Abstract
PURPOSE
We performed a population-based study comparing trends in perioperative outcomes and cost for open (OP), laparoscopic (LP), and robotic (RP) pediatric pyeloplasty. Specific billing items contributing to cost were also investigated.
MATERIALS AND METHODS
Using the Premier Perspective database, we identified 12,662 pediatric patients who underwent open, laparoscopic and robotic pyeloplasty (ICD-9 55.87) in the United States from 2003 – 2010. Univariate and multivariate statistics were used to evaluate perioperative outcomes, complications, and costs for the competing surgical approaches. Propensity weighting was employed to minimize selection bias. Sampling weights were used to yield a nationally representative sample.
RESULTS
A decrease in OP and a rise in minimally invasive pyeloplasty (MIP) was observed. All procedures had low complication rates. Compared to OP, LP and RP had longer median operating room (OR) times (240 minutes, p<0.0001 and 270 minutes, p<0.0001, respectively). There was no difference in median length of stay (LOS). The median total cost was lower among patients undergoing OP versus RP ($7,221 vs $10,780, p<0.001). This cost difference was largely attributable to robotic supply costs.
CONCLUSIONS
During the study period, OP made up a declining majority of cases. LP utilization plateaued, while RP increased. OR time was longer for MIP, while LOS was equivalent across all procedures. A higher cost associated with RP was driven by OR use and robotic equipment costs, which abrogated low room and board cost. This study reflects an adoption period for RP. With time, perioperative outcomes and cost may improve.
Keywords: minimally invasive pyeloplasty, laparoscopic pyeloplasty, robotic pyeloplasty, pediatric, ureteropelvic junction obstruction, comparative effectiveness
INTRODUCTION
Open pyeloplasty (OP) has long been the gold standard for operative management of ureteropelvic junction obstruction (UPJO) in children. With the advent of minimally invasive surgery, both laparoscopic (LP) and robotic (RP) pediatric pyeloplasty have gained popularity. These approaches for minimally invasive pediatric pyeloplasty (MIP) have been reported to have potentially shorter hospital stays for adolescents,1,2 equivalent or reduced use of postoperative pain medications,1,3–5 and superior parental satisfaction.6 Other investigators have also shown equivalent cost between OP and RP2 and equivalent charges for LP and RP.7
The contemporary data for pediatric pyeloplasty are derived largely from single-institution series describing the experience of high-volume surgeons and pediatric hospitals. It is unclear if the same favorable results are occurring on a national level. Therefore, the aim of our study was to perform a population-based assessment of the contemporary trends in utilization, perioperative complications, and costs for OP, LP, and RP in the United States. We hypothesized that our population, which includes both low- and high-volume hospitals, would demonstrate less advantageous perioperative outcomes for MIP as well as cost disparities among the three approaches.
METHODS
Data Source
Hospitalization data for pediatric patients (≤18 years of age) with a procedure code for pyeloplasty (ICD9 55.87) between January 1, 2003 and December 31, 2010 were extracted from the Premier Perspectives Database (Premier, Inc, Charlotte, NC). This database was created for national quality and utilization benchmarking and includes approximately 20% of discharges from non-federal institutions in the US. A patient identifier key allows tracking of an individual patient for each patient encounter thereby permitting longitudinal data analyses. All procedures and diagnoses were identified using the International Classification of Disease, 9th revision, Clinical Modification (ICD9). This study was exempt from institutional review board approval given the de-identified nature of the data.
Study Population
Relying on billing records representing 221–274 hospitals annually, 1,990 unique pyeloplasty hospitalizations were identified. Sampling weights were applied and generalized estimating equations were used to adjust for hospital clustering.8 This resulted in a national estimate of 12,662 patients. To minimize coding error, the procedure code was cross-referenced with the diagnosis codes of UPJO (ICD9 753.21) and/or hydronephrosis (ICD9 591).
Through a review of the charge master for each patient, regular expression matching techniques were used to find terms specific to robotic surgery based on a review of the Intuitive surgery catalogue (e.g. "ROBOTIC" or "DA VINCI" or "ENDOWRIST"). Using this method, nearly 3,000 individual robotic billing codes were used to distinguish robotic cases, including those prior to 2008. Similarly, patients with laparoscopic equipment were categorized as LP, while those without any laparoscopic or robotic supplies were categorized as OP.
Patient and Hospital Characteristics
Patient characteristics included age in years (<1, 1–2, 3–10, and 11–18), gender, race (White, Black, Hispanic, Other), and insurance status (Medicare, Medicaid, Private, Other). Hospital characteristics included teaching status (teaching or non-teaching), hospital size (<200, 200–399 or ≥400 beds), location (urban or rural), and US Census geographical region (Northeast, Midwest, West, or South).
Perioperative Outcomes
Perioperative outcomes included operating room time (OT), length of stay (LOS), and postoperative complications. Billing data was used to determine OT and represented total time in the operating room (OR). LOS was determined by calculating the difference between admission and discharge dates and reported in days. We identified post-operative complications using ICD9 codes, which were further classified using the Clavien system and subdivided into minor (Clavien grades 1–2) or major (Clavien grades 3–5) complications. Complications occurring during the index hospitalization and/or re-admission to the hospital within 90 days were included; outpatient complications were not included due to the inability to reliably capture these in the dataset.
Cost Calculations
The available data contained the direct cost of individual billing items for a hospitalization, which does not require conversion from hospital charges. Total cost was calculated by summing the cost of all individual billing items provided in the charge master for each procedure. Costs were tabulated for the 90 days following a pyeloplasty in order to include the medical expenditures associated with post-operative complications requiring re-hospitalization. These costs were further subdivided into OR use, OR supplies, room and board and other (which included laboratory, radiology, pharmacy and miscellaneous non-categorizable items). The proportion of overall cost attributable to each category was calculated. Fixed costs, including capital costs, annual maintenance fees and reimbursement for surgical assistants were not included. All costs were adjusted to 2010 United States dollars with the medical component of the Consumer Price Index.
Statistical Analysis
Medians and interquartile ranges were determined for continuous variables. Frequencies and proportions were calculated for categorical variables. Descriptive statistics with non-parametric analyses and Chi-square tests were used to assess medians and proportions. To predict the utilization and perioperative outcomes, we generated logistic and quantile regression models. All analyses incorporated sampling weights. For inferential statistical analyses, we combined projection weights with propensity weights based on patient and hospital characteristics.9 By using propensity weighting, we aimed to reduce selection bias inherent to administrative data.10 All statistical tests were two-sided and a p-value of <0.05 was considered statistically significant. Stata 13 (College Station, TX) was used for all statistical analyses.
RESULTS
Between 2003 and 2010, there were an estimated 12,662 hospitalizations for pediatric pyeloplasty in the US (10,545 OPs, 1,517 LPs and 690 RPs). Socio-demographic and hospital characteristics are presented in Table 1. The majority of patients were male (72%), Caucasian (67%) and under private insurance coverage (59%). Procedures were most frequently performed in urban hospitals (96%), and around half of the procedures were performed in teaching hospitals (47%). The groups were similar except in regards to age and teaching status. A significantly higher number of 11–18 year-olds underwent MIP (p<0.0001) and more LPs were performed in non-teaching institutions (p=0.02).
Table 1.
Population-weighted, unadjusted patient and hospital characteristics
| Open n = 10454 (82.5%) | Laparoscopic n = 1517 (12%) | Robotic n = 691 (5.5%) | |
|---|---|---|---|
| Patient Characteristics¶ | |||
| Age (years)* | |||
| <1 | 37.8 | 18.7 | 2.0 |
| 1–2 | 16.0 | 14.6 | 2.7 |
| 3–10 | 26.3 | 28.6 | 30.0 |
| 11–18 | 19.8 | 38.1 | 65.2 |
| Gender | |||
| Male | 72.8 | 69.0 | 67.2 |
| Female | 27.2 | 31.0 | 32.8 |
| Race | |||
| White | 65.8 | 68.1 | 84.2 |
| Black | 9.4 | 10.3 | 3.8 |
| Hispanic | 8.3 | 7.0 | 1.2 |
| Other | 16.6 | 14.6 | 10.6 |
| Insurance Status | |||
| Medicare | 0.2 | 0.0 | 0.0 |
| Medicaid | 36.2 | 31.2 | 22.5 |
| Private Insurance | 58.0 | 58.8 | 73.1 |
| Other | 5.6 | 9.9 | 4.4 |
| Hospital Characteristics¶ | |||
| Teaching Hospital** | |||
| Yes | 50.5 | 18.2 | 52.4 |
| No | 49.5 | 81.7 | 47.4 |
| Urban | |||
| Yes | 96.0 | 96.9 | 99.3 |
| No | 4.0 | 3.0 | 0.7 |
| Hospital Size (number of beds) | |||
| <200 | 13.2 | 6.8 | 2.0 |
| 200 to 399 | 36.9 | 20.0 | 45.1 |
| ≥400 | 49.9 | 73.1 | 52.9 |
| Region | |||
| Midwest | 17.0 | 9.4 | 29.3 |
| Northeast | 22.0 | 7.6 | 19.2 |
| South | 47.3 | 67.9 | 36.4 |
| West | 13.8 | 14.9 | 14.8 |
Chi-square analysis performed for patient and hospital variables.
p<0.0001 and
p<0=02.
Chi-square analysis performed to determine the distribution of patient and hospital variables across the modalities. Significant differences noted for patient age (p<0.0001) and hospital teaching status (p = 0.02) with adolescent more likely to undergo MIP and laparoscopic pyeloplasty more likely to be performed in a non-teaching hospital. All other variables fit within a normal distribution for each modality.
During the study period, the number of pediatric pyeloplasties per year remained relatively stable (1,634 in 2003 and 1,542 in 2010). However, the number of RPs increased while the number of OPs steeply decreased (Figure 1). The number of LPs appeared to plateau around 2006. A patient had greater odds of undergoing MIP in the urban setting and as hospital size increased. The odds of undergoing MIP were highest in the oldest age groups, with 11–18 year-olds being 40 times more likely to undergo RP compared to infants (OR 40.5, [8.6–191]).
Figure 1.
Median OT was significantly longer for MIP (p<0.0001), with RP having the longest OT at 270 minutes (Table 2). There was no difference in median hospital stay for any approach. Mean robotic LOS was significantly shorter than the open group (2.1 versus 2.8 days, p<0.05). The incidence of complications, both major (0.5%) and minor (5.6%), was relatively low and similar among the approaches (p = 0.2). Median OR cost was significantly higher for RP as compared to OP ($10,780 versus $7,221, p<0.0001). There was no significant difference in cost between OP and LP (p = 0.6).
Table 2.
Perioperative outcomes and cost for open, laparoscopic and robotic pediatric pyeloplasty, propensity score matched 2003–2010
| Open | Laparoscopic | Robotic | |
|---|---|---|---|
| Length of Stay (days) | |||
| Median (IQR) | 2 (2– 3) | 2 (1–3) | 2 (1–3) |
| Mean (95% CI) | 2.8 (2.5–3) | 2.3 (1.4–3.2) | 2.1* (1.7–2.5) |
| Complications § | |||
| None | 93.2% | 96.8% | 91.2% |
| Minor | 6.3% | 1.7% | 8.8% |
| Major | 0.5% | 1.4% | 0% |
| Operating Room Time (min) | |||
| Median (IQR) | 185 (150–235) | 240** (200–299) | 270** (210–300) |
| Mean (95% CI) | 207 (184–231) | 254* (226–283) | 264** (246–282) |
| Total Inpatient Cost | |||
| Median (IQR) | $7,221 (5,698–9,339) | $7,355 (4,904–11,058) | $10,780** (7,993– 15,600) |
| Mean (95% CI) | $8,069 (7,179–8,959) | $8,897 (5,319–12,474) | $12,077* (9,644–14,511) |
Weighted chi-square analysis showed no differences among the treatment modalities. Statistical significance compared with open pyeloplasty:
p<0.05,
p<0.0001
Given that more than half of RPs (63%) occurred during the last two years of the study period, a sub-group analysis was performed for pyeloplasties performed in 2009 and 2010 (Table 3). Median LOS for LP was significantly shorter at 1 day (p<0.0001) while OP and RP remained equivalent. Median OT was significantly longer for RP (p<0.0001) but was improve by 30 minutes in comparison to the overall study period. Finally, RP remained significantly more costly than OP (p<0.0001). LP was significantly less costly than OP during the last two study years.
Table 3.
Perioperative outcomes and cost for open, laparoscopic and robotic pediatric pyeloplasty, propensity score matched, 2009–2010
| Open | Laparoscopic | Robotic | |
|---|---|---|---|
| Length of Stay (days) | |||
| Median (IQR) | 2 (2 to 3) | 1** (1 to 2) | 2 (1 to 2) |
| Mean (95% CI) | 2.6 (2.2 to 2.9) | 1.4** (1.0 to 1.8) | 2.1 (1.5 to 2.7) |
| Complications § | |||
| No Complications | (87.9%) | (100%) | (88.6%) |
| Minor (Clavien I-II) | (11.7%) | (0%) | (11.4%) |
| Major (Clavien III-V) | (0.4%) | (0%) | (0%) |
| Operating Room Time (min) | |||
| Median (IQR) | 192 (150 to 225) | 210 (188 to 257) | 240** (204 to 295) |
| Mean (95% CI) | 196 (179 to 214) | 230 (182 to 279) | 257** (233 to 282) |
| Total Inpatient Cost | |||
| Median (IQR) | $7,946 (6331 to 10157) | $6,082** (4759 to 9245) | $10,737** (6926 to 16240) |
| Mean (95% CI) | $8,594 (7179 to 8959) | $7,566 (4267 to 10864) | $12,079 (8512 to 15647) |
Weighted chi-square analysis showed no differences among the treatment modalities. Statistical significance compared with open pyeloplasty:
<0.05,
p<0.0001
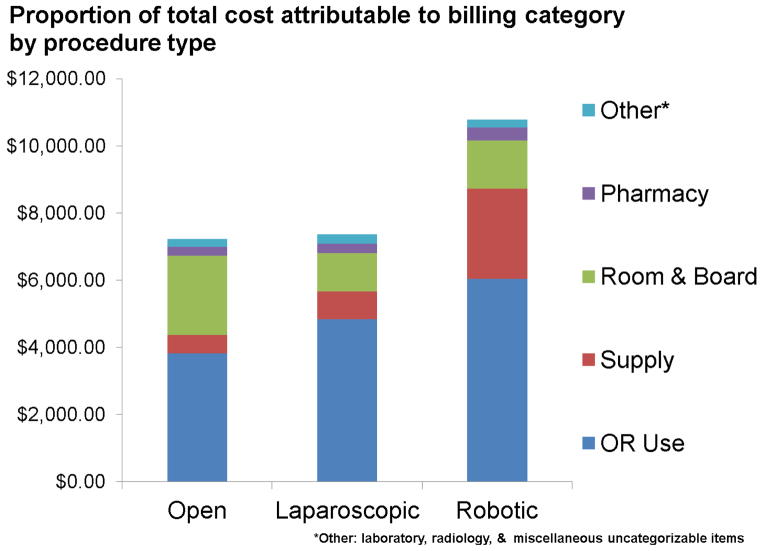
Figure 2 represents disaggregated costs for items associated with each procedure and hospitalization. OR cost was the greatest contributor to overall cost for all procedures. In the robotic group, both OR cost and supplies accounted for the largest proportion of the total cost ($6,034 and $2,681, respectively). For OP, room and board was the second largest contributor to cost ($2,360) and was higher in comparison to MIP (mean $1,285). No differences were observed for laboratory, radiology, or pharmacy costs across the modalities.
Figure 2.
DISCUSSION
Since Anderson and Hynes first reported the open dismembered pyeloplasty,11 the procedure has been modified for laparoscopy and more recently robotic-assisted laparoscopy. In contrast to prior high-volume, single institution studies reporting advantages of LP and RP over OP, we evaluated the pyeloplasty experience of a general medical community through a large contemporary population-based analysis. The current study confirms that pediatric MIP is safe, but does not show a clear advantage over the open procedure in terms of LOS and OT. Furthermore, a significantly higher cost was associated with the robotic procedure.
Capitalizing on the detailed billing data contained in our dataset, we were able to examine trends in robotic pyeloplasty prior to 2008 and compare to OP and LP (Figure 1). From 2003–2010, OP dramatically declined yet still comprised a substantial majority of procedures performed. After an initial growth period the performance of LP plateaued around 2006, a trends similar to that of adult laparoscopic radical prostatectomy, pyeloplasty and partial nephrectomy.12 We hypothesize that this relates to the technical difficulty associated with intracorporeal suturing using rigid laparoscopic instruments combined with the concomitant introduction of RP. Finally, RP use increased during the study period. This rise is largely attributable to pyeloplasties occurring in adolescents, who had 40 times the odds of undergoing RP compared to infants. A disproportionate rise in adolescent LP has similarly been observed and may stem from reduced postoperative pain medication use after MIP 5,13. This may also reflect surgeons favoring older patients with more intra-abdominal working space and a lower anesthesia risk when first learning RP.
Shorter hospitalization times and consequent cost savings and parental satisfaction are often touted to be one of the benefits of MIP. In the current study, our analysis demonstrated no differences in the median LOS among all three approaches. We observed a shorter mean LOS for RP compared with OP suggesting that there may be a greater proportion of patients with a prolonged hospitalization after open surgery. Although this mean difference (17 hours) did not confer an obvious financial advantage, there may be indirect benefits in the hours saved. For example, Behan et al were able to show a significant reduction in lost parental wages after the robotic procedure.14 In the case of pediatric hospitalizations, one or both parents can endure human capital losses and therefore even a modest difference in LOS matters for this population.
Previous studies assessing OT have reported conflicting results.2–4,7,13,15–21 In our study, median OT for LP and RP was 55 and 85 minutes longer than OP, respectively. Admittedly, our study period reflects an early phase of adoption for RP and presumably the efficiency of MIP improves as surgeons move along the learning curve. Minnillo et al demonstrated this when they found that after five years of experience, the average OT for RP decreased by approximately 60 minutes at their high-volume center.18 Interestingly, we found that OT disparity persisted in a subgroup analysis focusing on the last two study years, which was likely capturing a later part of the learning curve for MIP (Table 3). As such, we believe there is potential for an appreciable reduction in OT with increasing case volume, but this phenomenon may only be true for high-volume robotic centers.
The majority of past studies have shown MIP to be more expensive than the open approach,5,21–24 although two recent single-center studies have demonstrated a cost equivalence.2,7 Our study demonstrates a significant disparity in cost depending on the surgical approach. Compared with OP, LP costs are similar while RP is significantly more expensive ($10,780 versus $7,221, p<0.0001). Even in our subgroup analysis of the final two years of the study, RP remained statistically more costly than OP ($10,737 versus $7,946, p<0.0001), suggesting that cost may not change dramatically even at later stages of the learning curve. Disaggregating total cost reveals OR supply cost is the main driver for the higher cost of RP compared to OP ($2,681 versus $546). Importantly, the cost savings associated with RP room and board did not outweigh these supply costs. Until market forces are able to drive down the cost of robotic equipment, we suspect that RP will continue to have a substantial cost disadvantage compared to OP or LP from a population-based perspective.
An unexpected finding was the relative equivalence between LP and the open procedure. Interestingly, LP was actually the most cost-efficient procedure in terms of overall cost, LOS and OT in the sub-group analysis (Table 3). It seems contradictory then that after an initial growth period, the performance of LP plateaued. Given similar efficacy 4,13,16–19, 21,25 and complication rates across the procedures, our findings make it difficult to justify the higher cost of RP if the advantages of MIP can be met without any additional cost using a purely laparoscopic approach.
Although the current findings suggest RP may not yet be economical for surgeons outside of high-volume robotic centers, previous studies from centers where robotic adoption occurred early have demonstrated favorable results for LOS, OT and/or cost.2–4,7,18 Perhaps the current results and past studies hint at RP being most economical in high-volume robotic centers. Certainly the concept of regionalization of surgical procedures has been supported by literature demonstrating the positive affect of surgeon volume on perioperative outcomes.26 At the very least, the current findings suggest that RP is not superior to the other modalities when considered broadly.
There are several limitations to our study, the first being the possibility of misclassification and selection bias inherent to using administrative data. We attempted to minimize this by capitalizing on the granularity of the available billing data, cross-referencing diagnosis and procedure codes, and employing propensity score weighting. We were also unable to identify the number of freestanding pediatric institutions sampled by our data, so although we know our sample is heterogeneous we cannot determine to what degree. Complications were limited to inpatient complications, but we included 90-day data to capture complications that led to re-admission. We were also unable to assess efficacy with this dataset, although previous literature suggests equivalence across the procedures.4,13,16–19,21,25 In addition, OT included time devoted to non-operative procedures and would have captured time related to anesthesia, nursing, positioning, etc. However, given such a large aggregate of data, we assume our results to be generalizable and relevant. In regards to cost, our calculations derive from specific billing items. This does not account for the purchasing cost or maintenance fees associated with the robotic platform, and therefore cost may be underestimated. In contrast, the use of robotic supplies may be billed for each procedure yet amortized over 10 uses. In this case, cost would be overestimated.
CONCLUSIONS
Minimally invasive pediatric pyeloplasties represented a minority of the pyeloplasties performed in the United States from 2003–2010. The number of laparoscopic pyeloplasties plateaued while robotic pyeloplasties appeared to be slowly rising. Operating room time was longer for MIP, while LOS was equivalent across all procedures. RP was significantly more costly than OP with the cost difference due primarily to robotic equipment costs. LP represents a minimally invasive alternative to OP with similar perioperative outcomes while maintaining comparable or reduced costs.
Footnotes
Disclosure: none.
Conflicts of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Traxel EJ, Minevich EA, Noh PH. A Review: The Application of Minimally Invasive Surgery to Pediatric Urology: Upper Urinary Tract Procedures. Urology. 2010;76:122–133. doi: 10.1016/j.urology.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 2.Rowe CK, Pierce MW, Tecci KC, et al. A Comparative Direct Cost Analysis of Pediatric Urologic Robot-Assisted Laparoscopic Surgery Versus Open Surgery: Could Robot-Assisted Surgery Be Less Expensive? J Endourol. 2012;26:871–877. doi: 10.1089/end.2011.0584. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien ST, Shukla AR. Transition from open to robotic-assisted pediatric pyeloplasty: A feasibility and outcome study. J Pediatr Urol. 2012;8:276–281. doi: 10.1016/j.jpurol.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen MD, Delostrinos C, Johnson MH, et al. Comparison of the Learning Curve and Outcomes of Robotic Assisted Pediatric Pyeloplasty. J Urol. 2011;185:2517–2522. doi: 10.1016/j.juro.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka ST, Grantham JA, Thomas JC, et al. A Comparison of Open vs Laparoscopic Pediatric Pyeloplasty Using the Pediatric Health Information System Database—Do Benefits of Laparoscopic Approach Recede at Younger Ages? J Urol. 2008;180:1479–1485. doi: 10.1016/j.juro.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Freilich DA, Penna FJ, Nelson CP, et al. Parental Satisfaction After Open Versus Robot Assisted Laparoscopic Pyeloplasty: Results From Modified Glasgow Children's Benefit Inventory Survey. J Urol. 2010;183:704–708. doi: 10.1016/j.juro.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Casella DP, Fox JA, Schneck FX, et al. Cost Analysis of Pediatric Robot-Assisted and Laparoscopic Pyeloplasty. J Urol. 2013;189:1083–1086. doi: 10.1016/j.juro.2012.08.259. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe KK. Projection Methodology: National Projections from Premier's Perspective Hospital Inpatient Data (1999) Charlotte, NC: Premier Inc; 2001. pp. 1–27. [Google Scholar]
- 9.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JC, Hynes W. Retrocaval ureter: a case diagnosed pre-operatively and treated successfully by a Plastic Operation. BJU Int. 1949;21:209–214. doi: 10.1111/j.1464-410x.1949.tb10773.x. [DOI] [PubMed] [Google Scholar]
- 12.Yu H-Y, Hevelone ND, Lipsitz SR, et al. Use, Costs and Comparative Effectiveness of Robotic Assisted, Laparoscopic and Open Urological Surgery. J Urol. 2012;187:1392–1399. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 13.Lee RS, Retik AB, Borer JG, et al. Pediatric Robot Assisted Laparoscopic Dismembered Pyeloplasty: Comparison With a Cohort of Open Surgery. J Urol. 2006;175:683–687. doi: 10.1016/S0022-5347(05)00183-7. [DOI] [PubMed] [Google Scholar]
- 14.Behan JW, Kim SS, Dorey F, et al. Human Capital Gains Associated With Robotic Assisted Laparoscopic Pyeloplasty in Children Compared to Open Pyeloplasty. J Urol. 2011;186:1663–1667. doi: 10.1016/j.juro.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Atug F, Woods M, Burgess SV, et al. Robotic assisted laparoscopic pyeloplasty in children. J Urol. 2005;174:1440–1442. doi: 10.1097/01.ju.0000173131.64558.c9. [DOI] [PubMed] [Google Scholar]
- 16.Kutikov A, Resnick M, Casale P. Laparoscopic Pyeloplasty in the Infant Younger Than 6 Months—Is it Technically Possible? J Urol. 2006;175:1477–1479. doi: 10.1016/S0022-5347(05)00673-7. [DOI] [PubMed] [Google Scholar]
- 17.Piaggio LA, Franc-Guimond J, Noh PH, et al. Transperitoneal Laparoscopic Pyeloplasty for Primary Repair of Ureteropelvic Junction Obstruction in Infants and Children: Comparison With Open Surgery. J Urol. 2007;178:1579–1583. doi: 10.1016/j.juro.2007.03.159. [DOI] [PubMed] [Google Scholar]
- 18.Minnillo BJ, Cruz JAS, Sayao RH, et al. Long-Term Experience and Outcomes of Robotic Assisted Laparoscopic Pyeloplasty in Children and Young Adults. J Urol. 2011;185:1455–1460. doi: 10.1016/j.juro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Riachy E, Cost NG, Defoor WR, et al. Pediatric Standard and Robot-Assisted Laparoscopic Pyeloplasty: A Comparative Single Institution Study. J Urol. 2013;189:283–287. doi: 10.1016/j.juro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Yee DS, Shariat SF, Lowrance WT, et al. Impact of Previous Radiotherapy for Prostate Cancer on Clinical Outcomes of Patients With Bladder Cancer. J Urol. 2010;183:1751–1756. doi: 10.1016/j.juro.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penn HA, Gatti JM, Hoestje SM, et al. Laparoscopic Versus Open Pyeloplasty in Children: Preliminary Report of a Prospective Randomized Trial. J Urol. 2010;184:690–695. doi: 10.1016/j.juro.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Yee DS, Shanberg AM, Duel BP, et al. Initial comparison of robotic-assisted laparoscopic versus open pyeloplasty in children. Urology. 2006;67:599–602. doi: 10.1016/j.urology.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Vemulakonda VM, Cowan CA, Lendvay TS, et al. Surgical Management of Congenital Ureteropelvic Junction Obstruction: A Pediatric Health Information System Database Study. J Urol. 2008;180:1689–1692. doi: 10.1016/j.juro.2008.03.096. [DOI] [PubMed] [Google Scholar]
- 24.Monn MF, Bahler CD, Schneider EB, et al. Trends in Robot-assisted Laparoscopic Pyeloplasty in Pediatric Patients. Urology. 2013;81:1336–1341. doi: 10.1016/j.urology.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Cascio S, Tien A, Chee W, et al. Laparoscopic Dismembered Pyeloplasty in Children Younger Than 2 Years. J Urol. 2007;177:335–338. doi: 10.1016/j.juro.2006.08.145. [DOI] [PubMed] [Google Scholar]
- 26.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. New Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]