Abstract
Background
The rate of human immunodeficiency virus type 1 (HIV-1) infection in Iran has increased dramatically in the past few years. While the earliest cases were among hemophiliacs, injection drug users (IDUs) fuel the current epidemic. Previous molecular epidemiological analysis found that subtype A was most common among IDUs but more recent studies suggest CRF_35AD may be more prevalent now. To gain a better understanding of the molecular epidemiology of HIV-1 infection in Iran, we analyzed all Iranian HIV sequence data from the Los Alamos National Laboratory.
Methods
All Iranian HIV sequences from subtyping studies with pol, gag, env and full-length HIV-1 genome sequences registered in the HIV databases (www.hiv.lanl.gov) between 2006 and 2013 were downloaded. Phylogenetic trees of each region were constructed using Neighbor-Joining (NJ) and Maximum Parsimony methods.
Results
A total of 475 HIV sequences were analyzed. Overall, 78% of sequences were CRF_35AD. By gene region, CRF_35AD comprised 83% of HIV-1 pol, 62% of env, 78% of gag, and 90% of full-length genome sequences analyzed. There were 240 sequences re-categorized as CRF_AD. The proportion of CRF_35AD sequences categorized by the present study is nearly double the proportion of what had been reported.
Conclusions
Phylogenetic analysis indicates HIV-1 subtype CRF_35AD is the predominant circulating strain in Iran. This result differed from previous studies that reported subtype A as most prevalent in HIV- infected patients but confirmed other studies which reported CRF_35AD as predominant among IDUs. The observed epidemiological connection between HIV strains circulating in Iran and Afghanistan may be due to drug trafficking and/or immigration between the two countries. This finding suggests the possible origins and transmission dynamics of HIV/AIDS within Iran and provides useful information for designing control and intervention strategies.
Introduction
Important characteristics that contribute to the worldwide spread of HIV are its enormous genetic variability and rapid evolution, which makes the virus highly adaptable to selection pressures of new hosts. The high error rate of the reverse transcriptase which lacks a proofreading mechanism, high rates of virus production in vivo, persistent nature of infection and selective immune pressure are factors responsible for the high genetic variation of HIV. [1] The presence of viral RNA as a dimer and co-infection of a cell with more than one viral genotype also results in recombination and mixed genotypes. Together, this presents a complex picture of genetic variation of HIV-1 virus. [2]
The genetic variability of HIV affects pathogenesis, immune response and escape, vaccine development, transmission, disease progression, drug resistance and treatment response. [3], [4] Therefore, molecular epidemiology studies are extremely important to characterize the HIV-1 subtype distribution in a specific population/region which may significantly influence diagnostic and therapeutic strategies. [5]
HIV-1 has four distinct genetic groups: M, N, O, and P. When the genetic groups are presented on a phylogenetic tree, strains within group M form well-defined clusters. Nine distinct subtypes (A–D, F–H, J, and K) have been identified, along with 61 circulating recombinant forms (CRFs) which are inter-subtype recombinant strains. [6]
The rate of human immunodeficiency virus type1 (HIV-1) infection in Iran has increased dramatically in the past few years. While the earliest cases were among hemophiliacs, injection drug users (IDUs) fuel the current epidemic. According to the CDC, a total of 26,556 PLWH had been identified in Iran through June 2013.[7]–[9] The HIV transmission routes in all the cases registered since 1986, in order of magnitude, are sharing injection equipment among IDUs (68.4%), sexual intercourse (12.3%), blood transfusion (0.9%), and mother-to-child transmission (1.2%); the route of transmission for the remaining 17.2% is unknown. [7]
In Iran, previous molecular epidemiological analysis of HIV-1 gag and env gene segments found that the predominant strain circulating among IDUs was subtype A which was related to African Ugandan/Kenyan sub-Saharan isolates.[10]–[13] More recent studies of pol, gag and env gene segments reported that the predominant strain was CRF_35AD.[6], [14]–[16] To gain a better understanding of the molecular epidemiology of HIV-1 infection in Iran, we analyzed all Iranian HIV sequence data from the Los Alamos National Laboratory.
Methods
A secondary analysis was performed using all Iranian HIV sequences from subtyping studies with pol, gag, env and full-length HIV-1 genome sequences registered in the HIV databases at the Los Alamos National Laboratory (www.hiv.lanl.gov) between 2006 and 2013. The sequences were downloaded along with reference nucleotide sequences for those regions [accession numbers: AB703607-AB703616, AY693842-AY693971, DQ077824-DQ077851, DQ077854-DQ077871, DQ115645-DQ115707, DQ149128-DQ149133, DQ788541-DQ788560, EU881931, FJ178375, FJ178376, FJ392730-FJ392755, FJ790242, FJ807629, FJ807630, GQ243705-GQ243708, GQ273945-GQ273960, GQ274861-GQ274878, GQ853425-GQ853428, GU724804-GU724845, HQ233645, HQ735066, HQ735068, KF029508-KF029592, EF158040- EF158043, GQ477442-GQ477451, DQ676872, AB253421, AB253429, AF286238, GU201516, AF286237, K03455, AY523387, AY173951, AY331295, U52953, U46016, AF067155, AY772699, K03454, AY371157, AY253311, U88824, AF077336, AF005494, AF075703, AJ249238, AY371158, AJ249236, AJ249237, AF377956, AF084936, AF061641, U88826, AY612637, AF190127, AF190128, AF005496, FJ711703, EF614151, GU237072, AF082394, AJ249239, GQ477441, GU564221, U54771, AY271690, AB485636, L39106, AF193276, AF049337, AF119819, AF119820, AF076998, AF193253, AY227107, AF064699, AY535659, AB286851, EF368372, EF368370, AF286230, HM067748, AY008715, AJ866553, AY093605, AY093603, AY093607, AF289548-AF289550, AF492623, AF492624, AF408629, AF408630, AF385936, DQ845388, AF450096, AF450097, DQ354120, AF516184, AY945736, AF286239, EU581825, EU581827, EU581828, AF377959, AY586541, AY894993, AY588971, AY588970, AY894994, AY586545, AY945737, AF457051, AF457072, AY371159, GQ229529, AY900571, AY900572, AY900574, AY900575, AJ670526, EU693240, EU697906, EU697908, FM877780, FM877782, FM877777, AJ404325, AM851091, DQ085872, DQ085873, DQ085874, DQ085876, AY771590, DQ085871, EF091932, AY727526, AY727527, AY535660, AB547464, DQ366659, DQ366662, EF165541, EF158043, EF158040, EF158041, EF087995, EF087994, EF116594, AF377957, FJ213781, FJ213782, FJ213780, EU735534, EU735536, EU735535, EU735538, EU735540, EU735339, EU170155, EU697904, EU697907, EU697909, FJ358521, FN392874, FN392876, FN392877, DQ358801, DQ358802, HM026456, GQ372987, FJ670529, HQ385477, HQ385479, HQ385478, L20587, L20571, AY169812, AJ302647, AY532635, AJ006022, AJ271370, HQ179987, GU111555, U42720, DQ373066, AF103818]. Pol, gag and env genes have been shown to be reliable regions for HIV-1 subtyping. [8], [17]–[19]
Nucleotide sequences from each gene were aligned with the reference sequences using CLUSTAL W software. Phylogenetic trees were constructed using Neighbor-Joining (NJ) and Maximum Parsimony methods (1000 times bootstrap replicates) with Molecular Evolutionary Genetics Analysis (MEGA) software version 5. [20] The Kimura 2-parameter model was used with a transition/transversion ratio of 1.5 and statistical support of the tree structures was obtained with 1000 bootstrap replicates. Significance was based on bootstrap values of >70%. [20]
To confirm the results obtained using MEGA5, sequences were re-analyzed using REGA. To improve the accuracy of the characterization of recombinant forms, Maximum Likelihood and NJ trees were re-constructed using RDP v.4.35 software. Results from all the different analyses were compared to determine the final subtype characterizations.
Results
A total of 475 Iranian HIV-1 sequences were analyzed in this study, of which 174 sequences were for pol genes, 161 sequences for env genes, 130 sequences for gag genes, and 10 sequences for full-length genomes. Overall, CRF_35AD was the predominant subtype representing 78% of sequences. By region, CRF_35AD comprised 83% of HIV-1 pol sequences, 62% of env sequences, 78% of gag sequences, and 90% of full-length genome sequences analyzed.
Table 1 presents the distribution of subtypes in the present study. There were 240 sequences re-categorized as CRF_AD and 2 sequences re-categorized as CRF_29BF. The proportion of CRF_35AD sequences categorized by the current study is nearly double the proportion of what had been reported in the HIV databases. Of the HIV-1 pol gene sequences analyzed, 69 A1 sequences (39.6%) in the Baesi and Hamkar studies and 55 CRF_AD sequences (31.6%) in the Soheili and Hamkar studies were re-categorized as CRF_35AD in present study and 2 sequences (1.1%) which had been reported as subtype B in the Hamkar study were re-categorized as subtype CRF_29BF. [10], [14], [16] Of the HIV-1 env gene sequences, 15 sequences (9.3%) which had been reported as subtype A1 in the Bahmani and Khosravi studies were re-classified as subtype CRF_35AD. [20] Of the HIV-1 gag gene sequences, 101 A1 sequences (77.7%) in the Naderi and Sarami studies were re-categorized as subtype CRF35_AD. [11], [12]
Table 1. HIV-1 subtype classification by gene region of HIV-1 Iranian sequences from the HIV databases (www.hiv.lanl.gov).
| HIV-1 Genes | ||||
| Subtype Number (%) | pol | env | gag | Full-length genome |
| CRF_35AD 144 (82.8%) | CRF_35AD 100 (62.1%) | CRF_35AD 101 (77.7%) | CRF_35AD 9 (90.0%) | |
| CRF_AE 2 (1.1%) | CRF_AE 1 (0.6%) | – | AE 1 (10.0%) | |
| – | – | A1 2 (1.5%) | – | |
| B 25 (14.4%) | B 54 (33.5%) | B 27 (20.7%) | – | |
| CRF_29BF 2 (1.1%) | – | – | – | |
| C 1 (0.6%) | C 6 (3.7%) | – | – | |
| Total | 174 | 161 | 130 | 10 |
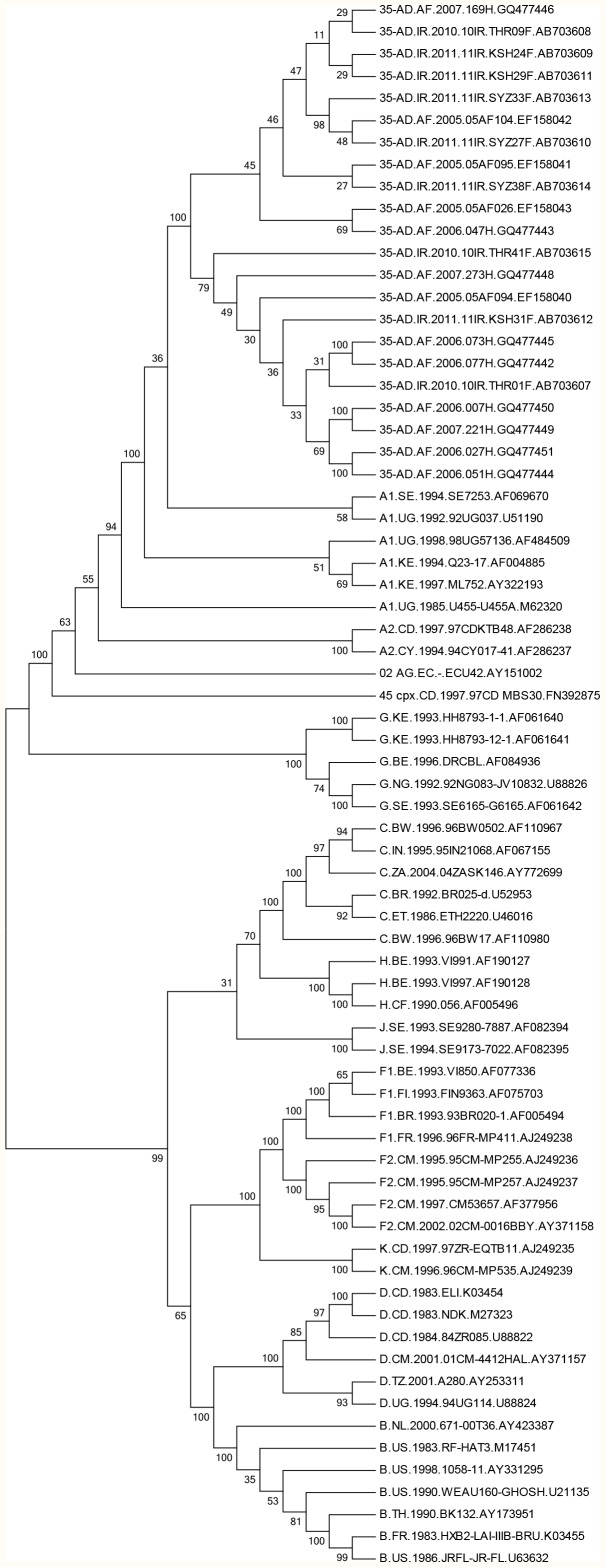
A phylogenetic tree of HIV whole genome sequences from Iran and Afghanistan is shown in Figure 1. The 13 Afghani isolates were from the mid- to late 2000’s whereas the 9 Iranian isolates were from the early 2010’s. In the three pairs that were comprised of an isolate from Afghanistan clustering with an isolate from Iran, the support values were low.
Figure 1. Un-rooted phylogenetic tree of CRF_AD whole genome sequences from Iran and Afghanistan, constructed using Kimura 2-parameter matrices and Neighbor-Joining method.
Discussion
In this phylogenetic analysis of sequences in the Iranian population, HIV-1 subtype CRF_35AD was found to be the dominant circulating strain. The result of this analysis differed from previous studies which reported subtype A as the most prevalent in HIV- infected patients in Iran but confirmed the results of other studies which reported the predominance of HIV-1 CRF_35AD among Iranian IDUs. The phylogenetic analysis also identified 2 cases of CRF_29BF. The observed differences between our findings and those of previous studies may be due to the unavailability of HIV reference sequences for certain subtypes, e.g., CRF_29BF, in the HIV databases in previous years or alignments performed using reference sequences that were not representative of all subtypes in Iran.
The identification of CRF_35AD and CRF_29BF strains circulating in Iran are likely the result of the importation of these strains from other countries. The observed epidemiological connection between HIV strains circulating in Iran and Afghanistan may be due to drug trafficking and/or immigration between these two countries. Iran is a major route for drug trafficking between Afghanistan and Europe. In addition, Iran has received a large number of Afghan refugees. Since CRF_35AD is also the dominant strain among Afghan IDUs, it is possible that the observed expansion of CRF_35AD is due in part to Afghan IDUs who immigrated to Iran. [6], [21], [22] CRF_29 strains in Iran may have originated from South America where this subtype is more commonly found.
Our findings suggest the possible origins and transmission dynamics of HIV/AIDS within Iran. Knowing the distribution of HIV variants alongside the corresponding epidemiologic factors will help assess the implications of any differences in transmissibility. The public health implications of such findings, including prevention and treatment strategies, are of special interest. According to the latest report from CDC, injection drug use remains the primary transmission route of HIV infection in the country. Therefore, current harm reduction programs for IDUs in Iran need to be strengthened to prevent further HIV transmission among IDUs and to other populations. This molecular epidemiological information will also be extremely relevant for guiding the development and implementation of diagnostic as well as preventive and therapeutic approaches in Iran.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All nucleotide sequences are available from the HIV databases at the Los Alamos National Laboratory (www.hiv.lanl.gov). Accession numbers: AB703607-AB703616, AY693842-AY693971, DQ077824-DQ077851, DQ077854-DQ077871, DQ115645-DQ115707, DQ149128-DQ149133, DQ788541-DQ788560, EU881931, FJ178375, FJ178376, FJ392730-FJ392755, FJ790242, FJ807629, FJ807630, GQ243705-GQ243708, GQ273945-GQ273960, GQ274861-GQ274878, GQ853425-GQ853428, GU724804-GU724845, HQ233645, HQ735066, HQ735068, KF029508-KF029592, EF158040- EF158043, GQ477442-GQ477451, DQ676872, AB253421, AB253429, AF286238, GU201516, AF286237, K03455, AY523387, AY173951, AY331295, U52953, U46016, AF067155, AY772699, K03454, AY371157, AY253311, U88824, AF077336, AF005494, AF075703, AJ249238, AY371158, AJ249236, AJ249237, AF377956, AF084936, AF061641, U88826, AY612637, AF190127, AF190128, AF005496, FJ711703, EF614151, GU237072, AF082394, AJ249239, GQ477441, GU564221, U54771, AY271690, AB485636, L39106, AF193276, AF049337, AF119819, AF119820, AF076998, AF193253, AY227107, AF064699, AY535659, AB286851, EF368372, EF368370, AF286230, HM067748, AY008715, AJ866553, AY093605, AY093603, AY093607, AF289548-AF289550, AF492623, AF492624, AF408629, AF408630, AF385936, DQ845388, AF450096, AF450097, DQ354120, AF516184, AY945736, AF286239, EU581825, EU581827, EU581828, AF377959, AY586541, AY894993, AY588971, AY588970, AY894994, AY586545, AY945737, AF457051, AF457072, AY371159, GQ229529, AY900571, AY900572, AY900574, AY900575, AJ670526, EU693240, EU697906, EU697908, FM877780, FM877782, FM877777, AJ404325, AM851091, DQ085872, DQ085873, DQ085874, DQ085876, AY771590, DQ085871, EF091932, AY727526, AY727527, AY535660, AB547464, DQ366659, DQ366662, EF165541, EF158043, EF158040, EF158041, EF087995, EF087994, EF116594, AF377957, FJ213781, FJ213782, FJ213780, EU735534, EU735536, EU735535, EU735538, EU735540, EU735339, EU170155, EU697904, EU697907, EU697909, FJ358521, FN392874, FN392876, FN392877, DQ358801, DQ358802, HM026456, GQ372987, FJ670529, HQ385477, HQ385479, HQ385478, L20587, L20571, AY169812, AJ302647, AY532635, AJ006022, AJ271370, HQ179987, GU111555, U42720, DQ373066, AF103818.
Funding Statement
The authors have no support or funding to report.
References
- 1. Baesi K, Ravanshad M, Ghanbarisafari M, Saberfar E, Seyedalinaghi S, et al. (2014) Antiretroviral drug resistance among antiretroviral-naïve and treatment experienced patients infected with HIV in Iran. J Med Virol. 86(7): 1093–1098. [DOI] [PubMed] [Google Scholar]
- 2. Hu DJ, Dondero TJ, Rayfield MA, George JR, Schochetman G, et al. (1996) The emerging genetic diversity of HIV. The importance of global surveillance for diagnostics, research, and prevention. JAMA. 275(3): 210–216. [PubMed] [Google Scholar]
- 3. Lessells RJ, Katzenstein DK, de Oliveira T (2012) Are subtype differences important in HIV drug resistance? Curr Opin Virol. 2(5): 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luft LM, Gill MJ, Church DL (2011) HIV-1 viral diversity and its implications for viral load testing: review of current platforms. Int J Infect Dis. 15(10): e661–670. [DOI] [PubMed] [Google Scholar]
- 5. Wainberg MA, Brenner BG (2012) The Impact of HIV Genetic Polymorphisms and Subtype Differences on the Occurrence of Resistance to Antiretroviral Drugs. Mol Biol Int. 2012: 256982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jahanbakhsh F, Ibe S, Hattori J, Manavari SH, Matsuda M, et al. (2013) Molecular Epidemiology of HIV Type 1 Infection in Iran: Genomic Evidence of CRF35_AD Predominance and CRF01_AE Infection Among Individuals Associated with Injection Drug Use. AIDS Res Hum Retroviruses. 29(1): 198–203. [DOI] [PubMed] [Google Scholar]
- 7.National AIDS Committee Secretariat, Ministry of Health and Medical Education (2013) Islamic Republic of Iran AIDS Progress Report: on Monitoring of the United Nations General Assembly Special Session on HIV and AIDS.
- 8. Jahanbakhsh F, Hattori J, Matsuda M, Ibe S, Monavari SH, et al. (2013) Prevalence of transmitted HIV drug resistance in Iran between 2010 and 2011. PLoS One. 8(4): e61864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mir-Nasseri MM, Mohammadkhani A, Tavakkoli H, Ansari E, Poustchi H (2011) Incarceration is a major risk factor for blood-borne infection among intravenous drug users. Hepat Monthly. 11(1): 19–22. [PMC free article] [PubMed] [Google Scholar]
- 10. Baesi K, Ravanshad M, Hosseini SY, Haji AAM, Shahzamani K (2010) Phylogenetic analysis of HIV-1 pol gene (RT sequences) among Iranian HIV infected patients. Sci J Iran Blood Transfus Org. 7(2): 94–100. [Google Scholar]
- 11. Naderi HR, Tagliamonte M, Tornesello ML, Ciccozzi M, Rezza G, et al. (2006) Molecular and phylogenetic analysis of HIV-1 variants circulating among injecting drug users in Mashhad-Iran. Infect Agent Cancer. 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarrami-Forooshani R, Das SR, Sabahi F, Adeli A, Esmaeili R, et al. (2006) Molecular analysis and phylogenetic characterization of HIV in Iran. J Med Virol. 78(7): 853–863. [DOI] [PubMed] [Google Scholar]
- 13. Tagliamonte M, Naderi HR, Tornesello ML, Farid R, Buonaguro FM, et al. (2007) HIV type 1 subtype A epidemic in injecting drug user (IDU) communities in Iran. AIDS Res Hum Retroviruses. 23(12): 1569–1574. [DOI] [PubMed] [Google Scholar]
- 14. Hamkar R, Mohraz M, Lorestani S, Aghakhani A, Truong HM, et al. (2010) Assessing subtype and drug-resistance-associated mutations among antiretroviral-treated HIV-infected patients. AIDS. 24: S85–S91. [DOI] [PubMed] [Google Scholar]
- 15. Mousavi SM, Hamkar R, Gouya MM, Safaie A, Zahraei SM, et al. (2010) Surveillance of HIV drug resistance transmission in Iran: experience gained from a pilot study. Arch Virol. 155(3): 329–334. [DOI] [PubMed] [Google Scholar]
- 16. Soheilli ZS, Ataiee Z, Tootian S, Zadsar M, Amini S, et al. (2009) Presence of HIV-1 CRF35_AD in Iran. AIDS Res Hum Retroviruses. 25(1): 123–124. [DOI] [PubMed] [Google Scholar]
- 17. Kurle S, Tripathy S, Jadhav S, Agnihotri K, Paranjape R (2004) Full-length gag sequences of HIV type 1 subtype C recent seroconverters from Pune, India. AIDS Res Hum Retroviruses. 20(10): 1113–1118. [DOI] [PubMed] [Google Scholar]
- 18. Lynch RM, Shen T, Gnanakaran S, Derdeyn CA (2009) Appreciating HIV type 1 diversity: subtype differences in Env. AIDS Res Hum Retroviruses. 25(3): 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tongo M, Martin DP, Zembe L, Mpoudi-Ngole E, Williamson C, et al. (2013) Characterization of HIV-1 gag and nef in Cameroon: further evidence of extreme diversity at the origin of the HIV-1 group M epidemic. Virology J. 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castro-Nallar E, Perez-Losada M, Burton GF, Crandall KA (2012) The evolution of HIV: Inferences using phylogenetics. Mol Phylogenet Evol. 62(2): 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanders-Buell E, Saad MD, Abed AM, Bose M, Todd CS, et al. (2007) A nascent HIV type 1 epidemic among injecting drug users in Kabul, Afghanistan is dominated by complex AD recombinant strain, CRF35_AD. AIDS Res Hum Retroviruses. 23(6): 834–839. [DOI] [PubMed] [Google Scholar]
- 22. Sanders-Buell E, Bose M, Nasir A, Todd CS, Stanekzai MR, et al. (2010) Distinct circulating recombinant HIV-1 strains among injection drug users and sex workers in Afghanistan. AIDS Res Hum Retroviruses. 26(5): 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All nucleotide sequences are available from the HIV databases at the Los Alamos National Laboratory (www.hiv.lanl.gov). Accession numbers: AB703607-AB703616, AY693842-AY693971, DQ077824-DQ077851, DQ077854-DQ077871, DQ115645-DQ115707, DQ149128-DQ149133, DQ788541-DQ788560, EU881931, FJ178375, FJ178376, FJ392730-FJ392755, FJ790242, FJ807629, FJ807630, GQ243705-GQ243708, GQ273945-GQ273960, GQ274861-GQ274878, GQ853425-GQ853428, GU724804-GU724845, HQ233645, HQ735066, HQ735068, KF029508-KF029592, EF158040- EF158043, GQ477442-GQ477451, DQ676872, AB253421, AB253429, AF286238, GU201516, AF286237, K03455, AY523387, AY173951, AY331295, U52953, U46016, AF067155, AY772699, K03454, AY371157, AY253311, U88824, AF077336, AF005494, AF075703, AJ249238, AY371158, AJ249236, AJ249237, AF377956, AF084936, AF061641, U88826, AY612637, AF190127, AF190128, AF005496, FJ711703, EF614151, GU237072, AF082394, AJ249239, GQ477441, GU564221, U54771, AY271690, AB485636, L39106, AF193276, AF049337, AF119819, AF119820, AF076998, AF193253, AY227107, AF064699, AY535659, AB286851, EF368372, EF368370, AF286230, HM067748, AY008715, AJ866553, AY093605, AY093603, AY093607, AF289548-AF289550, AF492623, AF492624, AF408629, AF408630, AF385936, DQ845388, AF450096, AF450097, DQ354120, AF516184, AY945736, AF286239, EU581825, EU581827, EU581828, AF377959, AY586541, AY894993, AY588971, AY588970, AY894994, AY586545, AY945737, AF457051, AF457072, AY371159, GQ229529, AY900571, AY900572, AY900574, AY900575, AJ670526, EU693240, EU697906, EU697908, FM877780, FM877782, FM877777, AJ404325, AM851091, DQ085872, DQ085873, DQ085874, DQ085876, AY771590, DQ085871, EF091932, AY727526, AY727527, AY535660, AB547464, DQ366659, DQ366662, EF165541, EF158043, EF158040, EF158041, EF087995, EF087994, EF116594, AF377957, FJ213781, FJ213782, FJ213780, EU735534, EU735536, EU735535, EU735538, EU735540, EU735339, EU170155, EU697904, EU697907, EU697909, FJ358521, FN392874, FN392876, FN392877, DQ358801, DQ358802, HM026456, GQ372987, FJ670529, HQ385477, HQ385479, HQ385478, L20587, L20571, AY169812, AJ302647, AY532635, AJ006022, AJ271370, HQ179987, GU111555, U42720, DQ373066, AF103818.