Abstract
Purpose
Medulloblastoma is the most common malignant brain tumor occurring in childhood and a significant cause of morbidity and mortality in pediatric oncology. More intense treatment strategies are recommended for patients displaying high-risk factors, however considerable variation in outcome remains, indicating a need for improved predictive markers. In this study, 1H magnetic resonance spectroscopy (MRS) was used to investigate non-invasive molecular biomarkers of survival in medulloblastoma.
Experimental Design
MRS was performed on a series of 35 biopsy confirmed medulloblastoma cases. One case was excluded due to poor quality MRS. The prognostic value of MRS detectable biomarkers was investigated using Cox-Regression retrospectively (N=15). A subsequent validation analysis (N=19) was also performed to reduce the chance of type I errors. Where available, high-resolution exvivo MRS of biopsy tissue was used to confirm biomarker assignments.
Results
The retrospective analysis revealed that creatine, glutamate and glycine were markers of survival (p<0.01). The validation analysis showed that glutamate was a robust marker, with a hazard ratio of 8.0 for the full dataset (p=0.0003, N=34). A good correlation between in-vivo and ex-vivo MRS glutamate / total-choline was found (p=0.001), validating the in-vivo assignment. Ex-vivo glutamate / total-choline was also associated with survival (p<0.01).
Conclusion
The identification of glutamate as a predictive biomarker of survival in pediatric medulloblastoma provides a clinically viable risk factor and highlights the importance of more detailed studies into the metabolism of this disease. Non-invasive biomarker detection using MRS may offer improved disease monitoring and potential for widespread use following multi-center validation.
Keywords: Metabolism, brain, tumor, MRS, survival
Introduction
Brain tumors present the highest cancer-related mortality rate in children, and medulloblastoma is the most common malignant brain tumor occurring in childhood (1). Whilst survival rates have improved, the prognosis for this disease remains relatively poor, and survivors often suffer a range of deficits due the limited specificity of available treatment options (2, 3). Current risk stratification for treatment of medulloblastoma primarily depends on: disease spread; the extent of residual disease following resection; patient age; and histologic sub-type (4). However considerable variation in survival is still found within these criteria (5), presenting a clear need for improved indicators of disease risk.
A number of new predictive markers of survival in medulloblastoma have been reported in recent years. Analysis of chromosomal aberrations have shown poor survival is correlated with 17p loss and 1q gain (6) and more recently, transcriptional analysis of medulloblastoma tissue has identified four distinct molecular subtypes (7). The WNT and Sonic Hedgehog (SHH) signaling pathways are thought to play a dominant role in the pathogenesis of the first two groups, having good and intermediate survival prospects respectively. The key molecular pathways of the third and fourth groups are less well established, however both are characterized by an over-representation of pathways involved in neuronal development (8) and a poorer outcome, with the c-Myc oncogene being frequently amplified and overexpressed in the third group. Together, these newly identified risk factors are likely to inform future treatment strategies of medulloblastoma.
Non-invasive molecular investigations offer great potential for improving disease management and stratification in medulloblastoma. Unlike conventional molecular markers that require biopsy tissue, non-invasive methods could be used to safely monitor patients at regular intervals, providing dynamic information on disease progression. 1H magnetic resonance spectroscopy (MRS) is a widely available technique, providing non-invasive measurement of tissue metabolite concentrations. Previous studies of pediatric brain tumors have found that MRS can detect a number of metabolites, which are predictive of survival across a range of tumor types (9, 10). However, prognostic biomarkers that are tailored to the tumor type have greater clinical value.
In this study we evaluate the potential of pre-treatment MRS to provide non-invasive predictive molecular biomarkers in a cohort of pediatric medulloblastoma. A simple protocol of single voxel MRS and fully automated spectral analysis was chosen to reduce barriers to widespread adoption of the method.
Materials and Methods
All patients with a confirmed diagnosis of medulloblastoma undergoing MR imaging at Birmingham Children’s Hospital were eligible to be enrolled on this study. Patients were enrolled on a consecutive basis without additional selection. The accrual period was between September 2003 and September 2011 and patients were followed up until July 2012. The accrual period was chosen to ensure at least 30 patients were included in the study, since an initial power calculation (two-sample comparison of proportions) showed that 15 patients in two equally sized groups with survival probabilities of 25% and 75% would have a power of 82% at a 5% significance level using a two-sided test. Survival time was defined as the period between the date of diagnosis, taken as the date of first tumor surgery, to the date of death; determined from the West Midlands tumor registry database and clinical records. The diagnosis of all tumors was established locally by two histopathologists (WHO 2007 classification (11)). The clinical and radiological features were also reviewed by the multidisciplinary team. Approval was obtained from the research ethics committee and informed consent given by parents/guardians. Investigators were not blinded to patient information, however since the MRS methodology chosen was fully-automated, and therefore user independent, interpretation bias was eliminated.
Since the study spanned a large time period, a number of different treatment protocols were used and are given in supplementary material. The treatment protocols followed national and international clinical trials or guidelines where available and local guidelines based on previous clinical trials where these were not available. The general principles were that a maximal surgical resection of the primary tumor was followed by adjuvant treatment. Children under 3 years of age were treated with intensive chemotherapy regimes and often focal radiotherapy. Older children received craniospinal radiotherapy and chemotherapy, with high-risk patients receiving higher doses of radiotherapy than those with standard risk. Standard risk patients were defined as being older than 3 years of age, M0 stage, no adverse histology and a complete or near complete resection of the primary tumor. Anaplastic or large cell medulloblastoma were both considered as adverse histological subtypes. Near complete resection was defined a residual tumor of less than 1.5cm2, measured from the imaging slice displaying the largest cross-section of tumor.
MRI and MRS were carried out at Birmingham Children’s Hospital, prior to the patient receiving treatment, on a 1.5T Siemens Symphony Magnetom with a single channel head coil and a 1.5T GE Signa Excite scanner equipped with an 8-channel head coil. Standard imaging included T1 and T2 weighted images of the brain followed by gadolinium contrast administration and then T1 weighted images of the head and spine where appropriate. The conventional imaging set was used to delineate the margins of the primary tumor from known characteristics (12) and the voxel for MRS was placed entirely within this region encompassing as much of the solid component of the lesion as possible.
Point resolved single voxel spectroscopy (PRESS) (13) was performed with an echo time of 30ms and a repetition time of 1500ms. Cubic voxels of either 2cm or 1.5cm length were used depending on the size of the tumor. Water suppressed data was acquired with 128 repetitions from the larger voxels and 256 repetitions from the smaller ones. A corresponding water unsuppressed spectrum was also acquired with 4 scans for use as a concentration reference. The TARQUIN (14) analysis package (version 4.2.11) was used to determine metabolite concentration and data quality parameters from the raw MRS data. The following metabolites were measured: alanine (Ala); citrate (Cit); creatine (Cr); gamma-Aminobutyric acid (GABA); glycerophosphocholine (GPC); glucose (Glc); glutamine (Gln); glutathione (Glth); glutamate (Glu); glycine (Gly); myo-inostial (Ins); lacate (Lac); N-acetylaspartate (NAA); N-acetylaspartylglutamate (NAAG); phosphocholine (PCh); scyllo-inositol (scyllo); taurine (Tau); total-choline (TCho=GPC+PCh); total-NAA (TNAA=NAA+NAAG) and total-lipids at 1.3ppm (TLip13). It is known that phosphocreatine produces a signal indistinguishable from creatine at a field strength of 1.5T. Therefore, future references to creatine or Cr are equivalent to creatine + phosphocreatine. Whilst all metabolites listed were included in the fitting process, Glc and GABA were excluded from further analysis due to limited evidence demonstrating they are reliably measured in brain tumor tissue. NAA and NAAG were not considered as individual measurements, since it is unlikely that these signals can be resolved at 1.5T given the high degree of overlap and low level of TNAA in this tumor group.
Each spectrum and its associated voxel placement were reviewed. Data were rejected if any of the following conditions were met: the voxel was placed closer than 4mm to lipid containing structures, greater than 5% of the voxel was estimated to contain non-involved brain, the baseline was unstable, obvious artefacts were present, the signal-to-noise ratio (SNR) was less than 4 or the overall metabolite linewidth exceeded 0.15ppm.
To reduce the risk of type I errors in the analysis, the cohort was split into two groups to allow a subsequent validation step: 1) patients diagnosed before January 2007 and 2) patients diagnosed after January 2007. These two groups are referred to as the initial and validation cohorts respectively. An exploratory statistical analysis was carried out on the initial cohort to establish potential non-invasive metabolite biomarkers of survival for medulloblastoma. Since MRS can provide a number of potential biomarkers, a more stringent significance value of p<0.01 was chosen to reduce errors associated with multiple comparisons. Kaplan-Meier survival analysis was performed on the initial cohort for each metabolite by dividing the cohort into two groups; cases with metabolite concentrations above and below a cut off value. The optimal cut off value was found for each metabolite by performing a chi-squared significance test over a range of values. Values that resulted in fewer than 5 cases in either group were not considered due to statistical instabilities. Hazard ratios were calculated at the determined cut-off using Cox-Regression. The metabolites associated with survival in the initial cohort were then tested pseudo-prospectively on the validation cohort by performing a Kaplan-Meir survival analysis using the same cut-off values.
Ex-vivo high-resolution magic angle spinning (hr-MAS) was performed on tumor tissue where available. Biopsy tissue was snap frozen in liquid nitrogen shortly after resection and stored at −80°C. Immediately prior to hr-MAS, tissue was thawed at room temperature and cut to approximately 15 mg where appropriate. Tissue was placed into a zirconia rotor and weighed. 4 μL of 3-(trimethylsilyl)proponic-2,2,3,3-d4 acid sodium salt (TSP) was dissolved in D2O at a concentration of 10 mM and was added to the rotor for referencing the ppm scale. The remaining volume of the rotor was filled with D2O. hr-MAS was performed on either a 600 MHz vertical bore spectrometer using a 4 mm gHX nanoprobe (Varian NMR Inc, Palo Alto, CA, USA) or a 500 MHz vertical bore spectrometer using a 4 mm hr-MAS 1H-13C NMR probe with a z-gradient (Bruker UK Limited, Coventry, UK). Standard pulse acquire MRS with water suppression was performed on each sample and 128 or 256 scans were collected depending on the sample weight. Sample temperature was maintained at 4°C throughout the experiment. Spectral processing and analysis was performed using in-house software. Data was manually phased, baseline corrected and frequency calibrated to the creatine resonance at 3.03ppm. Spectral integration was applied to the glutamate and choline region. The Glu/TCho ratio was used for comparison with the in vivo data since water reference data for absolute quantitation was not available for the ex-vivo data. The association between ex-vivo and in-vivo data was assessed graphically and a Pearson product-moment correlation coefficient was calculated.
Results
A total of 35 medulloblastoma patients were eligible for the study and 17 had died by the end of the study period. In one case, the in vivo MRS failed QC due to baseline distortion, making quantitation unreliable, and was excluded from further analysis. The baseline was poor for this particular case due to insufficient water suppression leaving residual signal; an example of this spectrum is given alongside a normal appearing baseline in supplementary Fig. S1. 16 cases were included in the initial cohort (diagnosed before January 2007) and 19 cases were included in the validation cohort (diagnosed after January 2007). The median observation time was 26 months, defined as time from diagnosis to either death or end of follow-up.
Optimal metabolite cutoff values and their respective hazard ratios and significances for the initial cohort (N=15) are given in Table 1. Three of the fourteen metabolites tested were found to be predictors of survival (p<0.01); creatine, glutamate and glycine. A subsequent survival analysis of creatine, glutamate and glycine on the validation cohort, using the same cutoff values determined from the initial cohort, found only glutamate to be significant (p=0.0096). Glutamate was found to have a hazard ratio of 8.0 with lower and upper confidence intervals of 2.2 and 29.0 respectively (likelihood ratio test p=0.0003) for the full cohort using a cutoff value of 2.77mM. Figure 1 shows Kaplan-Meier survival plots for glutamate for the A) initial cohort; B) validation cohort and C) full cohort.
Table 1. Table of optimized metabolite cutoff values for survival prediction in the initial cohort.
Hazard ratios were calculated using Cox-Regression and significance values represent the chisquare test for equality. TCho – total-choline; TNAA – total-NAA; TLip13 – total-lipid signal at 1.3PPM.
| Metabolite | Cutoff (mM) | Hazard ratio | P |
|---|---|---|---|
| Alanine | 0.24 | 0.31 | 0.0806 |
| Citrate | 0.22 | 0.57 | 0.3483 |
| Creatine | 2.80 | 0.19 | 0.0055 |
| Glutamine | 0.72 | 1.76 | 0.3545 |
| Glutathione | 0.65 | 0.20 | 0.0245 |
| Glutamate | 2.77 | 5.65 | 0.0077 |
| Glycine | 2.10 | 0.04 | 0.0001 |
| myo-Inositol | 0.11 | 1.31 | 0.6460 |
| Lactate | 2.68 | 1.82 | 0.3230 |
| scyllo-Inositol | 0.62 | 0.28 | 0.0503 |
| Taurine | 3.11 | 0.53 | 0.2872 |
| TCho | 3.21 | 0.39 | 0.1563 |
| TNAA | 1.37 | 0.64 | 0.4759 |
| TLip13 | 9.03 | 2.31 | 0.2064 |
Figure 1. Kaplan-Meier survival plots for glutamate levels in: A) initial cohort; B) validation cohort and C) full cohort.
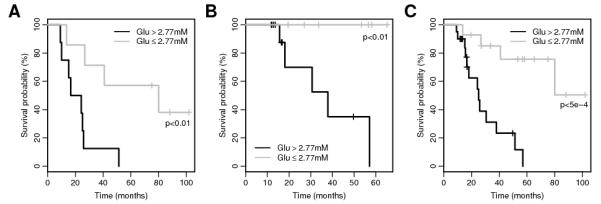
Significance values represent the chisquare test for equality.
The 35 cases included in this study were found to belong to the following histologic subtypes: classic medulloblastoma (N=31); desmoplastic/nodular medulloblastoma (N=2) and large-cell medulloblastoma (N=2). A summary of patient characteristics and common clinical prognostic markers are given in Table 2. The full cohort (excluding one case due to failed MRS QC) consisted of a relatively high proportion of boys to girls (2.4) with the population level expected to be between 1.5 and 2.0 (1) but no difference was found between the survival of boys and girls (p=0.5, chi-squared significance test). Age at diagnosis was found to predict survival in this cohort with patients under the age of five doing significantly worse than those who were older (p<0.002, chi-squared significance test), a finding consistent with previous studies (15). Both cases with large-cell medulloblastoma had high levels of glutamate. Cytogenetic analysis was routinely attempted on tumor tissue within the study period, and more recently interphase FISH was undertaken for Myc and chromosome 17 abnormalities. Overall seven cases were found to have chromosome 17 abnormalities in the tumor and one had c-Myc amplification. Five of the seven cases with chromosome 17 abnormalities were alive at the end of the study period. The patient with c-Myc amplification was 4 years and 11 months old at diagnosis; Chang stage M3; and in the high glutamate category. This patient died within two years of initial diagnosis.
Table 2. A summary of patient characteristics.
| High Glu (N=20) | Low Glu (N=14) | Initial cohort (N=15) | Validation cohort (N=19) | All (N=34) | |
|---|---|---|---|---|---|
| Male (%) | 75% | 71% | 73% | 74% | 74% |
| M0 (%) | 55% | 36% | 33% | 58% | 47% |
| Median age at diag (years, s.d) | 5.9 (4.1) | 6.7 (3.2) | 7.0 (3.8) | 6.1 (3.8) | 6.2 (3.7) |
| Median observation-time (months) | 17 | 55 | 26 | 27 | 26 |
| Sub-total resection of primary tumor (%) | 15% | 7% | 20% | 5% | 12% |
| Adverse histology (%) | 10% | 0% | 13% | 0% | 6% |
| Focal radiotherapy (%) | 20% | 7% | 27% | 5% | 15% |
| <30Gy craniospinal radiotherapy (%) | 30% | 36% | 13% | 47% | 32% |
| >30Gy craniospinal radiotherapy (%) | 40% | 57% | 53% | 42% | 47% |
No difference in survival was found between cases with non-metastatic (M0) and metastatic (M+) disease for children of all ages (p=0.5, chi-squared significance test). In addition, no association was found between glutamate levels and patient age (p=0.9, t-test); metastatic stage (p=0.5, Fisher’s Exact Test) or sub-total resection of primary tumor (p=0.6, Fisher’s Exact Test). Glutamate was also found to be a predictor (p<0.001, chi-squared significance test) of survival in the high-risk patients alone (N=18). Three of the cases treated on the standard risk protocol that had died within the study period were classified as having a high level of glutamate.
A scatter plot of glutamate values for all cases is given in Figure 2, with the optimal cutoff value found from the initial cohort represented as a horizontal line. No significant outliers are seen.
Figure 2. Distribution of glutamate for all cases.
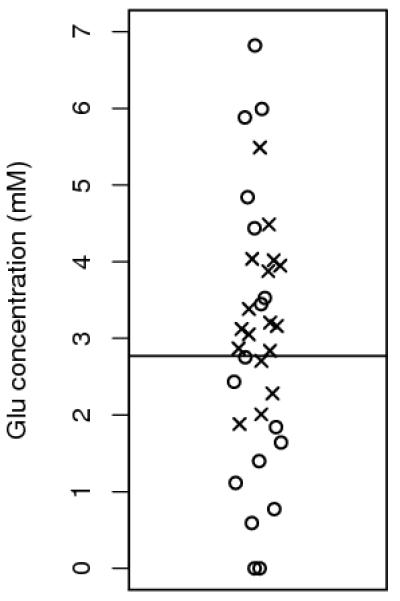
Horizontal line represents the cut off value used in Figure 1. Circles and crosses represent patients that survived or died respectively, during the follow-up period.
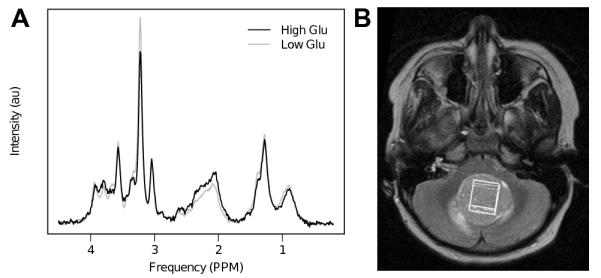
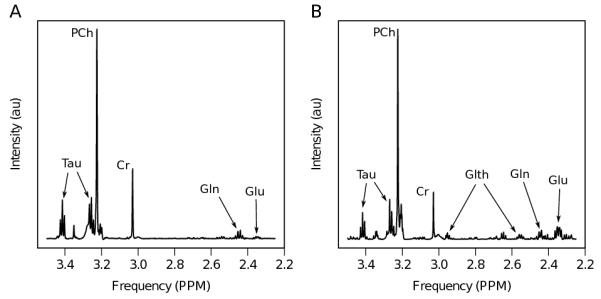
In addition to the sophisticated MRS analysis using TARQUIN (14), a simpler analysis was performed using spectral integration to help confirm the assignment of glutamate in the MRS. An integration of the main glutamate spectral region (between 2.1 and 2.5ppm) was found to be predictive of survival in the full cohort (p<0.05). Average spectra are shown in Figure 3 for cases with high (>2.77mM) and low (≤2.77mM) levels of glutamate.
Figure 3. A) Average spectra for cases with high (>2.77mM) and low (≤2.77mM) levels of glutamate. B) T2 weighted axial MRI, showing typical MRS voxel placement within the solid component of the tumor.
Matched hr-MAS data from biopsy tissue was available for 15 cases and a correlation (r=0.76, p=0.001) in the Glu/TCho ratio was found between in-vivo and ex-vivo results (Figure 4A), supporting in-vivo assignment and quantitation. Similar agreement between hr-MAS and in-vivo MRS has been reported previously (16, 17). Furthermore, a separate survival analysis of the hr-MAS data showed a similar trend to in-vivo MRS with a high Glu/TCho ratio inferring a poorer survival outcome (Figure 4B).
Figure 4. A) Correlation between in-vivo and ex-vivo MRS measurements of Glutamate/Total-choline, B) Kaplan-Meier survival plot for the available ex-vivo MRS samples (N=15).
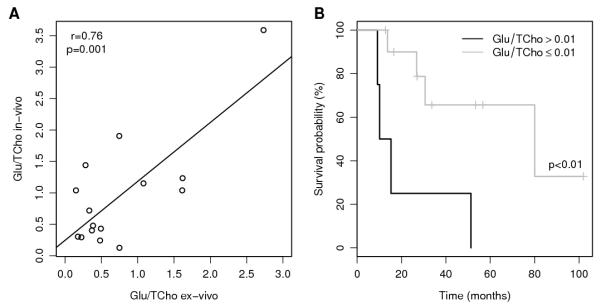
Figure 5 shows the typical data quality available from the hr-MAS technique, with the glutamate resonances being clearly identified well resolved from other metabolites. Figure 5 parts A) and B) show example hr-MAS data from two patients with low and high Glu/TCho ratios respectively. The patient with low glutamate was alive at the end of the study period, over 8 years from diagnosis, whereas the patient with high glutamate had died within one year of diagnosis.
Figure 5. Example ex-vivo MRS spectra from two medulloblastoma patients with low A) and high B) levels of Glutamate/Total-choline.
Prominent metabolite resonances are labeled as follows: Tau – taurine; PCh – phosphocholine; Cr – creatine; Gln – glutamine and Glu – glutamate.
Discussion
This study has shown that the concentration of glutamate, detected using in-vivo MRS, predicts the survival of children with medulloblastoma. The lack of a strong association between glutamate levels and other known clinical and radiological risk factors implies that this could be a new prognostic risk factor. Three patients with standard risk disease and high levels of glutamate died within the study period and may have benefited from more intensive treatment, in addition, high glutamate was predictive of poor survival in the sub-group of high risk cases. Glutamate could therefore provide improved treatment stratification for both high risk and standard risk medulloblastoma. In this study, the limited information available from tumor genetic testing failed to show an association with poor outcome but it is possible that glutamate mirrors molecular genetic subgroups of poor prognosis and this needs further exploration.
Unlike molecular markers obtained from tissue, MRS has the important property of being non-invasive, potentially offering new options for disease management. For example, with further improvements in adjuvant treatment, the pre-operative identification of lower risk disease may in future reduce the need for radical surgery and thereby reduce the risk of postoperative complications such as cerebellar mutism and ataxia (18). Outside the immediate post operative period, metabolite profiles measured by MRS have been shown to be useful in distinguishing tumor from post treatment abnormalities present on MRI (19) and tumor glutamate levels should be part of this assessment.
Glutamate was found to be significantly associated with survival in both the initial and validation cohorts despite an overall improvement in survival in the later cohort. Comparing the patient characteristics in the initial cohort and the validation cohort, Table 2 indicates that they are similar except for fewer patients with M0 disease in the initial cohort. In terms of treatment, children in the initial cohort were less likely to have a complete resection of the primary but more likely to have focal radiotherapy or less than 30Gy craniospinal radiotherapy. The trends in treatment are as expected with the emergence of protocols using lower doses of craniospinal radiotherapy for those with standard risk disease.
Since all the patients were not treated in a uniform manner, treatment could be a potential confounder in the analysis. However, the finding that glutamate is a strong marker of prognosis in two cohorts with consecutive time spans, and therefore treated with different protocols, implies that treatment effects do not account for the finding that high glutamate is associated with poor prognosis. Further studies of specific treatment groups should be undertaken to identify the patient groups for which glutamate level is most relevant. Indeed, the relatively high percentage of cases with metastatic disease and the high number of young children with M0 classic medulloblastoma make this a cohort with relatively poor prognosis - for which the discovery of novel biomarkers of prognosis is all the more important.
The role of glutamate metabolism in medulloblastoma is underexplored, however in recent years there has been particular interest in glutamate metabolism in adult gliomas. Studies of cell line and rodent glioma models have demonstrated an elevation in glutamate secretion (20) promoting neural degeneration in the tumor vicinity and disease spread (21). Glutamate metabolism has also been linked with the c-Myc oncogene in adult glioblastoma, where it has been shown that c-Myc transformed cells exhibit a reduced dependence on glucose and increased glutamine catabolism (22). These findings are relevant to medulloblastoma since glutamine is an immediate precursor to glutamate and Myc oncogenes are known prognostic factors (23), suggesting a potential link between Myc and glutamate/glutamine metabolism in this disease. In the cohort studied, the one case with confirmed c-Myc amplification also had poor outcome and high glutamate, providing anecdotal support for this hypothesis.
Recent improvements in the molecular subtyping of medulloblastoma (7) have led to great interest in the development of pre-clinical mouse models for the main molecular sub-groups (24). These new models, in combination with our findings and modern MRS techniques (25, 26) present a timely opportunity to investigate glutamine metabolism and the efficacy of therapeutic agents targeting this pathway (27-29) with clear translational potential.
Total-NAA, total-choline and total-creatine are reliably measured using MRS due to their relatively high concentration and distinctive narrow spectral appearance. However, the accurate detection of coupled resonances such as glutamate and glutamine is less well established due to difficulties associated with broader patterns overlapping with macromolecule resonances around 2ppm. Despite this, Figure 4 demonstrates a good correlation between in-vivo and ex-vivo MRS indicating that the combination of a short-echo time MRS protocol and automated spectral fitting (14) provides reliable quantitation. Furthermore, MRS quantitation accuracy in medulloblastoma is also aided by high quality data, due to minimal magnetic susceptibility issues in the cerebellum, and dense cellularity giving high signal-to-noise. A separate analysis of the available ex-vivo MRS showed that glutamate/total-choline ratio is predictive of survival. Total-choline will contribute to this finding if low values are associated with poor survival, although this was not found in-vivo.
Choline containing metabolites, such as glycerophosphocholine and phosphocholine, have been highlighted as potentially important biomarkers in cancer (30). Due to high spectral overlap at 1.5T, these signals were considered in combination as total-choline in this study, and not found to be related to survival. However, additional analysis of the individual signals found that glycerophosphocholine may be related to survival in the test cohort (p<0.01). Whist this observation should be considered as exploratory in nature, it does suggest further investigation with 31P MRS may be warranted.
Assessment of the multi-center reproducibility of MRS measurements, particularly for highly coupled metabolites, such as glutamate, remains an important future goal. The feasibility of this is indicated by a recent multi-center study which produced encouraging results for MRS metabolite profiles in aiding the diagnosis of the most common pediatric brain tumors (31). Overall, short echo time single voxel MRS is widely available and robust for the production of high quality data in most medulloblastomas. However, poor vendor support for the DICOM MRS data format can make multi-center studies difficult, as extra time and expertise is often required to perform proprietary data conversions to allow offline analysis with vendor neutral tools such as TARQUIN (14). Furthermore, a lack of standardization in hardware and MRS pulse sequences/shapes may introduce vendor specific bias.
Another important factor in the assessment of MRS biomarker detection is the growing clinical adoption of 3T MR systems, likely to lead to the improved detection of coupled metabolites due to reduced spectral overlap available at the higher field strength. Furthermore, optimizing MRS sequences for the detection of a particular molecule has been shown to be an effective strategy for detecting 2-hydroxyglutarate in adult brain tumors (32), a similar approach may yield improved glutamate detection.
The main limitations of this study were the relatively small numbers (N=35) available and multiple variables considered in the analysis, mitigated to a degree by setting a significance level of p<0.01 and performing validation on a test dataset. Whilst this study should be considered as exploratory in nature, multi-center validation is warranted.
In conclusion, the identification of glutamate as a non-invasive prognostic marker of survival in pediatric medulloblastoma offers the potential for improved disease management and highlights related molecular targets for therapy. Future work includes a comparison with established molecular subgroups and multi-center evaluation of this biomarker.
Supplementary Material
Translational relevance.
Medulloblastoma is the most common malignant brain tumor in children. Treatment intensification has led to improved survival rates but with a heavy burden of morbidity. The key clinical strategy is to personalize treatment through risk stratification in a manner which ensures the maximum chance of long term survival whilst minimizing morbidity. The identification of novel biomarkers of prognosis is an important part of this strategy and much progress has been made in defining molecular genetic subgroups. However, less progress has been made in identifying downstream molecular markers and this study presents a novel biomarker of poor prognosis; intra-tumoral gluatamate. This is of particular clinical relevance since it can be detected non-invasively allowing the result to be available prior to surgery and at follow-up if there is residual tumor. Incorporation in multi-centre clinical trials is required to allow its role in treatment decision making to be defined.
Acknowledgements
We would also like to thank the Birmingham Children’s Hospital Radiology Department, in particular Shaheen Lateef and Rachel Grazier for performing the spectroscopy and organizing the raw data. We would like to thank Dr Carole Cummins (University of Birmingham) for reviewing the statistical methodology and reporting.
Acknowledgements of research support: This work was funded by the Medical Research Council Grant Code G0601327, EU FP6 projects eTUMOUR and Health Agents and CR-UK& EPSRC Cancer Imaging Programme at the CCLG, in association with the MRC and Department of Health (England), NIHR Research Professorship, Birmingham Children’s Hospital Research Foundation and Poppyfields.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. 5th ed. Lippincott Williams & Wilkins; Philadelphia, Pa. ; London: 2006. [Google Scholar]
- 2.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. Journal of clinical oncology. 2001;19:3470–6. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 4.de Haas T, Hasselt N, Troost D, Caron H, Popovic M, Zadravec-Zaletel L, et al. Molecular risk stratification of medulloblastoma patients based on immunohistochemical analysis of MYC, LDHB, and CCNB1 expression. Clinical cancer research. 2008;14:4154–60. doi: 10.1158/1078-0432.CCR-07-4159. [DOI] [PubMed] [Google Scholar]
- 5.Polkinghorn WR, Tarbell NJ. Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol. 2007;4:295–304. doi: 10.1038/ncponc0794. [DOI] [PubMed] [Google Scholar]
- 6.McCabe MG, Backlund LM, Leong HS, Ichimura K, Collins VP. Chromosome 17 alterations identify good-risk and poor-risk tumors independently of clinical factors in medulloblastoma. Neuro-Oncology. 2011;13:376–83. doi: 10.1093/neuonc/noq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta neuropathologica. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. Journal of clinical oncology. 2011;29:1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson M, Cummins CL, MacPherson L, Sun Y, Natarajan K, Grundy RG, et al. Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur J Cancer. 2013;49:457–64. doi: 10.1016/j.ejca.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus KJ, Astrakas LG, Zurakowski D, Zarifi MK, Mintzopoulos D, Poussaint TY, et al. Predicting survival of children with CNS tumors using proton magnetic resonance spectroscopic imaging biomarkers. Int J Oncol. 2007;30:651–7. [PubMed] [Google Scholar]
- 11.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkovich AJ. Pediatric Neuroimaging. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 13.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–48. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, Peet AC. A constrained least-squares approach to the automated quantitation of in vivo (1)H magnetic resonance spectroscopy data. Magn Reson Med. 2011;65:1–12. doi: 10.1002/mrm.22579. [DOI] [PubMed] [Google Scholar]
- 15.Allen JC, Epstein F. Medulloblastoma and other primary malignant neuroectodermal tumors of the CNS. The effect of patients’ age and extent of disease on prognosis. J Neurosurg. 1982;57:446–51. doi: 10.3171/jns.1982.57.4.0446. [DOI] [PubMed] [Google Scholar]
- 16.Wilson M, Davies NP, Grundy RG, Peet AC. A quantitative comparison of metabolite signals as detected by in vivo MRS with ex vivo 1H HR-MAS for childhood brain tumours. NMR Biomed. 2009;22:213–9. doi: 10.1002/nbm.1306. [DOI] [PubMed] [Google Scholar]
- 17.Opstad KS, Wright AJ, Bell BA, Griffiths JR, Howe FA. Correlations between in vivo 1H MRS and ex vivo 1H HRMAS metabolite measurements in adult human gliomas. J Magn Reson Imaging. 2010;31:289–97. doi: 10.1002/jmri.22039. [DOI] [PubMed] [Google Scholar]
- 18.Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. Journal of neurosurgery. 2006;105:444–51. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 19.Gill SK, Wilson M, Davies NP, Macpherson L, English M, Arvanitis TN, et al. Diagnosing relapse in children’s brain tumors using metabolite profiles. Neuro-Oncology. 2014;16:156–64. doi: 10.1093/neuonc/not143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer research. 1999;59:4383–91. [PubMed] [Google Scholar]
- 21.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nature medicine. 2001;7:1010–5. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–93. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan SL, Schwalbe EC, Cole M, Lu Y, Lusher ME, Megahed H, et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta neuropathologica. 2012;123:501–13. doi: 10.1007/s00401-011-0923-y. [DOI] [PubMed] [Google Scholar]
- 24.Eberhart CG. Three down and one to go: modeling medulloblastoma subgroups. Cancer Cell. 2012;21:137–8. doi: 10.1016/j.ccr.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hekmatyar SK, Wilson M, Jerome N, Salek RM, Griffin JL, Peet A, et al. (1)H nuclear magnetic resonance spectroscopy characterisation of metabolic phenotypes in the medulloblastoma of the SMO transgenic mice. Br J Cancer. 2010;103:1297–304. doi: 10.1038/sj.bjc.6605890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin JL, Lehtimaki KK, Valonen PK, Grohn OH, Kettunen MI, Yla-Herttuala S, et al. Assignment of 1H nuclear magnetic resonance visible polyunsaturated fatty acids in BT4C gliomas undergoing ganciclovir-thymidine kinase gene therapy-induced programmed cell death. Cancer Res. 2003;63:3195–201. [PubMed] [Google Scholar]
- 27.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nature reviews Cancer. 2010;10:267–77. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 28.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6372–7. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocke KS, Staufner C, Luksch H, Geiger KD, Stepulak A, Marzahn J, et al. Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol Ther. 2010;9:455–68. doi: 10.4161/cbt.9.6.10898. [DOI] [PubMed] [Google Scholar]
- 30.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nature reviews Cancer. 2011;11:835–48. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vicente J, Fuster-Garcia E, Tortajada S, García-Gómez JM, Davies N, Natarajan K, et al. Accurate classification of childhood brain tumours by in vivo 1H MRS - a multi-centre study. Eur J Cancer. 2013;49:658–67. doi: 10.1016/j.ejca.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nature medicine. 2012;18:624–9. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


