor, R1 and R2 mRNA, were clearly detected in the kidney cortex (Figure 3A). The primer set used for GABAB receptor R1 mRNA detects all 7 known splice variants of the gene. To visualize the protein localization, immunohistochemistry was performed for both subtypes. In the cortex, R1 subtype was observed in the arteriolae and glomeruli. There was also diffuse signal in most of the tubules, with some stronger signal on the apical side of some of the tubules. GABAB receptor R2 subtype also showed diffuse signal in most of the tubules, with strong signal in the collecting-like tubules, similar to GABAA receptor π subunit, and moderate staining on the apical side of proximal-like tubules (Figure 3B).
Figure 3. GABAB receptor R1 and R2 subtypes are expressed in rat kidney.
A) RT-PCR results for GABAB receptor subtypes in rat kidney cortex. Appropriately-sized bands were detected for GABAB receptor R1 and R2 subtypes in at least five independent experiments. Rat brain RNA or commercial universal RNA was used as positive control. M: molecular marker. B) Immunostaining for GABAB receptor R1 and R2 subtypes in rat kidney cortex. Staining with normal IgG instead of primary antibody served as negative control.
GABAC Receptor ρ1 and ρ2 subunits were detected in rat kidney cortex
Messenger RNAs of GABAC receptor subunits ρ1 and ρ2 were detected by RT-PCR in WKY kidney cortex, but ρ3 was not detectable (Figure 4A). Immunoblotting for ρ1 subunit showed a similar pattern in the brain and kidney, with a major band around the theoretical 48 kDa. The kidney may express similar or greater amounts of ρ1 subunit than the brain. Immunoblotting for ρ2 subunit showed a major band around 50 kDa in both brain and kidney tissues (Figure 4B).
Figure 4. GABAC receptor subunits are expressed in rat kidney.
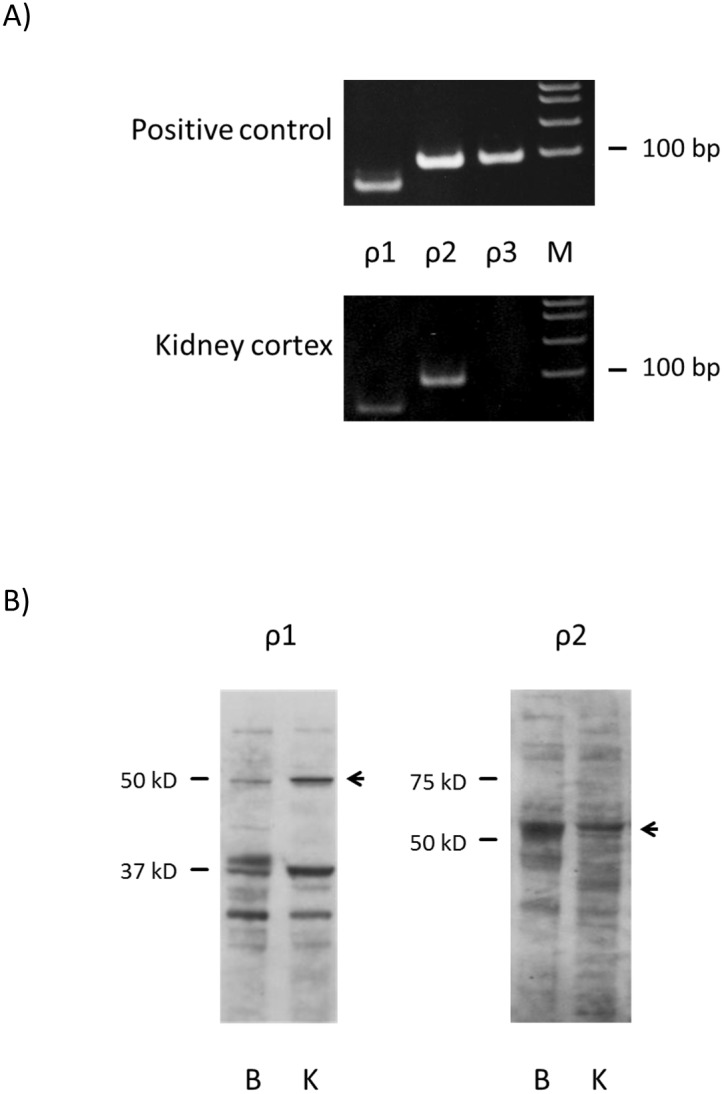
A) RT-PCR results for GABAC receptor subunits in rat kidney cortex. Appropriately-sized bands were detected for GABAC receptor ρ1 and ρ2 subunits in at least five independent experiments. Rat brain RNA or commercial universal RNA was used as positive control. M: molecular marker. B) Immunoblotting for GABAC receptor ρ1 and ρ2 subunits in rat kidney cortex. B: brain control, K: kidney cortex.
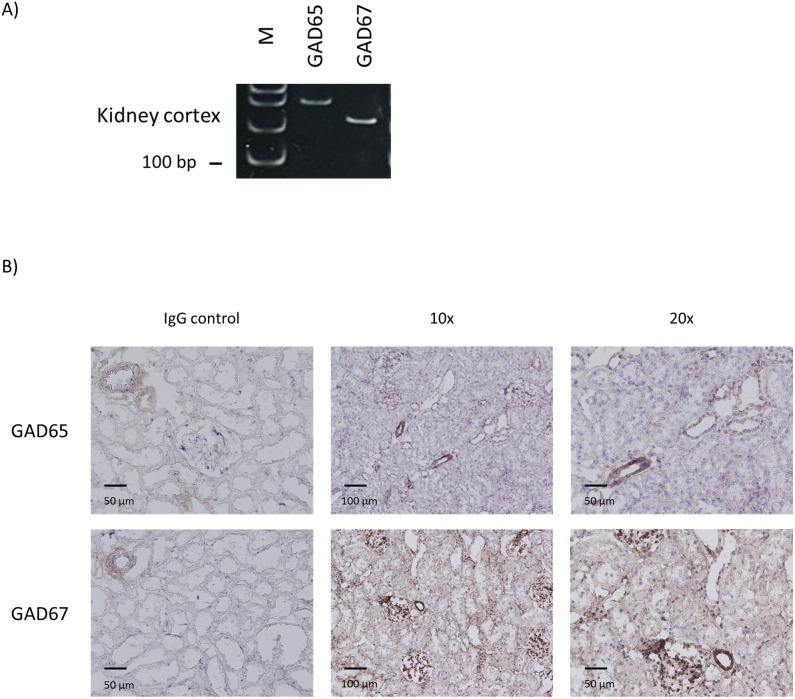
Two types of GABA-producing enzymes were detected in rat kidney cortex
Both types of GABA producing enzyme, GAD65 and GAD67 mRNA, were clearly detected in the kidney cortex (Figure 5A). By immunohistochemistry, GAD65 staining was mostly observed in glomeruli and arteriolae. Some weak staining of cortical tubules and stronger staining of vessels and glomeruli were observed for GAD67 (Figure 5B).
Figure 5. GABA-producing enzymes are expressed in rat kidney.
A) RT-PCR results for GAD65 and GAD67 in rat kidney cortex. Appropriately-sized bands were detected for GAD65 and GAD67 in at least five independent experiments. M: molecular marker. B) Immunostaining for GAD65 and GAD67 in rat kidney cortex. Staining with normal IgG instead of primary antibody served as negative control.
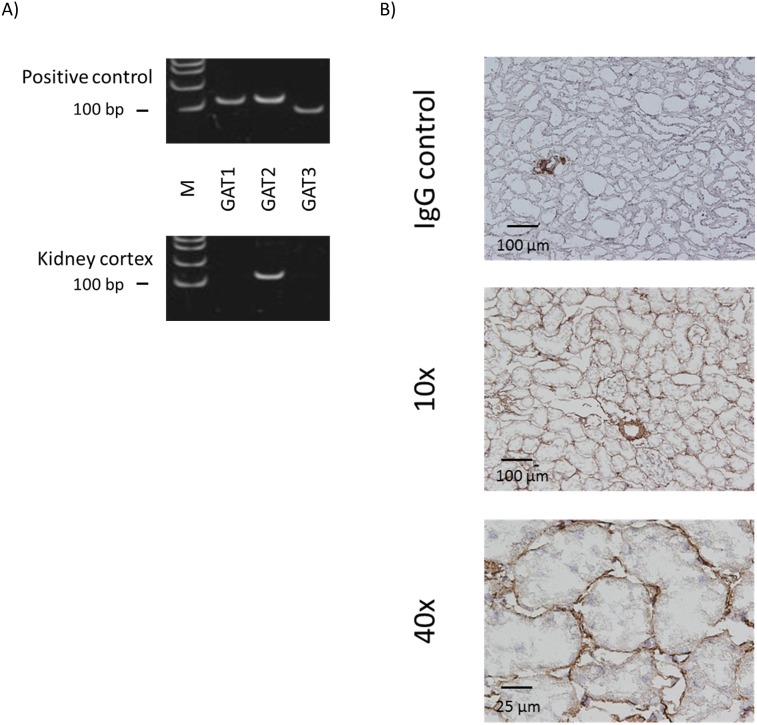
One type of GABA transporter, GAT2, was expressed in rat kidney cortex
Messenger RNAs of peripheral-type GABA transporter GAT2, were detected, but not of GAT1 and GAT3 (Figure 6A). To confirm the protein localization, immunohistochemistry was performed for GAT2. Specific staining was observed on the basolateral side of cortical renal tubules (Figure 6B).
Figure 6. GABA transporters are expressed in rat kidney.
A) RT-PCR results for GAT1, GAT2 and GAT3 in rat kidney cortex. Appropriately-sized bands were detected for GAT2 in at least five independent experiments. Rat brain RNA or commercial universal RNA was used as positive control. M: molecular marker. B) Immunostaining for GAT2 in rat kidney cortex. Staining with normal IgG instead of primary antibody served as negative control.
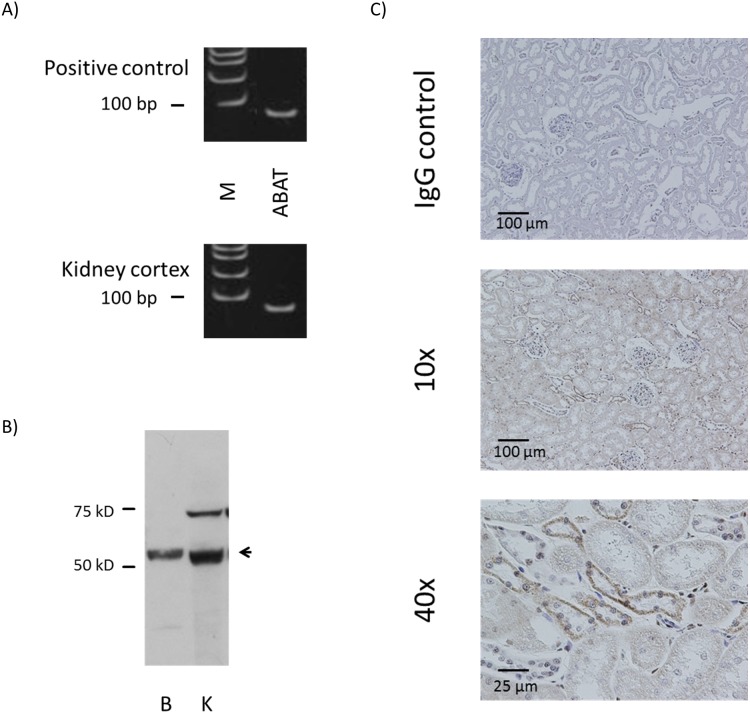
GABA degrading enzyme was detected in kidney cortex
Messenger RNA of GABA degrading enzyme ABAT was detected by RT-PCR (Figure 7A) in the kidney cortex using the primer set that amplifies all three known splice variants of the gene. Immunoblotting confirmed the protein expression of ABAT with the major band around 50 kDa for both brain and kidney, and a secondary band larger than the major band in the kidney (Figure 7B). Immunohistochemistry revealed that ABAT staining pattern was also similar to those of GABAA π subunit, present mostly in the distal-like tubules (Figure 7C).
Figure 7. GABA degrading enzyme is expressed in rat kidney.
A) RT-PCR results for ABAT in rat kidney cortex. Appropriately-sized bands were detected for ABAT in at least five independent experiments. Rat brain RNA or commercial universal RNA was used as positive control. M: molecular marker. B) Immunoblotting for ABAT in rat kidney cortex. B: brain control, K: kidney cortex. C) Immunostaining for ABAT in rat kidney cortex. Staining with normal IgG instead of primary antibody served as negative control.
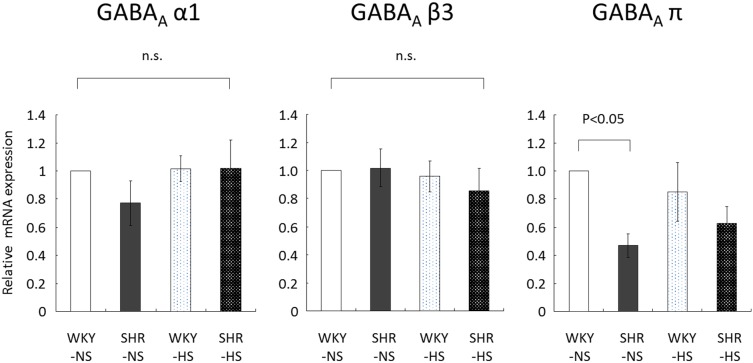
Renal GABAA receptor expression in hypertensive model rats with salt loading
To explore the possibility of GABA receptor alterations in hypertensive and/or salt-sensitive phenotype, we performed quantitative mRNA analysis and western blots of GABAA subunits using the kidney cortex of WKY and SHR on normal- and high-salt diet. GABAA receptor α1 and β3 subunit expressions in the kidney were not affected by stain or salt loading (Figure 8). In contrast, renal GABAA π subunit (Figure 8) mRNA expression was significantly lower in SHR than in WKY on normal salt diet. The level of GABAA π subunit mRNA in SHR kidney was about half of that in WKY kidney. On high-salt diet, GABAA π subunit mRNA expression tended to be lower in the SHR kidney compared to WKY kidney, but the difference was not significant. Salt loading did not significantly alter renal GABAA π subunit mRNA expression in WKY and SHR. We also performed western blot analysis for GABAA π subunit (WKY normal salt 100±12%, SHR normal salt 121±20%, WKY high salt 100±8%, SHR high salt 95±7%, relative expression normalized to actin, n = 8 rats/group). In contrast to the mRNA difference in WKY and SHR kidneys for GABAA π subunit, we found no significant difference in the expression level GABAA π subunit in the kidney cortex of four groups of rats, WKY and SHR with and without salt loading on the protein level.
Figure 8. Quantitative RT-PCR results for GABAA receptor subunits in rat kidney cortex.
WKY: Wistar-Kyoto rats, SHR: spontaneously hypertensive rats, NS: normal-salt diet, HS: high-salt diet, n.s.: not significant. Statistical significance was assumed at P<0.05.
Expressions of GABA-related molecules in human kidney
Next, we examined species differences on the expression of GABA-related molecules in the kidney using human kidney and brain RNA samples. All of the GABA-related molecules consistently detected in rat kidney, specifically, GABAA α1, β3 and π subunits, GABAB R1/R2 subtypes, GABAC subunits ρ1 and ρ2, GAD65 and 67, GAT2, and ABAT were also found in human kidney (data not shown).
Discussion
In this study, gene expressions of GABA-related molecules were examined in rat kidney cortex. Expressions of GABAA α1, β3, π receptor subunits, GABAB receptor R1 and R2 subtypes, GABAC ρ1 and ρ2 receptor subunits were found at both mRNA and protein levels. Enzymes involved in GABA synthesis, GAD67 and GAD65, GABA transporter, GAT2, and GABA metabolizing enzyme, ABAT, were also expressed in rat kidney.
Our results for GABAA receptor subunits differ in some extent to those previously reported by Sarang et al. They found the expressions of α1, α5, β1, β2, β3, γ1, and γ2 subunits but not of α2, α4, or δ subunits in the kidney cortex from Sprague-Dawley rats [20]. In addition, they found the expression of β2 and β3 subunits in microdissected rabbit proximal tubule S2 segment. We found the expression of α1 and β3 subunits similar to Sarang, et al., but we also found the renal expression of π subunit, which was not examined in their reports. Negative expression results for α5, β1, β2, γ1, and γ2 in our study may be due to either the difference in the detection methods, as Sarang et al used nested PCR whereas our method utilized single amplification, or the difference in rat strain, as we used WKY instead of Sprague-Dawley.
Our report is the first to show a concrete expression of GABAA receptor π subunit in rat and human kidney. To explore the differential expression, Nephromine, a web-based analysis engine freely accessible to academic users at http://www.nephromine.org, was used to study molecular expression in human renal diseases. A search for differential expression of GABAA pi subunit mRNA in human kidney using Nephromine revealed that it may be overexpressed approximately 2 fold in kidneys with diabetic nephropathy (n = 10) compared to those from healthy donors (n = 12). This difference was seen both in tubulointerstitium (fold change: 2.003, P-value: 4.99E-4) and glomeruli (fold change: 2.392, P-value: 0.008, data source [21]). However, GABAA pi subunit expression in hypertension data set did now show a significant difference between normal tissues from tumor nephrectomy (n = 4) and those with nephrosclerosis (n = 14, data source [22]).
We also found the expression of GABAB receptor R1 and R2 subtypes and GABAC receptor ρ1 and ρ2 subunits. As far as we know, this is the first report of GABAB and GABAC receptor protein expressions in the kidney.
It has been shown that GABAA receptor agonist muscimol and GABAB agonist baclofen increased fractional excretion of water and sodium in isolated rat kidney perfusion [16]. Our results suggest the possibility of a novel combination of GABAA receptor subunits, α1, β3 and π, in the kidney, and this combination may be relatively kidney-specific. Muscimol is a potent agonist of GABAC in addition to GABAA receptor [23]. When searching for kidney-specific modulation methods of GABAA receptor, it may be possible in the future to screen for substances that show specific agonistic activity on GABAA receptors with π subunits. Such findings may promote the development of tubule-specific natriuretic agent with less effect on the nervous system. Meanwhile, the function of renal GABAB receptor is not known. As GABAB receptor R1 subtype localizes to glomeruli and arterioles as well as tubules, it may control intra-glomerular perfusion pressure. Also, R2 subtype observed in the distal-like tubules may influence the effects of aldosterone or vasopressin as their co-expression in the principal cells is likely. It has been reported that intrarenal administration of baclofen, a GABAB receptor agonist, has renoprotective effect against ischemia reperfusion injury through the attenuation of renal sympathetic nerve activity [24]. Since overactivation of renal sympathetic nerve activity is associated with tissue renin-angiotensin-aldosterone system overactivity, GABAB activation in renal tubule may alter this phenomenon. Also, the activation of postsynaptic GABAB receptors enhances GABAA currents [25]. Chloride handing in distal tubule and collecting duct modulated by GABAA Cl− channel may be associated with sodium chloride cotransporter and/or epithelial sodium channel. Further study is required to depict a better picture and the precise mechanism of action on GABA receptors expressed in the kidney.
In addition to the GABA receptors, GABA-related enzymes and transporters were also examined in this study. The expression and activity of GAD in the kidney have been reported in the past. Liu et al. found GAD antigen in mouse proximal and distal tubules but not in glomeruli [26]. However, previous reports did not examine GAD subtypes separately. In this study, we found both GAD67 and GAD65 in the kidney tissue using antibodies specific for each subtype.
For GABA transporters, a high level of human GAT2 was found in the kidney and low levels in the brain and lung [27]. Rat GAT2 has been found not only in the brain and retina, but also in the liver, kidney, and heart [28]. GAT1 and GAT3 were not detected in rat kidney, similar to our results [28]. ABAT expression has been demonstrated in rat kidney, and the molecular mass of kidney ABAT was similar to that of liver ABAT and smaller than that of brain ABAT [29].
We have detected renal expressions of major GABA-related molecules, namely receptors, transporters, and synthesizing and metabolizing enzymes. All three GABAA subunits examined in this study, α1, β3, and π, showed staining in the renal tubules. Although the staining patterns are not identical, the presence of all three subunits in the same segment is probable, and this suggests that there may be functional receptor composed of α1β3π subunits, as GABAA receptors formed by α5β3π combination has shown to be functional in transfection experiments [1]. We also found expressions of GAD67 and GAD65 in the renal tubules, which suggest the production of GABA in the kidney. Dopamine in the kidney is a locally-functional, autocrine/paracrine factor known to play a role in the pathophysiology of hypertension [30], [31]. Proximal convoluting tubules produce dopamine which acts on mainly D1-like receptors in the proximal convoluting tubules to induce natriuresis. Renal dopamine synthesis and release is increased by dietary sodium intake and elevation in intracellular sodium concentration, and locally-secreted dopamine in the kidney is responsible for over 50% of sodium excretion during the state of sodium surfeit. Since the current study revealed that rat renal cortex expresses major molecules to account for the entire life cycle of GABA, locally-secreted GABA, similar to dopamine, may act in an autocrine/paracrine fashion in the kidney to induce natriuresis and to lower blood pressure.
In conclusion, rat kidney was found to harbor all major GABA-related components. This suggests the possible existence of a local GABAergic system with an intra-renal GABA production, secretion and function in an autocrine/paracrine manner. Further study is necessary to elucidate the precise localization and function of these renal GABA system molecules. As GABA has been suggested to elicit natriuresis and to lower blood pressure, the research on GABA-related molecules in the kidney is promising as a source of new important information on the pathophysiology of hypertension.
Acknowledgments
We thank Ms. Ayumi Haneda and Hiroko Ohashi for their technical assistance. The paper was presented at The 85th Annual Meeting of The Japanese Pharmacological Society. Renal Week 2012 of the American Society of Nephrology.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by KAKENHI (Grant-in-Aid for Young Scientists (B) (23790949)). URL: http://www.jsps.go.jp/english/index.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Neelands TR, Macdonald RL (1999) Incorporation of the pi subunit into functional gamma-aminobutyric Acid(A) receptors. Mol Pharmacol 56: 598–610. [DOI] [PubMed] [Google Scholar]
- 2. Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, et al. (1997) Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties [corrected; erratum to be published]. J Neurosci 17: 5027–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slugg RM, Zheng SX, Fang Y, Kelly MJ, Ronnekleiv OK (2003) Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly rectifying K+ conductance. J Physiol 551: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherif FM (1994) GABA-transaminase in brain and blood platelets: basic and clinical aspects. Prog Neuropsychopharmacol Biol Psychiatry 18: 1219–1233. [DOI] [PubMed] [Google Scholar]
- 5. Chintagari NR, Jin N, Gao L, Wang Y, Xi D, et al. (2010) Role of GABA receptors in fetal lung development in rats. PLoS One 5: e14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grigorenko EV, Yeh HH (1994) Expression profiling of GABAA receptor beta-subunits in the rat retina. Vis Neurosci 11: 379–387. [DOI] [PubMed] [Google Scholar]
- 7. Hedblom E, Kirkness EF (1997) A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem 272: 15346–15350. [DOI] [PubMed] [Google Scholar]
- 8. Erdo SL (1984) Identification of GABA receptor binding sites in rat and rabbit uterus. Biochem Biophys Res Commun 125: 18–24. [DOI] [PubMed] [Google Scholar]
- 9. Hu JH, He XB, Wu Q, Yan YC, Koide SS (2002) Subunit composition and function of GABAA receptors of rat spermatozoa. Neurochem Res 27: 195–199. [DOI] [PubMed] [Google Scholar]
- 10. Parramon M, Oset-Gasque MJ, Gonzalez MP, Stephenson FA (1994) Identification of GABAA receptor subunits expressed in bovine adrenal medulla. Neurosci Lett 168: 243–246. [DOI] [PubMed] [Google Scholar]
- 11. Berman JA, Roberts JL, Pritchett DB (1994) Molecular and pharmacological characterization of GABAA receptors in the rat pituitary. J Neurochem 63: 1948–1954. [DOI] [PubMed] [Google Scholar]
- 12. Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, et al. (2010) Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 59: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW (2008) Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 39: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, et al. (2004) Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr 92: 411–417. [DOI] [PubMed] [Google Scholar]
- 15. Yamakoshi J, Fukuda S, Satoh T, Tsuji R, Saito M, et al. (2007) Antihypertensive and natriuretic effects of less-sodium soy sauce containing gamma-aminobutyric acid in spontaneously hypertensive rats. Biosci Biotechnol Biochem 71: 165–173. [DOI] [PubMed] [Google Scholar]
- 16. Monasterolo LA, Trumper L, Elias MM (1996) Effects of gamma-aminobutyric acid agonists on the isolated perfused rat kidney. J Pharmacol Exp Ther 279: 602–607. [PubMed] [Google Scholar]
- 17. Parducz A, Dobo E, Wolff JR, Petrusz P, Erdo SL (1992) GABA-immunoreactive structures in rat kidney. J Histochem Cytochem 40: 675–680. [DOI] [PubMed] [Google Scholar]
- 18. Erdo SL, Dobo E, Parducz A, Wolff JR (1991) Releasable GABA in tubular epithelium of rat kidney. Experientia 47: 227–229. [DOI] [PubMed] [Google Scholar]
- 19. Sarang SS, Lukyanova SM, Brown DD, Cummings BS, Gullans SR, et al. (2008) Identification, coassembly, and activity of gamma-aminobutyric acid receptor subunits in renal proximal tubular cells. J Pharmacol Exp Ther 324: 376–382. [DOI] [PubMed] [Google Scholar]
- 20. Sarang SS, Plotkin MD, Gullans SR, Cummings BS, Grant DF, et al. (2001) Identification of the gamma-aminobutyric acid receptor beta(2) and beta(3) subunits in rat, rabbit, and human kidneys. J Am Soc Nephrol 12: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 21. Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, et al. (2011) Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neusser MA, Lindenmeyer MT, Moll AG, Segerer S, Edenhofer I, et al. (2010) Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am J Pathol 176: 594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frolund B, Ebert B, Kristiansen U, Liljefors T, Krogsgaard-Larsen P (2002) GABA(A) receptor ligands and their therapeutic potentials. Curr Top Med Chem 2: 817–832. [DOI] [PubMed] [Google Scholar]
- 24. Kobuchi S, Shintani T, Sugiura T, Tanaka R, Suzuki R, et al. (2009) Renoprotective effects of gamma-aminobutyric acid on ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol 623: 113–118. [DOI] [PubMed] [Google Scholar]
- 25. Tao W, Higgs MH, Spain WJ, Ransom CB (2013) Postsynaptic GABAB receptors enhance extrasynaptic GABAA receptor function in dentate gyrus granule cells. J Neurosci 33: 3738–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu ZH, Striker LJ, Hattori M, Yang CW, Striker GE (1996) Localization of glutamic acid decarboxylase in the kidneys of nonobese diabetic mice. Nephron 72: 662–666. [DOI] [PubMed] [Google Scholar]
- 27. Christiansen B, Meinild AK, Jensen AA, Brauner-Osborne H (2007) Cloning and characterization of a functional human gamma-aminobutyric acid (GABA) transporter, human GAT-2. J Biol Chem 282: 19331–19341. [DOI] [PubMed] [Google Scholar]
- 28. Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL (1992) Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system. Cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem 267: 21098–21104. [PubMed] [Google Scholar]
- 29. Kontani Y, Sakata SF, Matsuda K, Ohyama T, Sano K, et al. (1999) The mature size of rat 4-aminobutyrate aminotransferase is different in liver and brain. Eur J Biochem 264: 218–222. [DOI] [PubMed] [Google Scholar]
- 30. Hussain T, Lokhandwala MF (2003) Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142. [DOI] [PubMed] [Google Scholar]
- 31. Jose PA, Soares-da-Silva P, Eisner GM, Felder RA (2010) Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 1802: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.