Abstract
Background and Purpose
After stroke, many individuals lack resources to receive the intensive rehabilitation thought to improve upper extremity motor function. This case study describes the application of a telerehabilitation intervention using a portable robotic device combined with a home exercise program (HEP) designed to improve upper extremity function.
Case Description
The participant was a 54 year-old male, 22 weeks following right medullary pyramidal ischemic infarct. At baseline, he exhibited residual paresis of the left upper extremity resulting in impaired motor control consistent with a flexion synergistic pattern, scoring 22/66 on the Fugl-Meyer Motor Assessment (FMA).
Intervention
The participant completed 85 total hours of training (38 hours of robotic device and 47 hours of HEP) over the 8-week intervention period.
Outcomes
The participant demonstrated an improvement of 26 points on the Action Research Arm Test, 5 points on the Functional Ability Scale portion of the Wolf Motor Function Test (WMFT), and 20 points on the FMA, all of which surpassed the minimal clinically important difference (MCID). Of the 17 tasks of the WMFT, he demonstrated improvement on 11 of the 15 time-based tasks and both strength measures. The participant reported an overall improvement in his stroke recovery on the Stroke Impact Scale quality of life questionnaire from 40/100 to 65/100. His score on the Center for Epidemiologic Studies Depression Scale improved by 19 points.
Discussion
This case demonstrates that robotic-assisted therapy paired with a HEP can be successfully delivered within a home environment to a person with stroke. Robotic assisted therapy may be a feasible and efficacious adjunct to a HEP program to elicit substantial improvements in upper extremity motor function especially in those persons with stroke who lack access to stroke rehabilitation centers. Video Abstract available (See Video, Supplemental Digital Content 1.) for more insights from the authors.
Keywords: assistive technology, robotics, telerehabilitation, upper extremity, home exercise program
Introduction
Current evidence in upper extremity (UE) stroke rehabilitation emphasizes the value of intensive adaptive and repetitive task practice (RTP) to facilitate motor re-learning.1–3 These types of interventions typically require one-on-one therapist-directed treatment in traditional rehabilitation environments. However, many individuals with stroke, especially those living in urban and rural settings, do not have access to comprehensive stroke rehabilitation programs, thereby limiting functional recovery while contributing to long-term disability.4, 5 Telerehabilitation using long distance monitoring has been investigated as a potential model to provide rehabilitation to individuals who may benefit from home monitoring.6 In a recent systematic review, Johansson and Wild7 found telerehabilitation to be efficacious in improving the health of persons with stroke, including upper extremity function, while also being supportive of caregivers’ needs. Lum and colleagues8 described a model of remotely supervised constraint-induced (CI) movement therapy utilizing a device designed to automate the intensive training portion of CI therapy, while reducing direct therapist-patient interaction time.
Robotic-assisted devices that make use of motor learning principles can provide valuable repetitive practice to persons with stroke who might otherwise not have access to the intensive therapy necessary to elicit neuromotor recovery.9 Although the optimal dosage of practice required to elicit motor recovery after stroke is unknown, recent data suggest that 300–800 repetitions are required to learn a simple task.10 The robotic device used in this study (Hand Mentor Pro [HMP]; Kinetic Muscles, Inc. Tempe, Arizona) was designed to improve active flexion and extension range of motion in the wrist and fingers and improve motor control of the distal upper extremity. In a pilot study comparing combined RTP and robotic-assisted therapy using the HMP to dose-matched RTP alone, comparable gains in upper extremity function were shown in the two interventions.11, 12 Similar gains in both groups provide preliminary support for the use of the HMP as a component of a comprehensive outpatient upper extremity rehabilitation program. However, the feasibility of using this technology along with an exercise program in the home environment is unknown. This case study describes the clinical rationale, application and experience of a home-based robotic-assisted and exercise therapy program to improve UE motor function in an individual with subacute stroke.
Case Description
The participant was a married 54 year-old, right-hand dominant, African-American male who incurred a right medullary pyramidal ischemic infarct 5.5 months prior to enrollment. The participant had undergone outpatient physical and occupational therapy (24 visits of each) up until he was recommended for this study. Clinical examination revealed a 22/66 initial score on the Fugl-Meyer Assessment (FMA),13 characterized by residual paresis of the left upper extremity resulting in the inability to perform isolated movements of the shoulder, elbow, wrist, and hand; a finding consistent with a flexion synergistic pattern. This FMA score was within the range of minimal movement criteria for our larger clinical trial (11–55 on FMA).14 The participant was deemed to be an ideal candidate for robotic-assisted therapy with the HMP device for several reasons. He was making functional gains during his rehabilitation indicating a favorable prognosis for continued improvement, and expressed a desire to continue with therapy; however, a therapy visit cap limited his access to continued rehabilitation. The robotic device facilitates isolated movement at the distal UE by stabilizing the proximal UE and using goal-directed motor control programs to encourage the person with stroke to work out of synergistic patterns. The participant embraced the technologies associated with both robotic-assisted therapy and telerehabilitation, and was motivated to continue with stroke rehabilitation to improve the functional use of his UE. The institutional review board at the Cleveland Clinic approved this study and the participant provided informed consent to participate.
Examination
After enrollment, a clinical examination was completed to evaluate the participant’s impairments, motor function, quality of life, and mood. Examination revealed normal passive range of motion (ROM) in the affected UE, but he was not able to actively move through full range due to hemiparesis. Mild sensory loss was evident in the affected UE, as the participant scored a 1 on the sensory portion of the National Institute of Health Stroke Scale.15 His vision was intact and he did not show signs of inattention or neglect, as he missed only one on the Star Cancellation Test.16 His cognition and language skills were intact as he answered all questions correctly on the Short Portable Mental Status Questionnaire.17
Prior to the intervention, the participant underwent a battery of tests that was repeated at the conclusion of the 8-week intervention. The primary outcome measure was the total change in score (from baseline to post-intervention) of the affected upper extremity on the Action Research Arm Test (ARAT).18–20 Secondary outcome measures included the Wolf Motor Function Test (WMFT),21, 22 the Stroke Impact Scale (SIS),23 the FMA,13 the Centers for Epidemiologic Studies Depression Scale (CES-D)24 and Modified Ashworth Scale (MAS).25
The ARAT consists of four subscales that address grasp, grip, pinch, and gross motor movements (19 tasks) of both the affected and non-affected upper extremity. Movement is scored with an ordinal scale from 0–3 with a score of 3 indicating normal performance of task within 5 seconds and a score of 0 indicating the inability to perform any part of the task within 60 seconds. Higher scores indicate better movement capabilities. The ARAT is considered a valid and reliable tool for upper extremity deficits following stroke.18–20 The minimal clinically importance difference (MCID), defined here as the smallest difference in score that a person with stroke perceives as beneficial, is 12 points if the dominant UE is affected and 17 points if the non-dominant UE is affected.26 The minimal detectible change (MDC) is the smallest change in two scores that likely represents a true change, rather than error due to variability in the measurement tool.27 For the ARAT, the interrater MDC is 13.1 and test-retest MDC is 3.5.28 The participant’s baseline ARAT was 21 out of 57.
The WMFT consists of 15 timed tasks and 2 strength tasks of both the affected and non-affected upper extremities. Tasks progress from proximal to distal, beginning with isolated shoulder movements and progressing to fine-motor tasks of the hand. Participants are encouraged to perform each timed task as quickly as they can. Shorter times reflect better performance. Timed movements are also graded with a functional ability scale (FAS) for quality of movement. The WMFT has been validated for use with persons in the acute to chronic stages after stroke.21, 22, 29 For the cumulative timed portion of the test, MCID is 19 seconds for the dominant side, while the non-dominant side had no MDIC value due to the very small relationship between test score and participant perceived change rating. The FAS portion of the Wolf Motor Function test has a MCID of 1.0 and 1.2 in the dominant and non-dominant side, respectively.26 The interrater MDC is 20.2 points and the test-retest MDC is 12.0 points.28 The participant’s baseline FAS was 44, out of a possible 75 points.
The SIS is a quality of life questionnaire that addresses several domains following stroke including physical impairments, memory and cognition, mood, performance with activities of daily living, mobility, use of affected UE, and return to activities that have meaning to the person with stroke. The SIS has been shown reliable and valid in sub-acute to chronic stroke populations.30 The MDC and MCID for subsets of the test are as follows: strength 24.0 and 9.2 points, performance of ADLs 17.3 and 5.9 points, mobility 15.1 and 4.5 points, and use of affected UE 25.9 and 17.8 points.27 At baseline, the participant’s scores on the “ADL” and “use of affected UE” subsets were 35/50 and 6/25, respectively, while he self-rated recovery from his stroke at 40%.
The upper extremity FMA is an impairment-based measure for the upper extremity following stroke, consisting of 33 movements with higher scores indicating increased ability of the person with stroke to move out of synergistic patterns toward more isolated movements. Movement quality of the affected UE is compared to the movement quality of the non-affected UE on a 0–2 ordinal scale with 0 indicating no movement at all, 1 indicating partial movement of the affected extremity, and 2 indicating equal movement between affected and non-affected upper extremities. The FMA is a reliable and valid tool for measuring UE impairment following stroke.31, 32 The MDIC for the FMA is 10% of the total possible score, or 6.6 points.32 The interrater reliability MDC is 12.9 points and the test retest MDC is 5.2 points.28 Prior to the intervention, the participant’s FMA score was 22 out of 66.
The CES-D is a questionnaire used to screen for depressive symptomology. The test consists of 20 questions that capture how well a person is coping emotionally. Scores > 16 can indicate the person is at risk for depression. The CES-D has been found reliable and valid for the sub-acute stroke population.24, 33 At baseline the participant’s CES-D was 23 out of a possible 60.
The MAS is used to assess spastic hypertonus following stroke. Response to stretch is scored on a scale of 0–4 with higher scores indicating greater resistance to passive stretch. For this case report, wrist flexion, supination, and finger flexion were assessed. The reliability and validity of the MAS has been questioned, yet this measure is the most commonly used clinical tool to assess spasticity following stroke. A recent study found the MAS to be moderately reliable for upper and lower extremity muscle groups between raters.25 In terms of elbow and wrist flexors the MAS was 1+ while MAS for forearm pronators was 2.
Intervention
The HMP robotic device used in our study employs a pneumatic artificial muscle to facilitate movement about the wrist and fingers while providing visual biofeedback about the quality and quantity of wrist movements (Figure 1). The device consists of three components: control box, arm unit and data collection and communications module. The control box houses the electronics of the device (12”W × 12”L × 12”H, 14 lbs) and has a 10.4” color touch screen to facilitate user interface. The control box is connected to the arm unit by a pneumatic hose and data cable. The pneumatic actuator is the top portion of the arm unit and provides air into and out of the hose to simulate dorsal muscle contraction and relaxation. Voltage changes are detected from a potentiometer that is aligned to the wrist joint. These changes in voltage are calibrated to reflect active range of motion. Resistance to wrist and finger extension is measured by a pressure transducer housed within the arm unit.
Figure 1.
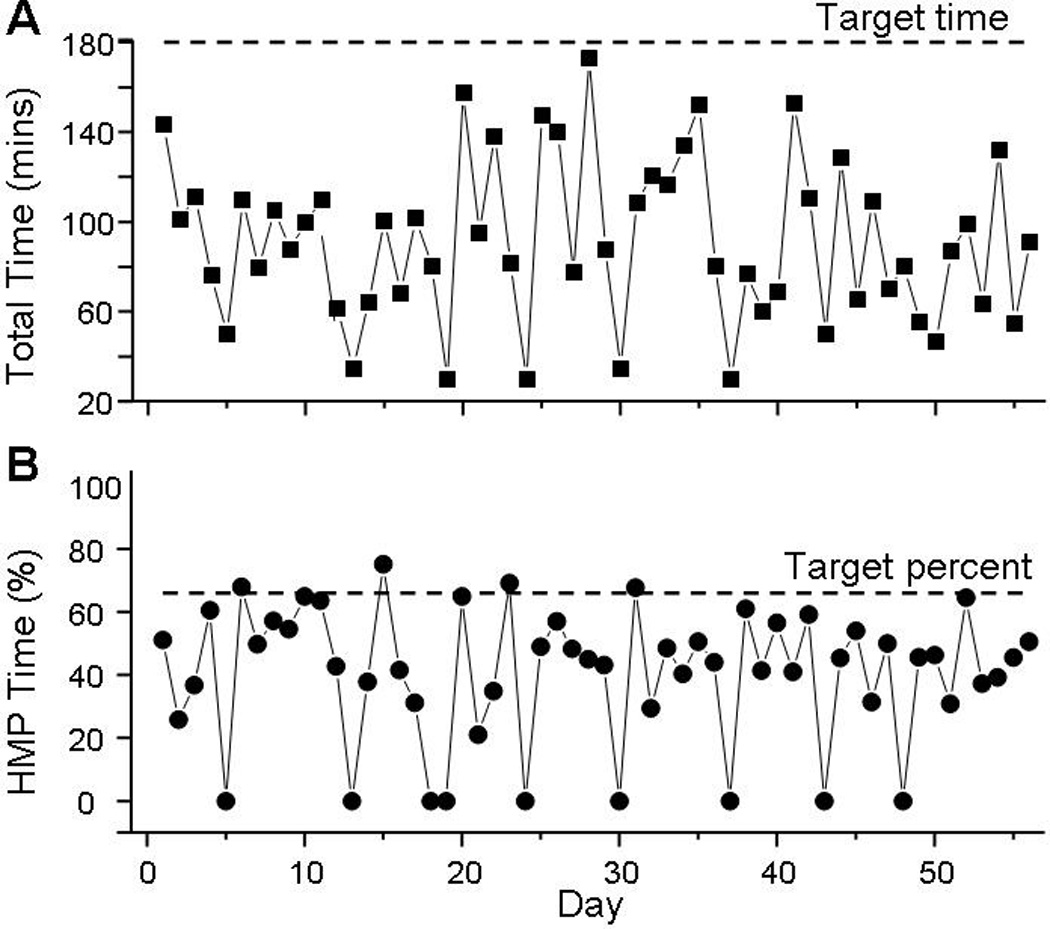
Illustration of the Hand Mentor Pro robotic device and its components. Indicated with arrows are: A) pneumatic hose and data cable, B) arm unit with pneumatic actuator, C) control box and touch-screen display
The robotic device we used has a data collection and communication module, which records the following variables: overall time of use, time of use in each module, number of attempted and successful repetitions, wrist angle, and pneumatic pressure. These data are stored in non-volatile memory and are encrypted and transmitted to a secure website (MentorHome) where it is accessible to the therapist involved in the care of this participant. Each time a participant completes a program, the summary for that session is displayed on-screen and stored in that participant’s coded electronic database. Data are automatically transmitted from the device controller to the web site via landline telephone, internet, or cellular connection to enable remote monitoring.
The robotic device we used has four training modules designed to improve active control of the wrist musculature and one spasticity reduction program. Video gaming design principles have been incorporated into the training modules in an effort to engage the participant. Two variations of the motor control program (Strongman Basic and Strongman Up) are intended to train flexion and extension or extension-only movement patterns, respectively. These programs use function-based games in which the goal is to move the hand to a target (e.g. pick up a barbell, corresponding to wrist flexion) and raise it first to waist height (neutral wrist), then overhead (corresponding to wrist extension) within a time specified by the therapist. The time to reach the target decreases as the participant experiences success. If the participant does not achieve the goal within the specified time, the air muscle is activated to assist the participant through the desired ROM. If the participant is successful in achieving the goal on at least 80%, the difficulty level is automatically increased to the next level (ten levels total for each program), requiring an increase in the distance the hand must move to achieve the target. Conversely, if the participant is successful on less than 20% of the trials, the difficulty level is decreased. The requirements to achieve more active flexion ROM increase by 1.5 degrees, while the active extension ROM requirements increase by 3 degrees for each level.
The advanced motor control programs of this robotic device incorporate timing-based training principles, requiring the participant to lift the hand at a precise time and velocity. In the Balloon game, a hot-air balloon moves up and down on the screen corresponding to active wrist extension and flexion, respectively. The participant must avoid objects as they scroll across the screen from right to left. In the second of these games, Therapong, the participant moves a paddle up and down to bounce a ball back to the other side of the playing field, which is controlled by the computer. As the participant completes successful trials, the active range of motion, timing, velocity, and strategic demands for the motor response are increased. The flexion and extension range of motion requirements for these advanced motor control games increase by three degrees for each level. There are ten levels, each containing three velocities.
The goal of the Spasticity Reduction program (Thermometer) is to decrease flexor tone of the fingers and wrist via visual biofeedback and assistive motion. The initial position of the hand is at approximately 30 degrees of flexion. The air muscle then inflates to bring the wrist to approximately half of the participant’s available passive ROM. The amount of force necessary to achieve this position is measured while a potentiometer measures wrist position. A thermometer is displayed on the screen with a green line showing the initial resistance; increased resistance is shown as yellow and then red. The line is reduced as flexor stiffness (resistance to passive motion) decreases.
Ten days following baseline testing, a two-hour home visit by the therapist was completed to instruct the participant on use of the HMP robotic device and home exercise program (HEP) intervention. Following this visit, he demonstrated proper and safe use of the robotic device and comprehension of the HEP. Previous evidence from constraint induced therapy studies describe 3–6 hours of repetitive task practice to produce desired improvements in UE function over a 2-week period.8, 34 Due to the 8-week duration of this case study and participant reported time constraints, the participant was asked to perform the intervention 5 days a week (2 hours HMP + 1 hour HEP) for a total intervention time of 120 hours. Expectations with respect to treatment intensity/dosage were outlined throughout the screening/enrollment process. Adherence was fostered through a behavioral contract, daily exercise log, and weekly follow-up phone calls with the therapist.
Based on the physical therapist evaluation, game modules were selected to address the participant’s impairments: spasticity, strength, motor planning, force generation, and movement timing. The participant was prescribed the following activities on the HMP device: 1) Spasticity reduction program (Thermometer Program) - 10 repetitions of passive wrist extension to 35 degrees with 60 second hold to provide an appropriate stretch; 2) Wrist extension (Strongman Up Program) – 10 repetitions with wrist beginning at neutral and requiring 3 degrees of extension to complete; 3) Wrist flexion to extension (Strongman Basic Program) – 10 repetitions beginning with wrist in neutral and requiring the participant to move from 1.5 degrees of flexion to 3 degrees of extension; 4) Motor control (Balloon and Therapong Program) – 15 minutes each game with wrist beginning in neutral and requiring with 3 degrees of flexion and 3 degrees of extension. The participant was instructed to repeat the prescribed sequence of tasks 2–3 times during the day to complete two hours of robotic-assisted intervention daily. The participant began at level 1, where gravity is the only resistance to movement, because although he had full passive ROM he was not able to move through the range due to weakness and spasticity. The robotic device program advanced the levels and ROM requirements according to the participant’s performance, as described above.
The HEP created for our larger clinical trial was designed to provide a database of progressive UE exercises to allow participants to incorporate basic movements and preparatory activities into functional tasks. It also emphasized training of the proximal UE not addressed by the HMP robotic device. The HEP included the following exercise domains/types: passive ROM and stretching activities, active-assisted ROM activities, weight-bearing exercises, AROM/strengthening exercises, goal-directed movement activities, functional / task-based activities, incorporation of impaired UE into ADL’s. For this participant, emphasis was placed on weight bearing activities to facilitate joint approximation, proprioception, and co-contraction, stretching and strengthening activities, and activities that focused on coordination, timing, and force of movement. Each exercise had clear instructions for performance and prescribed dosage, and was accompanied by a picture, consistent with current clinical practice standards. Each exercise page was labelled to correspond with a HEP diary, which the participant used to document the details of his daily exercise routine. The participant was asked to complete one hour of these activities daily and to incorporate the hemiparetic UE into daily functional tasks such as opening containers, sweeping the floor, self-feeding, and completing self-care/grooming tasks.
Remote monitoring was achieved by the therapist via review of data transmitted to the secured server from the HMP controller approximately twice weekly and through weekly phone calls to the participant. Data available on the server included calendar of use, daily duration of use, angles of flexion and extension achieved, level progression, and resistance to stretch to measure spasticity. Objective data transmitted from the device regarding adherence to the robotic intervention eliminated ambiguity of self-reported adherence and allowed the therapist to more openly address issues relating to the participant’s struggles with adhering to the 2-hour daily prescribed robotic-assisted therapy intervention.
Weekly follow-up phone calls were chosen based on the typical frequency of formal therapy sessions of a patient undergoing neurorehabilitation 6 months after stroke. A structured format was used for the follow-up phone calls, in which the following topics were assessed consistently: 1) detailed adherence to prescribed intervention; 2) formal assessment of pain; 3) adverse events including falls or changes in medical condition; 4) concurrent exercise / activities other than prescribed intervention; 5) issues preventing adherence; 6) changes observed in function of involved UE; 7) logistics with use of robotic device (ie: donning the hand piece, operating the device, etc). The therapist concluded each phone call by making modifications to the prescribed intervention based on information gathered during the call. Specific to this case, the weekly phone calls were used in conjunction with remote monitoring of the participant’s progress with the robotic device to manage the rehabilitation intervention.
After week 1 the participant reported an increase in stiffness and spasticity in his UE and results from the HMP revealed increased resistive forces generated with the spasticity reduction program. As a result, the therapist suggested increasing the number of repetitions and duration of stretch with the spasticity reduction program. During the weekly phone call following the completion of week 3, the therapist noticed there was some discrepancy between the data that was being transmitted from the robotic device and the participant’s self-reported functional progress. The participant was advancing through the levels; however, he was progressing at a rate beyond what would be expected in just 3 weeks of intervention. This prompted the therapist to make a home visit and discover that the device was set at levels higher than what the participant was able to achieve. Since the robotic device only advanced levels when the user is able to complete 80% of the tasks, the participant may have been using his unaffected hand to assist with level completion. After re-educating the participant on proper use and the purpose of the device, the therapist adjusted the device to appropriate levels, which was a level where he was able to achieve the task about 50–80% of the time so the participant was challenged, but able to achieve the target and advance through the programs.
The participant’s home exercise program was advanced to maintain appropriately challenging activities. In conjunction with ROM and strength improvements, the therapist encouraged him to incorporate that movement into his functional activity. For example, several weeks into the study, he reported increased finger flexion and extension. The therapist encouraged movements such as grasp and release of various shaped toys when he was playing with his grandchildren. The therapist also provided encouragement and strategies to improve exercise time management skills (adherence) during phone calls, as the participant voiced difficulty managing his responsibilities at home with his home rehabilitation program. Extensive patient education was provided during the weekly phone calls to optimize the participant’s understanding of how rehabilitation activities can improve motor recovery. Emphasis was placed on therapeutic intensity, active practice, and the incorporation of acquired movement into functional daily tasks such as grooming, feeding, and housekeeping.
Outcomes
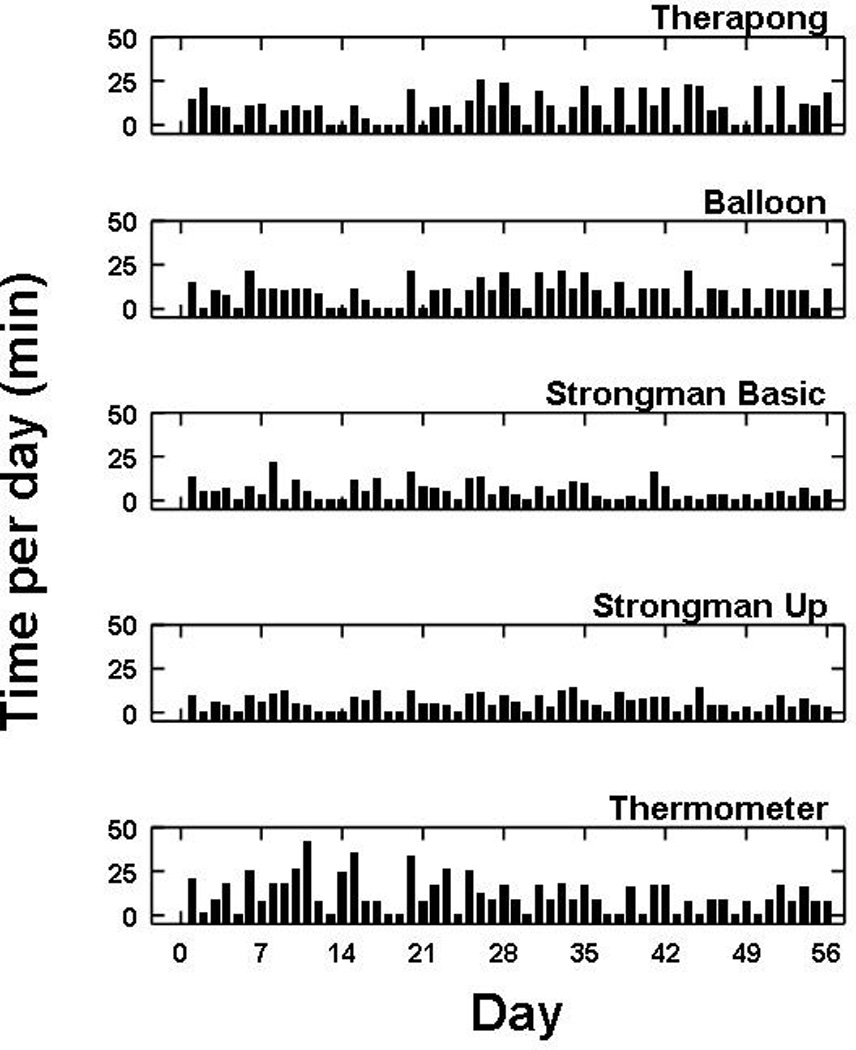
No adverse events were reported at any point of the study. The robotic device was used on 47/56 days and the HEP performed on 56/56 days (compared to prescribed 40 days). Total robotic use and HEP time are shown in Figure 2a. Overall, the participant completed 85 out of the prescribed 120 hours. The participant used the HMP 4–6 days per week and spent 20–100 minutes on the device each time. He spent an average of 355 minutes per week performing the HEP. The average time per day using the robotic device and HEP was approximately 100 minutes. The daily percent time of robotic device use is shown in Figure 2b (note: inverse of this line is time spent completing HEP). The time allotted to each specific program on the HMP robotic device is displayed in Figure 3.
Figure 2.
Total daily time spent completing combined robotic and HEP intervention (A) and daily percentage of exercise time using HMP robotic device (B). Note that daily percentage of HEP is the inverse of daily percentage of HMP time. Horizontal dashed lines depict total prescribed daily exercise time (180 minutes) (A) and relative percentage of HMP usage (66.6%) requested of participant in study protocol.
Figure 3.

Total daily time allotted to each program on the HMP robotic device over the 8-week trial.
The participant demonstrated an improvement on the ARAT from 21/57 at baseline to 37/57 post-intervention as shown in Table 1. This 16-point change in the ARAT approached the 17-point MCID value for the affected non-dominant UE following stroke26 and surpassed the MDC of 3.5 points.28 He also improved on the individual sub-scales of the ARAT from baseline to post-intervention: grasp (6/18 to 15/18), grip (5/12 to 8/12) pinch (6/18 to 8/18) and gross motor (4/9 to 6/9). The participant improved his FMA score by 20 points (22/66 to 42/66), which surpassed the MCID of 6.6 points32 and the test-retest MDC of 5.2 points.28 In terms of quality of life, the participant’s score on the ADL subset of the SIS improved from 35/50 to 42/50, surpassing the MCID value of 5.9 points. He reported total recovery from his stroke post-intervention at 65%, compared to 40% at baseline. His CES-D score, in which a higher score may be indicative of depressive symptomology,33 decreased from 23/60 to a 4/60.
Table 1.
Baseline and post-intervention outcomes: Action Research Arm Test (ARAT), Fugl-Meyer Assessment (FMA), Center for Epidemiologic Studies Depression Scale (CES-D), and the Stroke Impact Scale (SIS).
| Baseline | Post-Test | Difference | |
|---|---|---|---|
| ARAT | 21/57 | 37/57 | 16B |
| FMA | 22/66 | 42/66 | 20A,B |
| CES-D | 23/60 | 4/60 | 19 |
| SIS: Physical Problem/Strength | 10/20 | 9/20 | −1 |
| SIS: Memory | 27/35 | 25/35 | −2 |
| SIS: Feelings | 25/45 | 42/45 | 17 |
| SIS: Communication | 34/35 | 35/35 | 1 |
| SIS: ADL | 35/50 | 42/50 | 7A |
| SIS: Mobility | 31/45 | 30/45 | −1 |
| SIS: Hand Use | 6/25 | 8/25 | 2 |
| SIS: Meaningful Activity | 16/40 | 25/40 | 9 |
| SIS: Stroke Recovery | 40/100 | 65/100 | 25 |
indicates value ≥ MCID
indicates value ≥ MDC
Improvements in performance from baseline are identified with an asterisk (*).
Performance for individual timed tasks of the WMFT are provided in Table 2. The participant demonstrated improvements in 11 of the 15 timed tasks of the WMFT, essentially no change in two tasks, and worse performance in the remaining two tasks. Both strength measures improved subtanstially. The FAS of the WMFT improved by 5 points (44/75 to 49/75), which supassed the MCID of 1.2 points in the non-dominant side.26
Table 2.
Time and functional ability scale (FAS) score for individual tasks of the Wolf Motor Function Test (WMFT).
| WMFT Task | Baseline | Post-Test | Difference | |||
|---|---|---|---|---|---|---|
| Time (s) | FAS | Time (s) | FAS | Time (s) | FAS | |
| Forearm to table | 1.25 | 3 | 1.56 | 4 | 0.31 | 1 |
| Forearm to box | 2.50 | 3 | 1.22 | 4 | −1.28 | 1 |
| Extend elbow | 3.19 | 3 | 1.75 | 3 | −1.44 | 0 |
| Extend elbow with weight | 5.19 | 3 | 1.53 | 3 | −3.66 | 0 |
| Hand to table | 1.75 | 3 | 1.60 | 4 | −0.15 | 1 |
| Hand to box | 3.34 | 3 | 1.41 | 3 | −1.93 | 0 |
| Reach and retrieve | 1.75 | 4 | 1.66 | 4 | −0.09 | 0 |
| Lift can | 10.09 | 2 | 5.75 | 4 | −4.34 | 2 |
| Lift pencil | 5.44 | 3 | 6.91 | 3 | 1.47 | 0 |
| Lift paper clip | 6.72 | 3 | Unable to complete | 1 | N/A | −2 |
| Stack checkers | 55.00 | 2 | 24.30 | 3 | −30.70 | 1 |
| Flip cards | 28.70 | 3 | 27.80 | 3 | −0.9 | 0 |
| Turn key in lock | 18.10 | 3 | 42.00 | 2 | 23.9 | −1 |
| Fold towel | 10.68 | 3 | 10.21 | 4 | −0.47 | 1 |
| Lift basket | 22.50 | 3 | 5.59 | 4 | −16.91 | 1 |
| Weight to box (lb) | 5 | N/A | 8 | N/A | 3 | N/A |
| Average grip strength (kg) | 2.13 | N/A | 6.24 | N/A | 4.09 | N/A |
Improvements in performance from baseline are identified with an asterisk (*).
Discussion
Robotic-assisted therapy was utilized to deliver one component of the UE rehabilitation, as it incorporates principles of motor learning to facilitate cortical reorganization by providing repetitive, active (patient initiated), goal-directed movements of the affected limb, while providing feedback through knowledge of performance. Although repetitive, goal-directed practice appears to be important in fostering neuroplastic change post-stroke, such repetition should include functionally based training that embraces task-specific practice to drive functional motor recovery.2, 35 Since this consideration is not inherently embedded within the application of robotic devices, the therapeutic approach applied in this case study relies on the inclusion of a HEP in which a broad spectrum of appropriately challenging functional tasks is prescribed to the participant. Incorporating the involved UE into daily tasks appears instrumental in optimizing functional recovery.36 Because the participant described in this case was still displaying gains in therapy but was unable to access care due to insurance restrictions, the telerehabilitation model of robotic-assisted therapy overseen remotely by the therapist appeared to be a reasonable intervention to continue UE rehabilitation.
The participant was 70% adherent overall with the prescribed robotic training program and HEP. His self-reported adherence in performing the HEP activities was greater than activities using the robotic device whose software provided objective data regarding adherence. In fact, he completed 47 rather than the 40 hours of HEP. In contrast, the robotic device was used 38 out of 80 hours. The rationale for the selection of these prescribed HEP and robotic times was based on previous intervention times of CIMT and other repetitive task practice and robotic interventions that have been shown effective in clinical environments.8, 37 The participant reported that boredom with the device and family commitments were his greatest barriers to completing the prescribed 2-hour daily intervention time on the robotic device. The programs of the robotic device have been designed to be “game-like” and engaging; however, this participant did not find them to be sufficiently engaging to warrant two hours of daily use. We are unaware of any other studies that have examined or determined the optimal duration of robotic use in a home environment.
Our preliminary data from this case study suggest two hours of robotic therapy per day may be perceived as excessively burdensome, especially when coupled with one hour of HEP activities. Nevertheless, the improvements in upper extremity function demonstrated were comparable or exceeded those reported in the literature,10, 37 exceeding MCID values on the FMA and ADL subscale of the SIS, and 1 point less than the MCID on the ARAT. Thus, one hour per day of robotic therapy may be sufficient to facilitate favorable changes following stroke. Although adherence to the prescribed 2-hour daily robotic intervention is emphasized and encouraged during interactions with the participant, actual intervention times as recorded through the device may provide significant data regarding optimal dosage necessary to achieve motor recovery. A more reasonable intervention plan may be to reduce the total time of the daily activities to two hours per day rather than three and to divide the time between each mode of training equally, as this was the participant’s general use pattern. This alternative approach should be considered for future studies.
Strategies to facilitate adherence emphasized the incorporation of activities that the participant could complete with his family. These included function-based activities such as feeding himself with the affected UE and playing games that with his grandchildren that incorporated the involved UE. Jurkiewicz and Marzolini38 recently reported 55–76% adherence in HEP once formal outpatient stroke rehabilitation has ceased. This participant’s adherence may have been greater due to the remote involvement from the therapist, in addition to the emphasis on incorporating the involved UE into daily activities that involved his family. Through the weekly phone calls, the therapist was able to adapt the HEP to appropriately challenge the participant and to optimize his engagement. The incident in week 3, with the robotic device being set at a level higher than the participant could reasonably be expected to achieve, underscores the concept that use of a robotic device should be monitored by a therapist with in-depth knowledge related to typical motor recovery and familiarity with technology to ensure agreement between program progression and expected change in motor performance.
A barrier to the adaptation of any robotic device to a rehabilitation application is the technology itself. The patient must be willing to learn how to operate the device, and the therapist must have technical knowledge of the device. These concerns are important since technological difficulties in the various stages of installation and operation can negatively affect the effectiveness of telerehabililation.39 The participant in this case study embraced both the technology of the robotic device, and the distant monitoring component of this stroke rehabilitation model. Furthermore, although costs are minimal for direct therapy care, the cost of robotic devices has not traditionally been covered by third party payors. Further study will provide important data regarding the feasibility of telerehabilitation for stroke.
Another significant gap addressed by this case study is the investigation of the feasibility of using a robotic device as a component of a program for improving UE function in persons with significant paresis after stroke. While most UE rehabilitation interventions investigated in large-scale randomized controlled trials that have been found to be efficacious following stroke have had stringent movement requirements,34, 37 robotic therapy could include individuals who are not able to isolate movements of the wrist and hand. Future research investigating the efficacy of a home-based robotic-assisted intervention on the recovery of motor function in persons with stroke who have limited volitional movement will provide valuable data in directing rehabilitative care for this population.
Summary
Numerous barriers exist that prevent persons with stroke from obtaining the optimal dose of therapy to drive neural reorganization and promote motor recovery.19, 31 The current health care model in the US does not support the intensity of therapy documented in the literature as being necessary for improvement,40 primarily because most government and private insurance plans have therapy caps or visit limits. Upon discharge from therapy, people with stroke are traditionally instructed in home exercise programs to be undertaken independently, without the guidance of a physical or occupational therapist. They often experience a decline in function after discharge from therapy, due, in part, to poor long-term adherence to rehabilitation exercises.41 This case study addresses several of these barriers: access to stroke rehabilitation, quantity/dosage of practice, quality of practice, and adherence to the program. The outcomes of this case suggest that participants can complete the UE rehabilitation program independently in their home environment. As opposed to direct supervision of a therapist, remote monitoring may be sufficient to improve UE function in some cases. If such efforts can be reproduced in a clinical trial, the impact on health care costs and patient outcomes could be profound.
Supplementary Material
Supplemental Digital Content 1. Video Abstract..wmv
Acknowledgments
This study was supported by RC3NS070646 from the National Institute of Neurological Disorders And Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Dr. Wolf is Chairman of the Scientific Advisory Board for Kinetic Muscles Inc., and is a paid consultant for Kinetic Muscles Inc. Sharon Buchanan is a paid consultant for Kinetic Muscles Inc. No other authors have any financial relationship with Kinetic Muscles Inc.
References
- 1.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, et al. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the american heart association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 2.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hearing Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 3.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwamm LH, Audebert HJ, Amarenco P, Chumbler NR, Frankel MR, George MG, et al. Recommendations for the implementation of telemedicine within stroke systems of care: A policy statement from the american heart association. Stroke. 2009;40:2635–2660. doi: 10.1161/STROKEAHA.109.192361. [DOI] [PubMed] [Google Scholar]
- 5.Bennett K, Olatusi B, Probst JC. Health disparities: A rural-urban chartbook. 2008 [Google Scholar]
- 6.Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Physical therapy. 2012;92:463–468. doi: 10.2522/ptj.20110100. [DOI] [PubMed] [Google Scholar]
- 7.Johansson T, Wild C. Telerehabilitation in stroke care--a systematic review. J Telemed Telecare. 2011;17:1–6. doi: 10.1258/jtt.2010.100105. [DOI] [PubMed] [Google Scholar]
- 8.Lum PS, Uswatte G, Taub E, Hardin P, Mark VW. A telerehabilitation approach to delivery of constraint-induced movement therapy. The Journal of Rehabilitation Research and Development. 2006;43:391. doi: 10.1682/jrrd.2005.02.0042. [DOI] [PubMed] [Google Scholar]
- 9.Carignan CR, Krebs HI. Telerehabilitation robotics: Bright lights, big future? The Journal of Rehabilitation Research and Development. 2006;43:695. doi: 10.1682/jrrd.2005.05.0085. [DOI] [PubMed] [Google Scholar]
- 10.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabil Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutner NG, Zhang R, Butler AJ, Wolf SL, Alberts JL. Quality-of-life change associated with robotic-assisted therapy to improve hand motor function in patients with subacute stroke: A randomized clinical trial. Physical therapy. 2010;90:493–504. doi: 10.2522/ptj.20090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstein L, Ridgel AL, Thota A, Samame B, Alberts JL. Effects of combined robotic therapy and repetitive-task practice on upper-extremity function in a patient with chronic stroke. Amer J Occup Ther. 2008;62:28–34. doi: 10.5014/ajot.62.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient 1 A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 14.Linder SM, Rosenfeldt AB, Reiss A, Buchanan S, Sahu K, Bay CR, Wolf SL, Alberts JL. The home stroke rehabilitation and monitoring system trial: A randomized controlled trial. International journal of Stroke. doi: 10.1111/j.1747-4949.2012.00971.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 16.Bailey MJ, Riddoch MJ, Crome P. Evaluation of a test battery for hemineglect in elderly stroke patients for use by therapists in clinical practice. NeuroRehabilitation. 2000;14:139–150. [PubMed] [Google Scholar]
- 17.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 18.Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the fugl-meyer test, action research arm test and box and block test: A multicentre study. Clin Rehabil. 2005;19:404–411. doi: 10.1191/0269215505cr832oa. [DOI] [PubMed] [Google Scholar]
- 19.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- 21.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 22.Morris DM, Uswatte G, Crago JE, Cook EW, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 23.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0 : Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 24.Shinar D, Gross CR, Price TR, Banko M, Bolduc PL, Robinson RG. Screening for depression in stroke patients: The reliability and validity of the center for epidemiologic studies depression scale. Stroke. 1986;17:241–245. doi: 10.1161/01.str.17.2.241. [DOI] [PubMed] [Google Scholar]
- 25.Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the modified ashworth scale in the assessment of muscle spasticity: Limb and muscle group effect. NeuroRehabilitation. 2008;23:231–237. [PubMed] [Google Scholar]
- 26.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin KC, Fu T, Wu CY, Wang YH, Liu JS, Hsieh CJ, et al. Minimal detectable change and clinically important difference of the stroke impact scale in stroke patients. Neurorehabil Neural Repair. 2010;24:486–492. doi: 10.1177/1545968309356295. [DOI] [PubMed] [Google Scholar]
- 28.Lin JH, Hsu MJ, Sheu CF, Wu TS, Lin RT, Chen CH, et al. Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Physical therapy. 2009;89:840–850. doi: 10.2522/ptj.20080285. [DOI] [PubMed] [Google Scholar]
- 29.Winstein CJ, Miller JP, Blanton S, Taub E, Uswatte G, Morris D, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–152. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0 Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 31.Fugl-Meyer AR, Jaasko L, Norlin V. The post-stroke hemiplegic patientIiIncidence, mortality, and vocational return in goteborg, sweden with a review of the literature. Scand J Rehabil Med. 1975;7:73–83. [PubMed] [Google Scholar]
- 32.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 33.Parikh RM, Eden DT, Price TR, Robinson RG. The sensitivity and specificity of the center for epidemiologic studies depression scale in screening for post-stroke depression. International journal of psychiatry in medicine. 1988;18:169–181. doi: 10.2190/bh75-euya-4fm1-j7qa. [DOI] [PubMed] [Google Scholar]
- 34.Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The excite stroke trial: Comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winstein CJ, Wolf S. Chapter 17: Task oriented training to promote upper extremity recovery. In: Stein JZR, Harvey RF, Macho RV, Winstein CJ, editors. Stroke recovery and rehabilitation. Woodbridge, CT: Demos Publishing; 2009. [Google Scholar]
- 36.Christie L, Bedford R, McCluskey A. Task-specific practice of dressing tasks in a hospital setting improved dressing performance post-stroke: A feasibility study. Aust Occup Ther J. 2011;58:364–369. doi: 10.1111/j.1440-1630.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- 37.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. The New England journal of medicine. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurkiewicz MT, Marzolini S, Oh P. Adherence to a home-based exercise program for individuals after stroke. Topics in Stroke Rehabilitation. 2011;18:277–284. doi: 10.1310/tsr1803-277. [DOI] [PubMed] [Google Scholar]
- 39.Pramuka M, Van Roosmalen L. Telerehabilitation technologies: Accessibility and usability. Intl J Telerehabil. 2009;1:85–97. doi: 10.5195/ijt.2009.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page SJ, Levine P, Leonard A, Szaflarski JP, Kissela BM. Modified constraint-induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Physical therapy. 2008;88:333–340. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- 41.D'Alisa S, Baudo S, Mauro A, Miscio G. How does stroke restrict participation in long-term post-stroke survivors? Acta Neurol Scand. 2005;112:157–162. doi: 10.1111/j.1600-0404.2005.00466.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Video Abstract..wmv


