Abstract
Comorbidities are frequent in chronic obstructive pulmonary disease (COPD) and significantly impact on patients’ quality of life, exacerbation frequency, and survival. There is increasing evidence that certain diseases occur in greater frequency amongst patients with COPD than in the general population, and that these comorbidities significantly impact on patient outcomes. Although the mechanisms are yet to be defined, many comorbidities likely result from the chronic inflammatory state that is present in COPD. Common problems in the clinical management of COPD include recognizing new comorbidities, determining the impact of comorbidities on patient symptoms, the concurrent treatment of COPD and comorbidities, and accurate prognostication. The majority of comorbidities in COPD should be treated according to usual practice, and specific COPD management is infrequently altered by the presence of comorbidities. Unfortunately, comorbidities are often under-recognized and under-treated. This review focuses on the epidemiology of ten major comorbidities in patients with COPD. Further, we emphasize the clinical impact upon prognosis and management considerations. This review will highlight the importance of comorbidity identification and management in the practice of caring for patients with COPD.
Keywords: cardiovascular disease, prevalence, mortality, chronic bronchitis, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) represents a complex respiratory disorder characterized by chronic airflow limitation and an increased inflammatory response of the airways.1 Comorbidities are frequent in COPD and significantly impact on patients’ quality of life, exacerbation frequency, and survival.1,2 Although the mechanisms are yet to be defined, there is thought to be a chronic inflammatory state in COPD that accelerates the natural history of some comorbidities, and that these comorbidities merely reflect COPD as a systemic disorder.3
The distinction between comorbidities and systemic manifestations of COPD is, at present, unclear. Systemic features of COPD include cachexia, skeletal muscle abnormalities, osteoporosis, depression, anemia, and cardiovascular disease.1 For the purpose of this review, comorbidities are defined as concurrent diseases that occur in COPD with increased prevalence than in the general population and/or that significantly impact the management or prognosis of the patient with COPD.
Common problems in the clinical management of COPD include recognizing new comorbidities, determining whether the comorbidity or COPD itself is the cause of a patient’s symptoms, the concurrent treatment of COPD and comorbidities, and accurate prognostication. This paper focuses on the epidemiology of ten major comorbidities in COPD. Further, we highlight the clinical interaction between each comorbidity and COPD, with an emphasis on prognosis and treatment considerations.
Search methodology
The initial search was conducted using OVID Med-Line (from 1946) with the subject headings “pulmonary disease, chronic obstructive” and “comorbidities” (or “comorbidity” or “comorbidities”). This was repeated using the same subject headings on OvidMD. All abstracts were assessed for relevance, and articles of the relevant studies were retrieved. Subsequent searches utilized the following combinations of subject headings on PubMed: “pulmonary disease, chronic obstructive” or “cardiovascular disease” or “ischemic heart disease” or “congestive heart failure” or “pulmonary hypertension” or “lung cancer” or “diabetes” or “combined emphysema pulmonary fibrosis” or “chronic renal failure” or “chronic kidney disease” or “osteoporosis” or “depression” or “anxiety” or “gastroesophageal reflux” or “peptic ulcer disease”. For relevant titles, the abstracts were reviewed and, if still relevant, the article was retrieved. References within the selected articles were also reviewed for their relevance. In addition, for selected references, the “related citations” feature was explored using PubMed.
Comorbidities are common
Several studies have previously evaluated the impact of comorbidities on quality of life, mortality, and treatment options.2,4–7 Vanfleteren et al8 reviewed comorbidities in 213 patients with COPD in pulmonary rehabilitation as part of the CIRO Comorbidity (CIROCO) study. A total of 97.7% of patients in this cohort had one or more comorbidities, and 53.5% of patients were diagnosed with four or more comorbidities. Although this study actively investigated for comorbidities, it may nevertheless have underestimated the true prevalence of comorbidities, as patients with unstable COPD and/or certain comorbidities, such as acute myocardial infarction within the previous 6 months, were excluded from this trial.
Establishing the true prevalence of comorbidities in COPD and their relationship with COPD severity is confounded by several factors, including 1) shared risk factors for both COPD and several comorbidities,9 2) underdiagnosis of COPD,10,11 3) underdiagnosis of comorbidities, and 4) features of the comorbidity may overlap with features used to define the severity of COPD.12,13 Consequently, significant selection bias depending on the population reviewed is present in many studies investigating the incidence and prevalence of comorbidities in COPD. This bias, in part, explains the varied incidence and prevalence of common comorbidities between studies.
Despite these difficulties, the majority of studies agree that the most prevalent comorbidities include anxiety/depression, heart failure, ischemic heart disease (IHD), pulmonary hypertension (PHT), metabolic syndrome, diabetes, osteoporosis, and gastroesophageal reflux disease (GERD). We have also included lung cancer, pulmonary fibrosis, and chronic kidney disease (CKD) in this review, because of their clinical significance in COPD.
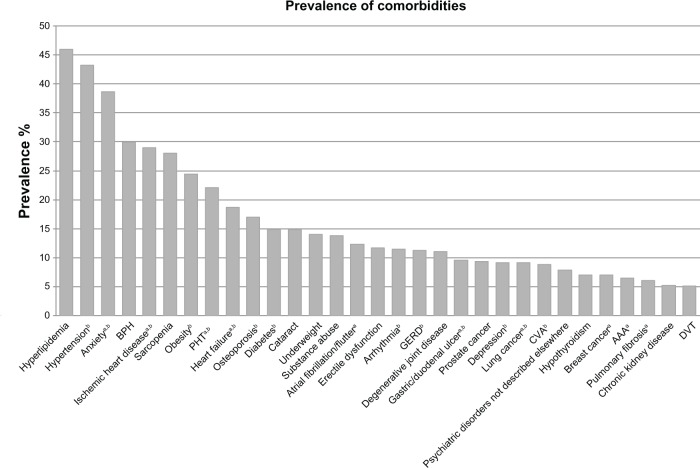
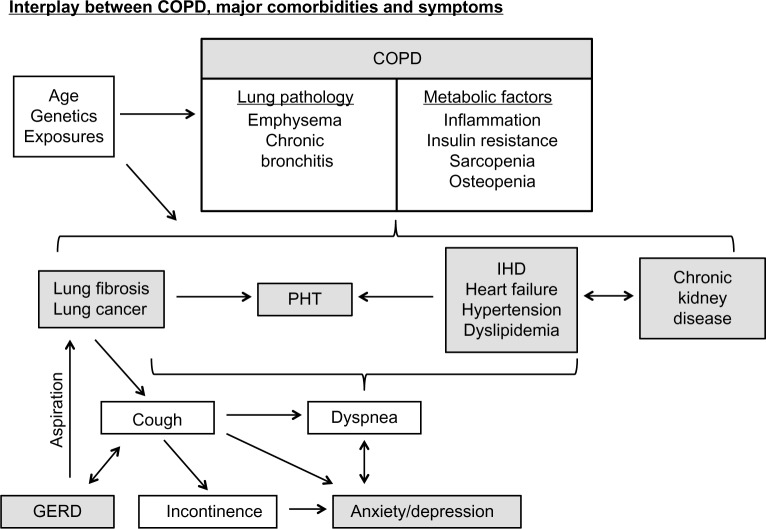
In Figure 1, we illustrate the prevalence of comorbidities in COPD, limited to those with a prevalence of greater than 5%. Figure 2 illustrates the interplay between COPD, major comorbidities, and symptoms.
Figure 1.
Prevalence of comorbidities in COPD.
Notes: Comorbidities with a prevalence of 5% or more are shown. Prevalence is calculated as a weighted average based on study sample size. aComorbidities with a significant increase in mortality risk compared with patients with COPD without the comorbidity; bcomorbidities with a significantly increased prevalence in patients with COPD compared with the general population. Data from many studies.3,8,27,28,30–32,35–40,48,50,51,86–91,122,126,127
Abbreviations: AAA, abdominal aortic aneurysm; BPH, benign prostatic hypertrophy; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DVT, deep vein thrombosis; GERD, gastroesophageal reflux disease; PHT, pulmonary hypertension.
Figure 2.
Interplay between COPD, major comorbidities, and symptoms.
Notes: Dyspnea and cough are central features of many comorbidities. The varied ways in which COPD and the comorbidities themselves interact together are displayed. Thus, clarifying the exact cause of these symptoms (or more likely which combination of comorbidities are involved and to what extent) in such patients can be challenging.
Abbreviations: COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease, IHD, ischemic heart disease; PHT, pulmonary hypertension.
Comorbidities decrease quality of life
Quality of life and self-reported health status decrease with an increasing number of comorbidities in patients with COPD.2,14,15 van Manen et al14 demonstrated that three or more comorbidities better correlated with health-related quality of life scores than forced expiratory volume in 1 second (FEV1) or dyspnea. Putcha et al2 investigated the impact of comorbidities on self-reported health status in 41,658 patients as part of the National Health and Nutrition Examination Survey (NHANES) survey between 2001 and 2008. For every additional comorbidity, the odds of poorer self-rated health were increased by 43%. Highly prevalent comorbidities such as heart failure, diabetes, arthritis, and urinary incontinence/prostatic disease were individually associated with a significant decrease in quality of life score, adjusted for age, sex, race, and other comorbidities.
Comorbidities increase exacerbations
Several comorbidities are associated with increased exacerbations, including GERD,16 anxiety,17 depression,17 pulmonary embolus,18 PHT,19 and cardiovascular disease.7 Furthermore, the number of comorbidities has been correlated with increased risk of exacerbation and hospitalization.20 Whether the comorbidities precipitate exacerbations, mimic COPD exacerbations, represent increased COPD severity, or perhaps a combination of the above, is still the subject of investigation.12 No matter the cause, increased comorbidities correlate with hospitalizations,20 length of stay, and mortality,7,20 both in hospital and once discharged.21
Comorbidities increase mortality
In patients with severe COPD, respiratory failure is the most common cause of death.5,22,23 However, in earlier stages of COPD, cardiovascular disease and lung cancer are the most common.5,23,24 The Towards a Revolution in COPD Health (TORCH) survival study investigators described the cause of death and whether this was related to COPD.25 Only 40% of deaths were judged to be related to COPD.25 Thus, rigorous investigation for comorbidities and management thereof could have been potentially lifesaving for the 60% of deaths that were related to other factors.
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) trial investigated 2,164 patients with COPD in an outpatient setting and compared these patients with smoking and nonsmoking controls.4 Comorbidities that were associated with a significantly increased mortality included heart failure (hazard ratio [HR]: 1.9), IHD (HR: 1.5), heart disease general (HR: 1.5), and diabetes (HR: 1.7). These were independent of FEV1, BODE index (body mass index, airflow obstruction, dyspnea and exercise); and exacerbation frequency.4 Additionally, the number of comorbidities is an independent predictor of all-cause mortality.4–7 ECLIPSE identified a mortality odds ratio (OR) of 2 when comparing three comorbidities versus none. The OR increased to 4.57 when comparing four comorbidities versus none.4 This may, in part, explain why low health-related quality of life scores were more reliable predictors of mortality than FEV1 severity.5,6
Divo et al3 investigated comorbidities in 1,664 patients with COPD for a median of 51 months. They used the 12 comorbidities with the highest HRs for mortality to construct a prognostic tool, the COPD-specific Comorbidity Test (COTE) index. Mortality from both COPD and non-COPD causes increased with increasing COTE scores, with a score of 4 or more significantly increasing the risk of mortality (HR: 2.3; 95% confidence interval [CI]: 2.00–2.75; P<0.001). Though further validation of this index is required (only 186 women were included in this study, making the breast cancer and anxiety factors less reliable), the underlying premise that a comorbidity index can predict mortality and be useful in clinical practice is promising.
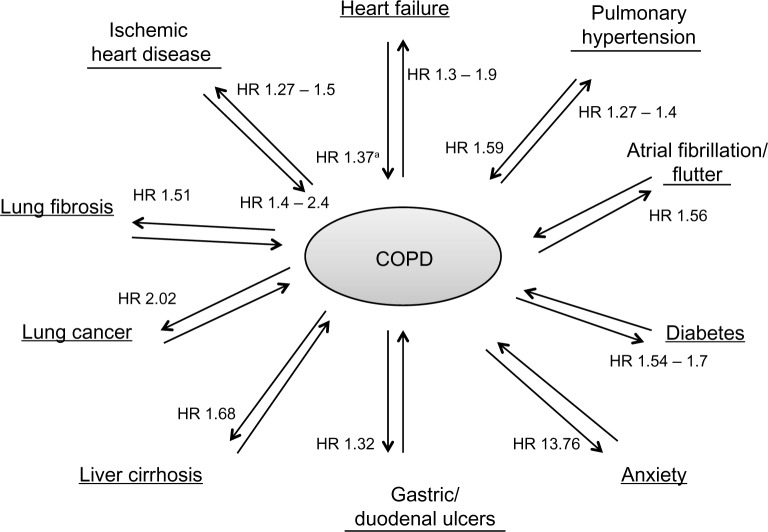
In Figure 3, we depict the mortality HR of major comorbidities in COPD. Comorbidities are discussed in further detail individually in the following sections.
Figure 3.
Impact of COPD and comorbidities on mortality.
Notes: The impact of each comorbidity on the mortality risk of patients with COPD is demonstrated. For example, for patients with COPD the HR of all-cause mortality associated with concurrent pulmonary hypertension (versus those with COPD alone) is 1.27–1.4. Where available for each comorbidity the reverse is also shown. For example, in patients with pulmonary hypertension the HR of all-cause mortality associated with concurrent COPD (versus those with pulmonary hypertension alone) is 1.59. aThis HR was not significant after adjustment for confounding risk factors. Data taken from multiple studies.3,4,105,145–147
Abbreviations: COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
Anxiety and depression
Epidemiology
Anxiety and depression are common comorbidities in COPD, with enormous variability in prevalence data, depending on study population and definition of these psychiatric comorbidities (self-reported, questionnaire, or clinical review, according to Diagnostic and Statistical Manual of Mental Disorders criteria). Anxiety prevalence ranges from 6% to 74%.3,8,26–32 However, a recent meta-analysis of studies with diagnostic clinical interview demonstrated prevalence of anxiety as 10%–55% (median 17%) in inpatients and 13%–46% in outpatients.33 Prevalence of depression ranges from 8% to 80% in patients with COPD.3,8,26,28,29,31,32,34–40 Notably, both anxiety and depression are more prevalent in COPD than in the general population.26,33,39,41 A temporal relationship between COPD and later depression diagnosis has been established,42 and a recent meta-analysis confirmed an increased risk of depression in COPD patients (risk ratio [RR]: 1.69; 95% CI: 1.45–1.96).43 Furthermore depression is more prevalent in COPD than in other chronic diseases such as diabetes, IHD, stroke, arthritis, hypertension, and cancer.40
Risk factors for anxiety and depression include physical disability, oxygen dependence, respiratory symptoms (mainly dyspnea), increased number of comorbidities, female sex, current smoking, low socioeconomic class, marital status (ie, widowed/divorced/never married), living alone, and poor quality of life.40,44 COPD severity by FEV1% predicted has been correlated with increased risk of depression.39,44 However, health-related quality of life scores generally predict risk of depression more reliably.39
Mechanisms of interaction
At present, the mechanisms of increased likelihood of anxiety and depression in COPD are unclear. There are obvious physical factors that can impact on the likelihood of reactive depression in terms of loss of independence, control over disease, and physical disability. However, these do not explain the increased prevalence over stroke and other chronic debilitating diseases. Other mechanisms that have been postulated include systemic inflammation, hypoxia, and smoking effects on brain function.45
With regard to anxiety, physical factors such as limited exercise tolerance, incontinence, fear of dyspnea, and requirement for constant medications can impact on the development of social phobias, as well as generalized anxiety disorders and panic attack disorder.33 However, physiological factors such as dynamic hyperinflation and hyperventilation related to dyspnea may also be part of the pathogenesis.33,41
Association of anxiety/depression and COPD
Anxiety or depression comorbidity in patients with COPD predicts decreased quality of life,41,43,45–47 reduced exercise capacity,39,41,43,45 increased hospitalizations/exacerbations17,41,43,47 with longer length of stay,17,45 and increased mortality.3,41,43 This poor prognosis is further exacerbated by anxiety and depression predicting decreased likelihood of smoking cessation41 and poor adherence to medications and pulmonary rehabilitation.43,47
Treatment of COPD, anxiety, and depression
Limited studies review treatments for COPD and anxiety or depression. At present, standard therapies according to local guidelines should be used, including cognitive behavioral therapy, antidepressants, and anxiolytics.41
Tricyclic antidepressants, mirtazapine, and benzodiazepines can precipitate respiratory drive depression and respiratory failure, making them particularly dangerous in carbon dioxide retainers and those with moderate or severe COPD.41
Pulmonary rehabilitation has been shown to reduce dyspnea, fatigue, symptoms of anxiety and depression, and to increase quality of life.41 This is despite poor adherence from patients with concurrent anxiety or depression. In patients with COPD, relaxation therapy with cognitive behavioral therapy reduced dyspnea and increased psychological well-being, and home-based palliative care services also improved psychological outcomes.41
Heart failure
Epidemiology
The prevalence of heart failure in COPD cohorts varies between 5.3% and 24.4%.48–51 This disparity is most notable when comparing the inpatient, outpatient, and national databases populations. This is due to several reasons, including 1) patients with significant heart failure are more likely to be hospitalized or seen in specialty outpatient clinics, 2) stable outpatients with COPD are less likely to have active investigation for comorbidities, and 3) accurately diagnosing heart failure in patients with COPD can be difficult. For example, adequate echocardiographic views are limited by hyperinflation in 10%–35% of patients.52 Similarly hyperinflation can mask increased cardiac size and remodeling of the pulmonary vascular bed may make the usual markings of interstitial edema difficult to perceive on chest roentography.52 Furthermore, obstructive spirometry may be seen in acute decompensated heart failure.52 Brain natriuretic peptides (BNPs) are helpful in the acute setting to establish a diagnosis of heart failure. However, these are not specific for left ventricular (LV) failure and are often raised in COPD.53 Nevertheless, heart failure is more prevalent in COPD cohorts than in the general population, and this is independent of smoking.48–51
Mechanisms of interaction
Shared etiological factors such as increased age and smoking, together with the high prevalence of hypertension and IHD in patients with COPD, confer much of the increased risk of heart failure in COPD patients. Systemic inflammation is thought to accelerate atherosclerosis and thereby increase the risk of heart failure.53 In the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial there was an increase in cardiac failure at 30 days (RR: 14.08) and 180 days (RR: 10.71) post-exacerbation.54 This risk was conferred even in those without known cardiovascular disease at baseline. Similarly, the increase in systemic inflammation and myocardial infarcts in this period provides a biologically plausible link between uncontrolled COPD and increased heart failure. Furthermore, decreased LV filling resulting in decreased cardiac output was correlated with computed tomography-based severity of emphysema.55,56 In severe emphysema, pulmonary hyperinflation (reducing systemic venous return and thus preload),57 alveolar hypoxia (inducing increased pulmonary vascular resistance), and ventricular interdependence are all likely to contribute to reduced LV filling55 and thus decrease cardiac output. However, the reduced LV filling was also demonstrated in mild emphysema where these factors are unlikely to be involved.55 Further work in this area is required to clarify the mechanism by which this interaction occurs.
Association of heart failure and COPD
Heart failure significantly decreases quality of life and health status (OR: 3.07; P<0.001) independent of age, sex, race, and other comorbid conditions statistically associated with self-rated health.2 Furthermore, the ECLIPSE trial demonstrated that concurrent heart failure conveyed an increased mortality (HR: 1.9), dyspnea score, and BODE score and a decrease in 6-minute walk distance.4
Reduced FEV1 has been shown in multiple epidemiological studies to be associated with increased cardiovascular mortality.53,58–60 However, a restrictive spirometric pattern (with a reduced FEV1 and FVC) is common in patients with heart failure, and hence FEV1 is not a good surrogate marker of COPD severity in this context.61,62
Ischemic heart disease
Epidemiology
The prevalence of IHD in COPD patients ranges between 16.1% and 53%3,35,51 and includes various descriptions (coronary artery disease, angina, and myocardial infarction). The majority of studies have found a statistically significant increase in IHD in patients with COPD.48,50 Studies have demonstrated the increased risk of myocardial ischemia in stable COPD50 during exacerbations63,64 and post-exacerbation.65 A large study by Curkendall et al48 demonstrated a significant RR for both angina (RR: 2.02; 95% CI: 1.82–2.25) and myocardial infarction (RR: 1.99; 95% CI: 1.72–2.32) in patients with COPD after adjustment for cardiovascular risk factors, previous history of cardiovascular disease, sex, and time frame of follow-up. Furthermore, Finkelstein et al50 reviewed outpatient data from the National Health Interview Survey (NHIS) and demonstrated a similar OR for IHD (OR: 2.0; 95% CI: 1.5–2.5) and were able to adjust for smoking history. Similar to heart failure, IHD is often under-recognized in COPD.64
Mechanisms of interaction
The mechanism by which IHD is increased in prevalence in patients with COPD is complex and incompletely understood. A combination of increased risk factors in patients with COPD,45 chronic systemic inflammation accelerating atherosclerosis, vascular endothelial dysfunction,66,67 physiological stress from comorbidities, and acute inflammation following exacerbation are likely to be involved.65
Arterial stiffness (measured by aortic pulse wave velocity), an independent predictor of cardiovascular events and mortality, is increased in patients with COPD and was correlated with computed tomography-quantified emphysema and airflow obstruction.66 Furthermore, Eickhoff et al67 demonstrated that both endothelium-dependent and endothelium-independent vasodilation was significantly impaired in patients with COPD when compared with nonsmoking and smoking controls. Evidence is conflicting at present as to the cause of arterial stiffness, with a recent study demonstrating no correlation between this and systemic inflammatory markers.68 Lahousse et al69 demonstrated that not only was there an increase in carotid artery thickening and plaque formation in patients with COPD but also they were more likely to develop lipid cores, a marker of vulnerable plaques that were more likely to rupture.
Though the exact mechanisms are yet to be elucidated, the temporal relationship of ischemic events with acute exacerbations65 and correlation of systematic inflammatory markers such as C-reactive protein and fibrinogen, with increased IHD70 implicate inflammation as a significant contributor.
Association of ischemic heart disease and COPD
Patel et al71 recently demonstrated that COPD patients with ischemic changes on their ECG have a significant decrease in their 6-minute walk distance and were more likely to have a 6-minute walk test of <350 m, which correlates with increased mortality.72 Though there was no increase in exacerbations found in this study, there was a significant increase in time to resolution of symptoms following an exacerbation, which may confer the decrease in health status.71 This same population also had significantly higher dyspnea scores (Modified Medical Research Council); increased Charlson Comorbidity Index; higher BODE or age, dyspnea, obstruction (ADO) scores irrespective of FEV1; and worse health status (St George’s Respiratory Questionnaire).71
In both the ECLIPSE trial and investigation of the BODE cohort, IHD was associated with significantly increased mortality.3,4 Furthermore, using a scoring system called Cardiac Infarction Injury Score (CIIS) in COPD outpatients, Brekke et al64 verified previous work (in patients without COPD) that a CIIS score of >20 was an independent predictor of mortality. The CIIS is a score based on features of ischemia on ECG, and demonstrated an adjusted HR of 1.52 (95% CI: 1.14–2.03)64 for the first year following COPD exacerbation. Similarly, markers of cardiac dysfunction, such as troponin and BNP, have been shown to predict 30-day mortality following COPD exacerbations.73
Therefore, it is clear that IHD is common, clinically relevant in terms of impact on quality of life and mortality, and under-recognized. Simple measures such as ECG, troponin, and BNP have been shown to be useful predictors of mortality and can inform physicians of the potentially reversible risk of IHD.
Treatment of cardiovascular disease in patients with COPD
β-blockers
COPD is often the cited reason for under-treatment of IHD and heart failure with β-blockers.74,75 In patients with COPD, the benefits of β-blockers in the treatment of IHD and heart failure are substantial in terms of both mortality and morbidity.53,74–76 Cardioselective β-blockers such as bisoprolol and metoprolol are safe for use in stable COPD, as long as adverse effects are considered and addressed if present.53,74,75,77 However, Ekstrom et al78 demonstrated increased mortality in patients with severe oxygen-dependent COPD treated with β-blockers. In contrast, in a study of 35,082 patients with an acute exacerbation of COPD who continued on their previously prescribed β1-selective β-blockers for IHD, heart failure, or hypertension, there was no increase in in-hospital mortality, 30-day readmission, or late mechanical ventilation rates.74 This is particularly pertinent given the increased risk of cardiac event periexacerbation.65,74 Nonselective β-blockers did confer an increased rate of readmission at 30 days in this study.74 Further studies are required to determine the safety of commencing β-blockers at the time of acute exacerbation, as late mechanical ventilation rates were increased in this group.74
In summary, in patients with stable COPD, cardioselective β-blockers should be used to treat chronic heart failure, IHD, and hypertension, as per local cardiovascular guidelines, with appropriate monitoring of side effects.53,74,75 Furthermore, patients chronically prescribed cardioselective β-blockers do not require these to be withheld during acute exacerbations of COPD.74 Until further evidence is available, cardioselective β-blockers should be used in preference to nonselective β-blockers in patients with COPD, and cardioselective β-blockers should be commenced with caution during acute exacerbation or in oxygen-dependent patients.74,78
Angiotensin-converting enzyme inhibitors
Angiotensin-converting enzyme (ACE) inhibitors have been associated with reduced exacerbations and mortality in COPD.45,79 Furthermore, lowering of ACE levels has been postulated to decrease lung inflammation and improve respiratory muscle function.45,75,79 At present, this data is mainly limited to observational studies. Therefore, guidelines suggest their use in COPD and cardiovascular disease but not yet for COPD alone.45,75,79
Statins
Statins have been proven effective in treating patients with IHD and heart failure through their pleiotropic effects.75 Statins have a combination of cholesterol-lowering, anti-inflammatory, immunomodulatory, and antioxidant effects.53,75 Observational studies have correlated statin use with reduced COPD and all-cause mortality, C-reactive protein levels, exacerbation risk, and improved quality of life.45,53,75,79–81 At present, evidence supports the use of statins in COPD patients with cardiovascular disease or hyperlipidemia, not purely for COPD alone.75
Treatment of COPD in patients with cardiovascular disease
Both short- and long-acting β-agonists have been associated with increased myocardial infarction, angina, arrhythmias, and heart failure.75 It is anticipated that the Study to Understand Mortality and Morbidity in COPD (SUMMIT), which is comparing fluticasone furoate/vilanterol (100/25 μg), fluticasone furoate (100 μg), vilanterol (25 μg), and matched placebo, will help delineate this issue.82 In this study, patients will have both COPD and a history of cardiovascular disease or significant risk factors for the development of cardiovascular disease, and drug effects on mortality will be reviewed.82 While awaiting these results, prudent use of the minimum β-agonist required to maintain COPD control is recommended.
Initial concerns with regard to cardiovascular risk increase with anticholinergic bronchodilators such as ipratropium and tiotropium have not been supported by larger studies.75,83 Indeed, UPLIFT demonstrated a reduction in all-cause and cardiovascular mortality.83 Thus, it is advisable to use long-acting anticholinergics as first-line therapy rather than long-acting β-agonists in patients with concurrent cardiovascular disease.75
Pulmonary hypertension
Epidemiology
The prevalence of PHT in COPD is difficult to ascertain. This is primarily due to the absence of right heart catheter information from large cohorts of COPD patients, due to the expense and potential risk. Current literature determining prevalence is highly variable, as it comes from heterogeneous populations of COPD subjects and uses nonuniform definitions of PHT. As a result, the reported prevalence of PHT in moderate to severe COPD ranges from 10.2% to 91%.3,84–91 The prevalence of PHT increases with the severity of COPD, with the majority of patients with COPD having mild to moderate PHT.84,85 There is, however, a subgroup of patients with severe PHT considered “out of proportion” to the severity of their COPD.87,92 Although this represents only a small subset of the COPD population, the prevalence of severe PHT in this context is nevertheless significantly greater than in the general population.
Mechanisms of interaction
Hypoxia is a critical precipitant in the pathogenesis of COPD-associated PHT19,84 and, at least in part, explains the correlation between severity of COPD and the development of PHT. Hypoxia induces pulmonary vasoconstriction and pulmonary vascular remodeling in the form of intimal thickening and muscularization of arterioles, thereby increasing pulmonary vascular resistance. Acidemia, dynamic pulmonary hyperinflation, endothelial dysfunction, polycythemia, inflammation, and parenchymal destruction have also been implicated in the pathogenesis of increased pulmonary vascular resistance in patients with COPD. Furthermore, comorbidities of COPD such as heart failure, sleep-disordered breathing, and pulmonary thromboembolism further compound the effects of COPD in increasing the pulmonary vascular resistance.19,84 At present, the impact each risk factor contributes and why a small but significant group of COPD patients develop severe PHT is unclear. Genetics clearly influence this,19,92 with recent studies of polymorphisms in endothelial nitric oxide synthase, 5-hydroxytryptamine, and interleukin genotypes providing further insight into the potential factors at play.19,84
Association of PHT and COPD
The presence of PHT in COPD patients is associated with decreased exercise function,91 increased hospitalizations,89 and increased mortality.93–95 PHT is a more reliable predictor of both mortality and exacerbations than FEV1.89,93
Although clinical assessment of PHT in COPD is challenging and investigations have their limitations, diagnosing PHT in this context will have a bearing on patient management. Clinical signs of right ventricular failure occur late in PHT.96 Clinical suspicion of PHT should be aroused in patients with reduced 6-minute walk distance, significant oxygen desaturation, or disproportionately low gas transfer.85,96,97 Echocardiography is considered the best first-line investigation,19,85 and despite the well-documented limitations of this modality,98–100 screening can be improved by incorporating an assessment of right ventricular size and function. Right heart catheter remains the gold standard for diagnosis of PHT,101 but careful consideration of risks and benefits needs to be given for each patient. The presence of PHT in COPD may also have a bearing on advanced therapies for COPD, including lung volume reduction surgery,102 bronchoscopic lung volume reduction,103 and lung transplantation.104
Treatment of COPD and pulmonary hypertension
The presence of COPD in patients with PHT impacts considerably on prognosis105 and treatment considerations. Consequently, clinicians need to ascertain the relative clinical significance of COPD and PHT in each patient and determine whether symptoms are driven by severity of ventilation (severe COPD, mild PHT), severity of circulatory/cardiovascular impairment (mild to moderate COPD, severe PHT), or both (severe COPD and PHT).85 Cardiopulmonary exercise testing may assist with this assessment.106
Generally, specific pulmonary vasodilator therapy does not help patients who are ventilatory limited and where parenchymal airway disease is the predominant pathology, as these medications interfere with compensatory hypoxic pulmonary vasoconstriction. In contrast, patients with severe PHT, circulatory limitation, and only mild to moderate COPD (FEV1 >60% predicted and mild airway/lung parenchymal pathology) should be treated as per current guidelines for pulmonary arterial hypertension.85,107 Patients with both clinically significant COPD and PHT require investigation into other causes of PHT along with referral to a specialist center for trial of therapy, entry into a clinical trial, or lung transplantation.84,85,92
Lung cancer
Epidemiology
A recent study of 1,664 patients with COPD from pulmonary clinics demonstrated a lung cancer prevalence of 9.1%.3 Incidence density has been reported as high as 16.7 cases per 1,000 person-years in patients with COPD.108
In patients with lung cancer, the prevalence of COPD is much higher. Young et al109 demonstrated a 50% prevalence of COPD109 versus 8% in the controls (matched for smoking). Not only was there a significant (six-fold) increase in the diagnosis of COPD (Global Initiative for Chronic Obstructive Lung Disease stage ≥2) in patients with lung cancer but also this increase was consistent over lung cancer stage and different histological group.109 Mannino et al10 demonstrated an HR of 2.8 (adjusted for smoking, age, sex, race, education) in patients with moderate to severe COPD. There is conflicting evidence with regard to the link between severity of FEV1 and risk of lung cancer.10 Nonetheless, COPD has been identified as an independent risk factor for lung cancer.10,110,111
Mechanisms of interaction
The mechanism of increased lung cancer risk in COPD is unclear. There appears to be a significant genetic component involved, and several candidate genes have been proposed.112 Neuronal nicotinic acetylcholine receptor subunits at 15q25 are some of the most well reviewed.112,113 Smoking behavior genes and COPD have also been linked to 15q loci.112,113 Whether this represents the effect of nicotine or true susceptibility is yet to be determined.112,113
Chronic inflammation from smoking and COPD is also central to the pathogenesis of lung cancer. Proinflammatory cytokines and, particularly, nuclear factor κB activation have been implicated in the pathogenesis of COPD and tumor development.45,112 A further mutual mechanism is that of matrix-degrading enzymes such as matrix metalloproteinase-1 that are involved in both degradation of elastin in emphysema and degradation of extracellular matrix-promoting tumor invasion.112 Further investigation of all of these proposed mechanisms may lead to more specific chemotherapeutic agents.
Association of lung cancer and COPD
The major impact of lung cancer in COPD is increased mortality, with lung cancer one of the most common causes of death, particularly in patients with mild to moderate COPD.5,23,24 Divo et al3 reported an HR of 2.02 (95% CI: 1.63–2.51; P<0.001) comparing mortality in COPD patients with and without lung cancer. Furthermore, the presence of COPD significantly increases mortality in patients with lung cancer.110
Treatment of COPD and lung cancer
In patients with concurrent lung cancer and COPD, reduced lung function may limit surgical operability or require limited resection.114 All patients should at least have spirometry and carbon monoxide-diffusing capacity measurements. Those with reduced lung function usually require further assessment, such as a shuttle test, 6- or 12-minute walk test, cardiopulmonary exercise testing, or nuclear perfusion lung scan.114 Equally, radiation therapy options may be limited by the patient’s lung function.
Paradoxically, FEV1 may not be as reduced post-lobectomy as expected in patients with COPD, as beneficial reductions in hyperinflation and ventilation/perfusion mismatching may occur.115 Further work in this area is required to predict those patients who, despite poor lung function, will benefit from curative surgical options.114 Deciding who is suitable for radical treatment of lung cancer is complex, and for this reason it is best done by a specialized multidisciplinary team.114 Treatment of COPD in patients with lung cancer is generally unchanged.
Pulmonary fibrosis
Epidemiology
Although first described in 1990,116 combined pulmonary fibrosis and emphysema (CPFE) is a clinical syndrome that is still being characterized. Divo et al3 reported the prevalence of concurrent COPD and pulmonary fibrosis as 6.1%, and this conveyed a significant increase in mortality with an HR of 1.51 (95% CI: 1.13–2.03; P<0.006). This was similar to a cohort of 1,143 lung cancer patients in which CPFE had a prevalence of 8.9%.117 The slightly elevated prevalence in this group is likely explained by the increased prevalence of lung cancer in CPFE.118–120 Recently, CPFE has been described as present in 35% of idiopathic pulmonary fibrosis (IPF) patients.121
Mechanisms of interaction
The pathogenesis of CPFE remains unclear, and efforts to elucidate this are likely hampered by the broad definition of CPFE. It is unclear whether patients develop both pulmonary fibrosis and COPD merely due to coincidence or the phenotype represents a pathological response in those with a genetic susceptibility.119,120 Smoking is a key risk factor for CPFE, as 98% of patients are current or former smokers. Agrochemicals have also been implicated as a risk factor.120 Tumor necrosis factor-α (TNF-α) overexpression in mice resulted in similar pathological changes to CPFE,118–120 thus providing a plausible link between inflammatory response and development of CPFE.
Association of pulmonary fibrosis and COPD
CPFE is associated with an increased prevalence of lung cancer, PHT (up to 50%), and acute lung injury when compared with patients with emphysema or pulmonary fibrosis alone.118,119 Prognosis is also unique to CPFE. Cottin et al120 demonstrated a median survival of 6.1 years in patients with CPFE. Though better than the standard 35 months for IPF, this is significantly reduced when compared with COPD alone.119,120 PHT is the best predictor of mortality. However, similar to COPD and IPF, PHT medications are ineffective, with oxygen the only treatment option.119
Treatment of COPD and pulmonary fibrosis
There is little evidence for any specific treatment for CPFE. Therefore, standard COPD treatment is currently recommended.119 Lung transplantation is the only real therapeutic option in this group.119
Metabolic syndrome
Epidemiology, mechanisms of interaction, and impact of metabolic syndrome on COPD
COPD increases the risk of developing diabetes (OR: 1.4–1.5)7 and hypertension (OR: 1.6).7 In patients with COPD, the prevalence ranges between 10% and 25% for diabetes,3,35–38,48,51,122 35.2% and 55% for hypertension,3,8,35,37,38,48,51 36% and 52% for hyperlipidemia,3,8,37 and 23% for obesity,8,48 making metabolic syndrome common in patients with COPD. The exact mechanism of the increased prevalence remains unclear. However, TNF-α and interleukin (IL)-6 are raised in both obesity and COPD, which is correlated with increased insulin resistance.45 Thus, systemic inflammation is likely to have a role. Decreased physical activity and frequent use of corticosteroids (both oral and inhaled)123 likely contribute. Diabetes is known to increase mortality in patients with concurrent COPD (HR: 1.5–1.7).3,4,7 Mannino et al7 also demonstrated increased mortality and hospitalizations with diabetes, hypertension, and cardiovascular disease, with all three combined having the worst prognosis.
Treatment of COPD and metabolic syndrome
There are no specific changes to the treatment of hypertension, hyperlipidemia, obesity, or diabetes in patients with concurrent COPD, aside from judicious use of corticosteroids and the aforementioned caution with non-cardioselective β-blocker use.
Osteoporosis
Epidemiology
In COPD, the prevalence of osteoporosis varies greatly between 8.4% and 69%.3,8,36–38,124–126 This variance is explained by the study population demographics. Graat-Verboom et al124 demonstrated that the mean prevalence of osteoporosis was significantly higher in patients with COPD than healthy controls (32.5% versus 11.4%; P<0.001) in their systematic review. Furthermore, when compared with other chronic respiratory illnesses such as asthma, IPF, and PHT, the prevalence of osteoporosis was significantly higher in the COPD patient group.124 Cystic fibrosis was the only respiratory group with higher prevalence.124 Prevalence of osteopenia ranged from 27% to 67% in the same systematic review.
Mechanisms of interaction
Patients with COPD often have many of the traditional risk factors for osteoporosis, including age, female sex, early menopause, smoking, alcohol use, reduced dietary calcium intake, previous fracture (in adulthood with low trauma), maternal hip fracture, physical inactivity, low body mass index, and corticosteroid use.8,45,127,128 Despite the association between COPD severity and bone mineral density loss,129 the true relationship between severity of COPD and likelihood of osteoporosis is unclear due to the presence of many confounders. Nevertheless, there is increasing evidence that the systemic inflammation of COPD and decreased lung function themselves are implicated in the development of osteoporosis.45,124 It has been proposed that several inflammatory mediators that are increased in COPD, such as matrix metalloproteinase-9, TNF-a, IL-1β, and IL-6, interact with the OPG/RANK/RANKL system, which controls osteoclast differentiation, maturation, and activation.45,130,131 Other bone remodeling pathways may also be involved.132
COPD patients who have frequent exacerbations are particularly susceptible to osteoporosis.133 Kiyokawa et al133 demonstrated the annual reduction from baseline bone mineral density to be 5.41% in those with exacerbations versus 0.60% (P=0.02) in those with no exacerbations. This was adjusted for age, smoking, and degree of airflow limitation. Steroid use was not accounted for, as in this small study only one of the 13 patients required systemic corticosteroids. In this study, hypoxia was also an independent predictor of bone mineral density loss.
Association of osteoporosis and COPD
The presence of osteoporosis in patients with COPD has negative prognostic bearing, in that thoracic vertebral compression fractures due to osteoporosis are associated with pain, increased dyspnea,4 worsening kyphosis,134 decrease in vital capacity,134 and reduced exercise tolerance.4 In fact, for every vertebral fracture, forced vital capacity is reduced by 9%.134
Treatment of COPD and osteoporosis
There is no specific treatment for osteoporosis in patients with COPD, and local guidelines should be followed with regard to calcium and vitamin D supplementation, bisphosphonate, strontium, denosumab, or teriparatide use. Most importantly, risk factors for osteoporosis, where possible, should be addressed, including smoking cessation, increasing physical activity, and minimizing use of systemic corticosteroids. COPD can complicate treatment of osteoporotic fractures, as patients with COPD are higher-risk candidates for surgical intervention and have increased postoperative pulmonary complications.
The impact of inhaled corticosteroids on osteoporosis risk is unclear. The majority of studies demonstrate an increase in osteoporosis risk. However, this appears to be modest and dose related.135 Furthermore, a recent trial of 544 patients revealed no increase in osteoporosis in patients with COPD treated with low-dose budesonide (320 μg/day), and a beneficial effect on osteoporosis in the chronic bronchitis subgroup.136 While further trials are conducted to elucidate any dose response, it would seem prudent to use the minimum dose required to effectively control exacerbations of COPD.
Gastroesophageal reflux disease and peptic ulcer disease
Epidemiology, mechanisms of interaction, and association of GERD and peptic ulcer disease with COPD
In studies reliant on self-reporting of diagnosis or symptoms, the prevalence of GERD in COPD patients ranged between 7.7% and 30%.3,36,38,51,137 However, Casanova et al138 used 24-hour pH monitoring to assess acid GERD prevalence and demonstrated that 62% of patients with severe COPD (FEV1 range 20%–49%) versus 19% of controls had acid GERD. Importantly, 58% of the COPD patients with GERD were asymptomatic. As previously discussed, GERD is implicated in increasing exacerbations of COPD.16,38,47,139 At least one study has implicated GERD in decreased quality of life scores. However, the significance of this is questionable in the small sample population.140
Peptic ulcer disease has a prevalence ranging between 5.2% and 32% in patients with COPD.3,35,36,38,51 Recent studies have shown that COPD is an independent risk factor for peptic ulcers35,36,51 and nonvariceal ulcer bleeding.141 Huang et al141 demonstrated an HR of COPD of 1.93 (1.73–2.17; P<0.001) after adjustment for age, sex, presence of comorbidities, history of peptic ulcer disease, and use of medication known to precipitate ulcers. Recently, Divo et al3 reported increased mortality and an HR of 1.32 (1.05–1.66; P<0.02) in patients with peptic ulcer disease and COPD. Siva et al142 revealed that with increased severity of COPD, prevalence of peptic ulcer disease and Helicobacter pylori also increased. They proposed that Helicobacter may precipitate inflammation of the lung and thus increased response to inhaled stimuli such as smoking. In their study, patients often presented with peptic ulcers prior to respiratory symptoms. However, two alternative hypotheses include 1) that the Helicobacter pylori/microaspiration precipitates exacerbations and thereby accelerates decline in lung function, and 2) that the increased systemic inflammation caused by COPD itself produces peptic ulcers.
Treatment of COPD, GERD, and peptic ulcer disease
Treatment of the GERD is not altered by the presence of COPD, except to say that it should be treated more aggressively. A small study by Sasaki et al143 revealed a significant reduction in relative risk of COPD exacerbation (0.23; 95% CI: 0.08–0.62; P<0.004) when treated with lansoprazole for a year. Patients with significant symptoms or known GERD or ulcers were excluded. However, given that there was no pH monitoring, there may have been uneven distribution of silent reflux in the case and control groups. Treatment of the COPD should attempt to minimize unnecessary steroid use. Further work is required to assess the efficacy of motility agents in this setting, as nonacid reflux is also likely to increase inflammation of the lungs.47
Regarding treatment of the peptic ulcer disease in the context of COPD, no alteration to standard acid suppression therapy is required. The severity of COPD may, however, complicate the ability to perform endoscopic or surgical procedures in terms of anesthetic safety. With regard to the treatment of COPD, steroids can delay the healing of ulcers, and thus minimization of oral steroids in the context of recent ulcer is prudent.
Chronic kidney disease
Epidemiology and impact of chronic kidney disease on COPD
Prevalence of CKD in patients with COPD is reported between 1.5% and 43%.3,8,35,144 Incalzi et al144 demonstrated 20.8% of patients have low muscle mass and normal or low creatinine levels, which masks their underlying low glomerular filtration rate. The risk of CKD, both overt (OR: 1.94; 95% CI: 1.01–4.66) and with normal creatinine (OR: 2.19, 95% CI: 5 1.17–4.12), was significantly increased. Low albumin was a useful marker of decreased muscle mass and therefore hidden CKD. The presence of concurrent CKD was an important negative prognostic factor in patients with COPD. CKD may impede acid–base balance compensation for hypercarbia.
Treatment of chronic kidney disease
CKD may affect drug treatments for COPD exacerbations (eg, antibiotic options and doses) and the treatment of comorbidities.75,144
Summary
Comorbidities are frequent in COPD and significantly impact on patients’ quality of life, exacerbation frequency, and survival. The epidemiology and clinical impact of major comorbidities in COPD are summarized in Table 1. Table 2 summarizes the reverse, the impact of COPD on its comorbidities, as well as the treatment options with concurrent disease.
Table 1.
Summary of major comorbidities in COPD
| Comorbidity | Prevalence | Mechanism | Clinical impact |
|---|---|---|---|
| Anxiety/depression | 6%–80% | Shared risk factors, dyspnea, limited independence | Decreases quality of life Increases exacerbations Decreases survival |
| Heart failure | 5%–24% | Shared risk factors, systemic inflammation, dynamic hyperinflation | Decreases quality of life Increases exacerbations Possibly decreases survival |
| IHD | 16%–53% | Systemic inflammation, vascular endothelial dysfunction | Possibly decreases quality of life Decreases survival |
| Pulmonary hypertension | 10%–91% | Hypoxia, endothelial dysfunction, pulmonary arterial remodeling | Decreases survival |
| Lung cancer | 9%–17% | Shared risk factors, systemic inflammation (especially NF-κβ) | Decreases survival |
| Pulmonary fibrosis | 6% | Shared risk factors, systemic inflammation | Decreases survival |
| Metabolic syndrome | Systemic inflammation, insulin resistance, corticosteroid use | ||
| • Diabetes | 10%–25% | Decreases quality of life, decreases survival | |
| • Hypertension | 32%–55% | ||
| • Dyslipidemia | 36%–52% | Not established | |
| • Obesity | 23% | Not established | |
| Osteoporosis | 8%–69% | Shared risk factors, systemic inflammation, nutritional deficiencies, corticosteroid use, hypoxia | |
| GERD/peptic ulcer disease | 8%–62% | Chronic cough | Increases exacerbations Decreases survival |
| CKD | 2%–43% | Shared risk factors | Decreases survival |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; IHD, ischemic heart disease; NF-κβ, nuclear factor κB.
Table 2.
Impact of COPD on comorbidities
| Comorbidity | Prevalence of COPD in this population | Impact of COPD on comorbidity | Treatment considerations |
|---|---|---|---|
| Anxiety/depression | • Worsens symptoms: dyspnea, lethargy • Delays diagnosis of anxiety/depression • Limits some treatment options |
• Standard therapy for anxiety/depression recommended, including CBT, antidepressants, and anxiolytics • Pulmonary rehabilitation in this group decreases anxiety and depression • Tricyclic antidepressants, mirtazapine, and benzodiazepines can precipitate respiratory drive depression and respiratory failure. These should be avoided, particularly in hypercapnic patients |
|
| Heart failure | ~15% | • COPD increases the difficulty of diagnosing heart failure: ○ Inadequate cardiac views on echocardiography, due to hyperinflation in 10%–35% ○ Chest X-ray signs unreliable due to hyperinflation and pulmonary vascular bed remodeling ○ BNP is less reliable in COPD patients • Increases dyspnea • Increased mortality HR 1.37a |
• Cardioselective β-blockers preferred over non-cardioselective • All other treatment for heart failure is unchanged • Use the minimum β-agonist possible to still maintain good control of COPD • Utilize long-acting anticholinergics as first-line therapy rather than long-acting β-agonists |
| IHD | • Increased risk of developing AMI: OR 2.2 (95% CI: 1.7–2.8) |
• Delays diagnosis due to overlapping symptom profles • Increased mortality HR 1.4–2.4 |
|
| Pulmonary hypertension | ~16.8% | • Limits treatment options • Increased mortality HR 1.59 |
• Supplemental oxygen is the mainstay for PHT • Pulmonary vasodilators only in expert centers, though specific vasodilator therapy is generally unhelpful |
| Lung cancer | 50% | • Worsens symptoms: dyspnea, lethargy • May limit modes of invasive investigation and/or treatment options |
• Reduced lung function may limit surgical operability or require limited resection • Radiation therapy may also be limited by lung function • Standard therapy for COPD |
| Pulmonary fibrosis | 35% | • Worsens symptoms: dyspnea, lethargy • Improved mortality when compared with IPF alone |
• Treatment is unchanged for both COPD and pulmonary fibrosis |
| Metabolic syndrome • Diabetes • Hypertension • Dyslipidemia • Obesity |
• Increased risk of cardiovascular disease than with metabolic syndrome alone | • Minimize oral corticosteroid use for COPD | |
| Osteoporosis | • May complicate any surgical interventions for fracture in terms of increased surgical risk and postoperative pulmonary complications | • Minimize oral and inhaled corticosteroid use for COPD • No change to osteoporosis treatment |
|
| GERD/peptic ulcer disease | • Worsens symptoms: cough • May limit endoscopic procedure safety |
• Aggressive treatment of GERD recommended • Minimize corticosteroid use for COPD |
|
| Chronic kidney disease | • Increased risk of cardiovascular disease than with chronic kidney disease alone | • Aggressive management of cardiovascular risk factors is suggested • Advanced kidney disease may affect antibiotic options for COPD |
Note:
HRs were no longer significant after adjustment for confounding risk factors.
Abbreviations: AMI, acute myocardial infarction; BNP, brain natriuretic peptide; CBT, cognitive behavioral therapy; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; HR, hazard ratio; IHD, ischemic heart disease; IPF, idiopathic pulmonary fibrosis; OR, odds ratio; PHT, pulmonary hypertension.
There is strong evidence that cardiovascular disease (IHD, heart failure, and atrial fibrillation), PHT, lung cancer, pulmonary fibrosis, diabetes, peptic ulcer disease, and CKD are poor prognostic factors.3,8,27,28,30–32,35–40,48,50,51,86–91,122,126,127 The evidence is slightly less robust for anxiety, given the small population of women in which this was investigated by Divo et al.3 Though very prevalent, hypertension and hyper-lipidemia have not been shown to impact on survival.
GERD is strongly associated with increased exacerbations.16,38,47,139 Cardiovascular disease, pulmonary embolism, depression, and anxiety have also been associated with increased hospitalizations. Whether these represent a true increase in susceptibility for exacerbations, misdiagnosis of an exacerbation of COPD where, for example, pulmonary embolism is the true cause, or rather that patients with more comorbidities are less likely to manage an exacerbation at home is unclear.
Quality of life is difficult to measure, and the studies attempting to quantify this have used multiple assessment tools, making comparisons between the studies tenuous. Heart failure, diabetes, arthritis, and urinary incontinence/prostatic disease have all been shown to decrease health status in one larger study by Putcha et al.2 IHD was shown to decrease quality of life in one similar study by Patel et al.71 However, it was not significant in the former. Despite these difficulties with comparing studies, all agree that increased number of comorbidities is associated with decreased quality of life.
There are several limitations that need to be considered when assessing the current literature on COPD and its comorbidities. Firstly, much of the data are from observational studies. Secondly, establishing the true prevalence of comorbidities in COPD in these studies is potentially confounded by 1) under-diagnosis of COPD in the community, 2) under-diagnosis of comorbidities, 3) overlapping symptoms between comorbidities, and 4) varied methods of comorbidity diagnosis amongst the studies. Consequently, significant selection bias depending on the population reviewed is present in many studies.
Notwithstanding these difficulties, the literature is clear that increased number of comorbidities is associated with decreased survival.
Screening for comorbidities
Recognizing comorbidities in COPD is critical because 1) comorbidities have prognostic significance, 2) many comorbidities have effective therapeutic options, and 3) the culmination of multiple comorbidities has a far more significant impact on patient survival and quality of life when considered as a whole entity. Clinicians should therefore be aware of common comorbidities, and screening for these at routine clinic visits should be considered.
Such screening protocols should be limited to comorbidities that are prevalent, clinically significant, have effective therapeutic options, and/or alter prognosis significantly.
Although further work is required as to the appropriate timing of screening for specific comorbidities, we anticipate the development of screening programs in COPD, similar to those that are standard of care for diabetes management in primary care settings. Although local guidelines for screening comorbidities such as osteoporosis (particularly specific to oral corticosteroid exposure) already exist for primary care settings, these need to be evaluated and potentially adjusted for COPD patients. In the interim, we recommend a checklist similar to Figure 4, which includes additional comorbidities grouped by organ system.
Figure 4.
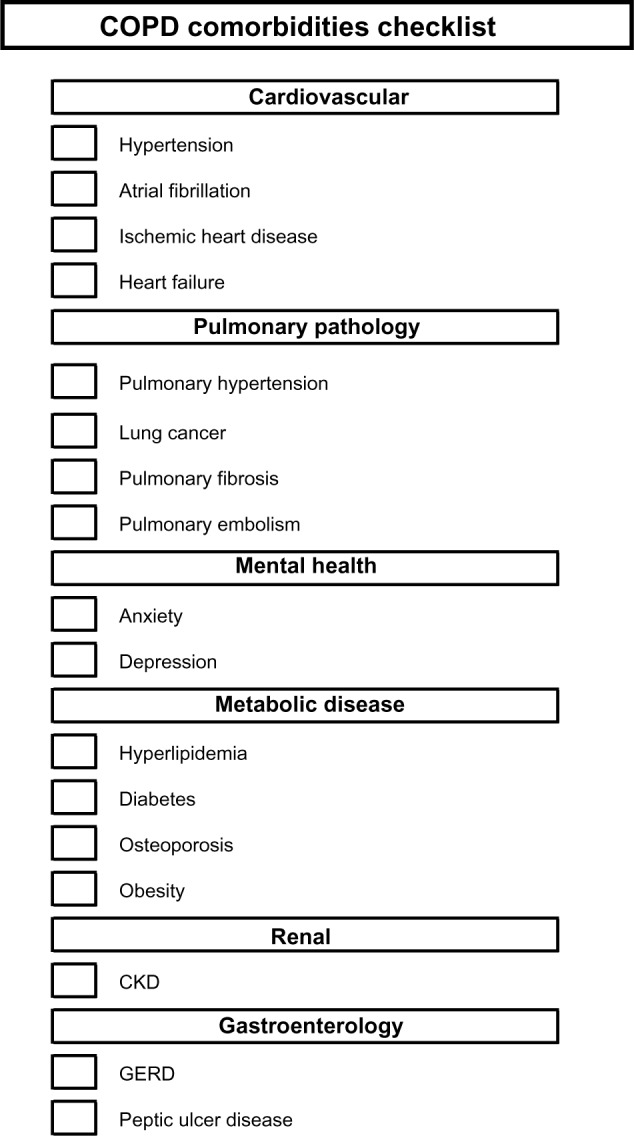
Comorbidities checklist.
Notes: This list includes the major comorbidities, as defined by those of high prevalence or significant impact on quality of life or mortality. It may assist the physician in screening for comorbidities in the outpatient setting.
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease.
Conclusion
In summary, comorbidities are highly prevalent and are a significant cause of morbidity and mortality in patients with COPD. Further research is required to fully understand the relationships and underlying mechanisms between COPD and the multitude of comorbidities. Similarly, further research is required to determine optimal treatment of comorbidities in the COPD patient group specifically. It is apparent that COPD clinicians should focus their attention not only to the management of COPD itself but also to the investigation and management of COPD comorbidities. Screening tools may be of benefit in the future to focus these investigations.
Footnotes
Disclosure
Miranda Caroline Smith received travel assistance from GlaxoSmithKline for attendance at a Thoracic Society of Australia and New Zealand advanced training course. Jeremy P Wrobel has received speaker fees from Actelion and GlaxoSmithKline and travel assistance to attend conferences from Pfizer, GlaxoSmithKline, Bayer, and Actelion.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for diagnosis, management, and prevention of COPD. 2014. [Accessed June 5, 2014]. Available from: http://www.goldcopd.org/
- 2.Putcha N, Puhan MA, Hansel NN, Drummond MB, Boyd CM. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001–2008. COPD. 2013;10(3):324–332. doi: 10.3109/15412555.2012.744963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divo M, Cote C, de Torres J, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 4.Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384. doi: 10.1016/j.rmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 6.Almagro P, Calbo E, Ochoa de Echaguen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 8.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data from the first National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163(12):1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 11.Balcells E, Anto JM, Gea J, et al. Characteristics of patients admitted for the first time for COPD exacerbation. Respir Med. 2009;103(9):1293–1302. doi: 10.1016/j.rmed.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Beghe B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41(4):993–995. doi: 10.1183/09031936.00180812. [DOI] [PubMed] [Google Scholar]
- 13.Fabbri LM, Beghe B, Agusti A. Cardiovascular mechanisms of death in severe COPD exacerbation: time to think and act beyond guidelines. Thorax. 2011;66(9):745–747. doi: 10.1136/thoraxjnl-2011-200406. [DOI] [PubMed] [Google Scholar]
- 14.van Manen JG, Bindels PJ, Dekker EW, et al. Added value of co-morbidity in predicting health-related quality of life in COPD patients. Respir Med. 2001;95(6):496–504. doi: 10.1053/rmed.2001.1077. [DOI] [PubMed] [Google Scholar]
- 15.Yeo J, Karimova G, Bansal S. Co-morbidity in older patients with COPD: its impact on health service utilisation and quality of life, a community study. Age Ageing. 2006;35(1):33–37. doi: 10.1093/ageing/afj002. [DOI] [PubMed] [Google Scholar]
- 16.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 17.Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918–923. doi: 10.1164/rccm.201105-0939PP. [DOI] [PubMed] [Google Scholar]
- 18.Rizkallah J, Man SF, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786–793. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- 19.Wells JM, Dransfield MT. Pathophysiology and clinical implications of pulmonary arterial enlargement in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:509–521. doi: 10.2147/COPD.S52204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts CM, Stone RA, Lowe D, Pursey NA, Buckingham RJ. Co-morbidities and 90-day outcomes in hospitalized COPD exacerbations. COPD. 2011;8(5):354–361. doi: 10.3109/15412555.2011.600362. [DOI] [PubMed] [Google Scholar]
- 22.McGarvey LP, Magder S, Burkhart D, et al. Cause-specific mortality adjudication in the UPLIFT(R) COPD trial: findings and recommendations. Respir Med. 2012;106(4):515–521. doi: 10.1016/j.rmed.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7(5):375–382. doi: 10.3109/15412555.2010.510160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA, TORCH Clinical Endpoint Committee Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25(12):1209–1221. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 27.Vogele C, von Leupoldt A. Mental disorders in chronic obstructive pulmonary disease (COPD) Respir Med. 2008;102(5):764–773. doi: 10.1016/j.rmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- 29.Kunik ME, Veazey C, Cully JA, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: a randomized controlled trial. Psychol Med. 2008;38(3):385–396. doi: 10.1017/S0033291707001687. [DOI] [PubMed] [Google Scholar]
- 30.Kuhl K, Schurmann W, Rief W. Mental disorders and quality of life in COPD patients and their spouses. Int J Chron Obstruct Pulmon Dis. 2008;3(4):727–736. doi: 10.2147/copd.s3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414–419. doi: 10.1183/09031936.05.00078504. [DOI] [PubMed] [Google Scholar]
- 32.Gudmundsson G, Gislason T, Janson C, et al. Depression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countries. Respir Med. 2006;100(1):87–93. doi: 10.1016/j.rmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858–866. doi: 10.4187/respcare.01862. [DOI] [PubMed] [Google Scholar]
- 34.Anecchino C, Rossi E, Fanizza C, et al. Prevalence of chronic obstructive pulmonary disease and pattern of comorbidities in a general population. Int J Chron Obstruct Pulmon Dis. 2007;2(4):567–574. [PMC free article] [PubMed] [Google Scholar]
- 35.Mapel DW, Picchi MA, Hurley JS, et al. Utilization in COPD: patient characteristics and diagnostic evaluation. Chest. 2000;117(5 Suppl 2):346S–353S. doi: 10.1378/chest.117.5_suppl_2.346s. [DOI] [PubMed] [Google Scholar]
- 36.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Lee JH, Kim Y, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013;13(1):51. doi: 10.1186/1471-2466-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanania NA, Mullerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 40.Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. 2008;23(11):1757–1762. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cafarella PA, Effing TW, Usmani ZA, Frith PA. Treatments for anxiety and depression in patients with chronic obstructive pulmonary disease: a literature review. Respirology. 2012;17(4):627–638. doi: 10.1111/j.1440-1843.2012.02148.x. [DOI] [PubMed] [Google Scholar]
- 42.van den Bemt L, Schermer T, Bor H, et al. The risk for depression comorbidity in patients with COPD. Chest. 2009;135(1):108–114. doi: 10.1378/chest.08-0965. [DOI] [PubMed] [Google Scholar]
- 43.Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766–777. doi: 10.1378/chest.12-1911. [DOI] [PubMed] [Google Scholar]
- 44.Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(Suppl 4):43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 46.Burgel PR, Escamilla R, Perez T, et al. Impact of comorbidities on COPD-specific health-related quality of life. Respir Med. 2013;107(2):233–241. doi: 10.1016/j.rmed.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 49.de Lucas-Ramos P, Izquierdo-Alonso JL, Rodriguez-Gonzalez Moro JM, et al. Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study) Int J Chron Obstruct Pulmon Dis. 2012;7:679–686. doi: 10.2147/COPD.S36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis. 2009;4:337–349. doi: 10.2147/copd.s6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mapel DW, Dedrick D, Davis K. Trends and cardiovascular comorbidities of COPD patients in the Veterans Administration medical system,1991–1999. COPD. 2005;2(1):35–41. doi: 10.1081/copd-200050671. [DOI] [PubMed] [Google Scholar]
- 52.Mascarenhas J, Azevedo A, Bettencourt P. Coexisting chronic obstructive pulmonary disease and heart failure: implications for treatment, course and mortality. Curr Opin Pulm Med. 2010;16(2):106–111. doi: 10.1097/MCP.0b013e328335dc90. [DOI] [PubMed] [Google Scholar]
- 53.de Miguel Diez J, Chancafe Morgan J, Jimenez Garcia R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305–312. doi: 10.2147/COPD.S31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4-year UPLIFT(R) trial. Lung. 2011;189(4):261–268. doi: 10.1007/s00408-011-9301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grau M, Barr RG, Lima JA, et al. Percent emphysema and right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-Lung and Multi-Ethnic Study of Atherosclerosis-Right Ventricle Studies. Chest. 2013;144(1):136–144. doi: 10.1378/chest.12-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watz H, Waschki B, Magnussen H. Emphysema, airflow obstruction, and left ventricular filling. N Engl J Med. 2010;362(17):1638–1639. doi: 10.1056/NEJMc1002018. author reply 1640–1631. [DOI] [PubMed] [Google Scholar]
- 58.Stavem K, Aaser E, Sandvik L, et al. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur Respir J. 2005;25(4):618–625. doi: 10.1183/09031936.05.00008504. [DOI] [PubMed] [Google Scholar]
- 59.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2(1):8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 60.Iversen KK, Kjaergaard J, Akkan D, et al. The prognostic importance of lung function in patients admitted with heart failure. Eur J Heart Fail. 2010;12(7):685–691. doi: 10.1093/eurjhf/hfq050. [DOI] [PubMed] [Google Scholar]
- 61.Kee K, Naughton MT. Heart failure and the lung. Circ J. 2010;74(12):2507–2516. doi: 10.1253/circj.cj-10-0869. [DOI] [PubMed] [Google Scholar]
- 62.Guder G, Rutten FH, Brenner S, et al. The impact of heart failure on the classification of COPD severity. J Card Fail. 2012;18(8):637–644. doi: 10.1016/j.cardfail.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 63.McAllister DA, Maclay JD, Mills NL, et al. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J. 2012;39(5):1097–1103. doi: 10.1183/09031936.00124811. [DOI] [PubMed] [Google Scholar]
- 64.Brekke PH, Omland T, Smith P, Soyseth V. Underdiagnosis of myocardial infarction in COPD: Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbation. Respir Med. 2008;102(9):1243–1247. doi: 10.1016/j.rmed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi: 10.1378/chest.09-2029. [DOI] [PubMed] [Google Scholar]
- 66.McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(12):1208–1214. doi: 10.1164/rccm.200707-1080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eickhoff P, Valipour A, Kiss D, et al. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(12):1211–1218. doi: 10.1164/rccm.200709-1412OC. [DOI] [PubMed] [Google Scholar]
- 68.Vanfleteren LE, Spruit MA, Groenen MT, et al. Arterial stiffness in patients with COPD: the role of systemic inflammation and the effects of pulmonary rehabilitation. Eur Respir J. 2014;43(5):1306–1315. doi: 10.1183/09031936.00169313. [DOI] [PubMed] [Google Scholar]
- 69.Lahousse L, van den Bouwhuijsen QJ, Loth DW, et al. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Am J Respir Crit Care Med. 2013;187(1):58–64. doi: 10.1164/rccm.201206-1046OC. [DOI] [PubMed] [Google Scholar]
- 70.Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(10):982–988. doi: 10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 71.Patel AR, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141(4):851–857. doi: 10.1378/chest.11-0853. [DOI] [PubMed] [Google Scholar]
- 72.Cote CG, Casanova C, Marin JM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31(3):571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 73.Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 74.Stefan MS, Rothberg MB, Priya A, Pekow PS, Au DH, Lindenauer PK. Association between beta-blocker therapy and outcomes in patients hospitalised with acute exacerbations of chronic obstructive lung disease with underlying ischaemic heart disease, heart failure or hypertension. Thorax. 2012;67(11):977–984. doi: 10.1136/thoraxjnl-2012-201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsiligianni IG, Kosmas E, Van der Molen T, Tzanakis N. Managing comorbidity in COPD: a difficult task. Curr Drug Targets. 2013;14(2):158–176. doi: 10.2174/1389450111314020004. [DOI] [PubMed] [Google Scholar]
- 76.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339(8):489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 77.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4):CD003566. doi: 10.1002/14651858.CD003566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ekstrom MP, Hermansson AB, Strom KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):715–720. doi: 10.1164/rccm.201208-1565OC. [DOI] [PubMed] [Google Scholar]
- 79.Matera MG, Calzetta L, Rinaldi B, Cazzola M. Treatment of COPD: moving beyond the lungs. Curr Opin Pharmacol. 2012;12(3):315–322. doi: 10.1016/j.coph.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Wang MT, Lo YW, Tsai CL, et al. Statin use and risk of COPD exacerbation requiring hospitalization. Am J Med. 2013;126(7):598–606. doi: 10.1016/j.amjmed.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 81.Janda S, Park K, FitzGerald JM, Etminan M, Swiston J. Statins in COPD: a systematic review. Chest. 2009;136(3):734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 82.Vestbo J, Anderson J, Brook RD, et al. The Study to Understand Mortality and Morbidity in COPD (SUMMIT) study protocol. Eur Respir J. 2013;41(5):1017–1022. doi: 10.1183/09031936.00087312. [DOI] [PubMed] [Google Scholar]
- 83.Celli B, Decramer M, Kesten S, et al. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(10):948–955. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 84.Wrobel JP, Thompson BR, Williams TJ. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic review. J Heart Lung Transplant. 2012;31(6):557–564. doi: 10.1016/j.healun.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 85.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(Suppl 25):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 86.Scharf SM, Iqbal M, Keller C, et al. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027. [DOI] [PubMed] [Google Scholar]
- 87.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 88.Matthay RA, Schwarz MI, Ellis JH, Jr, et al. Pulmonary artery hypertension in chronic obstructive pulmonary disease: determination by chest radiography. Invest Radiol. 1981;16(2):95–100. doi: 10.1097/00004424-198103000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 90.Keller CA, Shepard JW, Jr, Chun DS, Vasquez P, Dolan GF. Pulmonary hypertension in chronic obstructive pulmonary disease. Multivariate analysis. Chest. 1986;90(2):185–192. doi: 10.1378/chest.90.2.185. [DOI] [PubMed] [Google Scholar]
- 91.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136(2):412–419. doi: 10.1378/chest.08-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 93.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107(5):1193–1198. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 94.Doi M, Nakano K, Hiramoto T, Kohno N. Significance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemia. Respir Med. 2003;97(8):915–920. doi: 10.1016/s0954-6111(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 95.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104(12):1877–1882. doi: 10.1016/j.rmed.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Andersen CU, Mellemkjaer S, Nielsen-Kudsk JE, Bendstrup E, Hilberg O, Simonsen U. Pulmonary hypertension in chronic obstructive and interstitial lung diseases. Int J Cardiol. 2013;168(3):1795–1804. doi: 10.1016/j.ijcard.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 97.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 98.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 99.Fisher MR, Criner GJ, Fishman AP, et al. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J. 2007;30(5):914–921. doi: 10.1183/09031936.00033007. [DOI] [PubMed] [Google Scholar]
- 100.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl 25):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 102.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 103.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 104.Wrobel JP, Thompson BR, Snell GI, Williams TJ. Preoperative echocardiographic-defined moderate-severe pulmonary hypertension predicts prolonged duration of mechanical ventilation following lung transplantation for patients with COPD. Lung. 2012;190(6):635–643. doi: 10.1007/s00408-012-9423-7. [DOI] [PubMed] [Google Scholar]
- 105.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144(1):169–176. doi: 10.1378/chest.11-3241. [DOI] [PubMed] [Google Scholar]
- 106.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–1174. doi: 10.1378/chest.11-2798. [DOI] [PubMed] [Google Scholar]
- 107.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(Suppl 25):D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 108.de Torres JP, Marin JM, Casanova C, et al. Lung cancer in patients with chronic obstructive pulmonary disease – incidence and predicting factors. Am J Respir Crit Care Med. 2011;184(8):913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 109.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 110.Kiri VA, Soriano J, Visick G, Fabbri L. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J. 2010;19(1):57–61. doi: 10.4104/pcrj.2009.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodriguez LA, Wallander MA, Martin-Merino E, Johansson S. Heart failure, myocardial infarction, lung cancer and death in COPD patients: a UK primary care study. Respir Med. 2010;104(11):1691–1699. doi: 10.1016/j.rmed.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 112.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13(4):233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 113.Yang IA, Holloway JW, Fong KM. Genetic susceptibility to lung cancer and co-morbidities. J Thorac Dis. 2013;5(Suppl 5):S454–S462. doi: 10.3978/j.issn.2072-1439.2013.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(Suppl 3):iii1–iii27. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- 115.Sekine Y, Iwata T, Chiyo M, et al. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2003;76(2):356–361. doi: 10.1016/s0003-4975(03)00489-2. discussion 362. [DOI] [PubMed] [Google Scholar]
- 116.Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med. 1990;84(5):365–369. doi: 10.1016/s0954-6111(08)80070-4. [DOI] [PubMed] [Google Scholar]
- 117.Usui K, Tanai C, Tanaka Y, Noda H, Ishihara T. The prevalence of pulmonary fibrosis combined with emphysema in patients with lung cancer. Respirology. 2011;16(2):326–331. doi: 10.1111/j.1440-1843.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 118.Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15(2):265–271. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- 119.Papiris SA, Triantafillidou C, Manali ED, et al. Combined pulmonary fibrosis and emphysema. Expert Rev Respir Med. 2013;7(1):19–31. doi: 10.1586/ers.12.80. quiz 32. [DOI] [PubMed] [Google Scholar]
- 120.Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 121.Cottin V. The impact of emphysema in pulmonary fibrosis. Eur Respir J. 2013;22(128):153–157. doi: 10.1183/09059180.00000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 123.Caughey GE, Preiss AK, Vitry AI, Gilbert AL, Roughead EE. Comorbid diabetes and COPD: impact of corticosteroid use on diabetes complications. Diabetes Care. 2013;36(10):3009–3014. doi: 10.2337/dc12-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34(1):209–218. doi: 10.1183/09031936.50130408. [DOI] [PubMed] [Google Scholar]
- 125.Jorgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med. 2007;101(1):177–185. doi: 10.1016/j.rmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 126.Ferguson GT, Calverley PM, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the Towards a Revolution in COPD Health study. Chest. 2009;136(6):1456–1465. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 127.Romme EA, Rutten EP, Smeenk FW, Spruit MA, Menheere PP, Wouters EF. Vitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary disease. Ann Med. 2013;45(1):91–96. doi: 10.3109/07853890.2012.671536. [DOI] [PubMed] [Google Scholar]
- 128.Graat-Verboom L, Spruit MA, van den Borne BE, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009;103(8):1143–1151. doi: 10.1016/j.rmed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 129.Ohara T, Hirai T, Muro S, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134(6):1244–1249. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 130.Zhang PF, Pan L, Luo ZY, Zhao HJ, Cai SX. Interrelationship of circulating matrix metalloproteinase–9, TNF-alpha, and OPG/RANK/RANKL systems in COPD patients with osteoporosis. COPD. 2013;10(6):650–656. doi: 10.3109/15412555.2013.813928. [DOI] [PubMed] [Google Scholar]
- 131.Bai P, Sun Y, Jin J, et al. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res. 2011;12:157. doi: 10.1186/1465-9921-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hardy R, Cooper MS. Bone loss in inflammatory disorders. J Endocrinol. 2009;201(3):309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 133.Kiyokawa H, Muro S, Oguma T, et al. Impact of COPD exacerbations on osteoporosis assessed by chest CT scan. COPD. 2012;9(3):235–242. doi: 10.3109/15412555.2011.650243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR. Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res. 2007;22(3):447–457. doi: 10.1359/jbmr.061202. [DOI] [PubMed] [Google Scholar]
- 135.Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax. 2011;66(8):699–708. doi: 10.1136/thx.2011.160028. [DOI] [PubMed] [Google Scholar]
- 136.Mathioudakis AG, Amanetopoulou SG, Gialmanidis IP, et al. Impact of long-term treatment with low-dose inhaled corticosteroids on the bone mineral density of chronic obstructive pulmonary disease patients: aggravating or beneficial? Respirology. 2013;18(1):147–153. doi: 10.1111/j.1440-1843.2012.02265.x. [DOI] [PubMed] [Google Scholar]
- 137.Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119(4):1043–1048. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 138.Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23(6):841–845. doi: 10.1183/09031936.04.00107004. [DOI] [PubMed] [Google Scholar]
- 139.Sakae TM, Pizzichini MM, Teixeira PJ, da Silva RM, Trevisol DJ, Pizzichini E. Exacerbations of COPD and symptoms of gastro-esophageal reflux: a systematic review and meta-analysis. J Bras Pneumol. 2013;39(3):259–271. doi: 10.1590/S1806-37132013000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rascon-Aguilar IE, Pamer M, Wludyka P, Cury J, Vega KJ. Poorly treated or unrecognized GERD reduces quality of life in patients with COPD. Dig Dis Sci. 2011;56(7):1976–1980. doi: 10.1007/s10620-010-1542-5. [DOI] [PubMed] [Google Scholar]
- 141.Huang KW, Luo JC, Leu HB, et al. Chronic obstructive pulmonary disease: an independent risk factor for peptic ulcer bleeding: a nationwide population-based study. Aliment Pharmacol Ther. 2012;35(7):796–802. doi: 10.1111/j.1365-2036.2012.05028.x. [DOI] [PubMed] [Google Scholar]
- 142.Siva R, Birring SS, Berry M, Rowbottom A, Pavord ID. Peptic ulceration, Helicobacter pylori seropositivity and chronic obstructive pulmonary disease. Respirology. 2013;18(4):728–731. doi: 10.1111/resp.12075. [DOI] [PubMed] [Google Scholar]
- 143.Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57(8):1453–1457. doi: 10.1111/j.1532-5415.2009.02349.x. [DOI] [PubMed] [Google Scholar]
- 144.Incalzi RA, Corsonello A, Pedone C, et al. Chronic renal failure: a neglected comorbidity of COPD. Chest. 2010;137(4):831–837. doi: 10.1378/chest.09-1710. [DOI] [PubMed] [Google Scholar]
- 145.van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16(1):103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 146.Wu C, Camacho FT, King SB, 3rd, et al. Risk stratification for long-term mortality after percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(1):80–87. doi: 10.1161/CIRCINTERVENTIONS.113.000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campo G, Guastaroba P, Marzocchi A, et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013;144(3):750–757. doi: 10.1378/chest.12-2313. [DOI] [PubMed] [Google Scholar]