Abstract
Liver stem/progenitor cells (LPCs) are defined as cells that supply two types of liver epithelial cells, hepatocytes and cholangiocytes, during development, cellular turnover, and regeneration. Hepatoblasts, which are fetal LPCs derived from endoderm stem cells, robustly proliferate and differentiate into hepatocytes and cholangiocytes during fetal life. Between mid-gestation and the neonatal period, some cholangiocytes function as LPCs. Although LPCs in adult livers can be enriched in cells positive for cholangiocyte markers, their tissue localization and functions in cellular turnover remain obscure. On the other hand, it is well known that liver regeneration under conditions suppressing hepatocyte proliferation is supported by LPCs, though their origin has not been clearly identified. Recently many groups took advantage of new techniques including prospective isolation of LPCs by fluorescence-activated cell sorting and genetic lineage tracing to facilitate our understanding of epithelial supply in normal and injured livers. Those works suggest that, in normal livers, the turnover of hepatocytes mostly depends on duplication of hepatocytes. It is also demonstrated that liver epithelial cells as well as LPCs have great plasticity and flexible differentiation capability to respond to various types of injuries by protecting or repairing liver tissues.
Keywords: liver progenitor cell, bipotential, hepatoblast, cholangiocyte, hepatocyte, oval cell
Introduction
Tissue stem/progenitors of an epithelial organ continuously supply multiple types of epithelial cells to support organogenesis, cellular turnover, and regeneration throughout life. In skin and intestine, tissue stem cells are genetically labeled and proved to continuously supply all cell lineages in both cellular turnover and tissue regeneration.1,2
The liver performs important physiological functions including metabolic reactions, energy storage, serum protein production, bile secretion, and defense against pathogenic infections. The functional abnormalities of the liver directly threaten life. Therefore, liver functions are properly established during development and maintained throughout life. Moreover, they are quickly recovered when the mass of tissue is damaged or lost. Thus, it is important to understand how hepatocytes and cholangiocytes are supplied during development, and in normal and injured adult livers. During liver organogenesis, hepatoblasts, which are fetal LPCs, proliferate and differentiate into hepatocytes and cholangiocytes. Adult LPCs are shown to exist in normal liver based on expression of surface markers including epithelial cell adhesion molecule (EpCAM),3 CD13, and CD133.4 However, their roles in cellular turnover are still controversial because self-duplication of hepatocytes and cholangiocytes is likely the major mechanism to maintain homeostasis. Even when a partial loss of liver tissue occurs, it is promptly compensated by duplication of hepatocytes and/or cholangiocytes. It is well known that after 70% liver tissue resection (PHx) in rodents and acute damages induced by hepatotoxins, such as carbon tetrachloride and acetaminophen, the liver mass returns nearly to the original size within a week.5 Moreover, a recent finding indicates that hypertrophy and proliferation of hepatocytes are well coordinated to restore liver functions efficiently.6
In contrast to acute liver injury, LPCs are implicated in liver regeneration when proliferative capabilities of hepatocytes and/or cholangiocytes are abrogated.7,8 The response to severe injuries, known as “ductular reactions” (DRs), is associated with activation and expansion of LPCs, which eventually differentiate to mature hepatocytes (MHs) or cholangiocytes to repair damaged tissues. In addition to self-duplication and differentiation from LPCs, the lineage conversion between hepatocytes and cholangiocytes is implicated in liver regeneration.7 In particular, cholangiocytes derived from hepatocytes have been recognized in injured livers associated with DRs.9,10 In this review, we summarize recent works about LPCs in developing and adult livers as well as those about lineage conversion to illustrate how multiple ways of epithelial cell supplies contribute to establishing, maintaining, and regenerating liver tissues.
LPCs in developing liver
Fetal liver
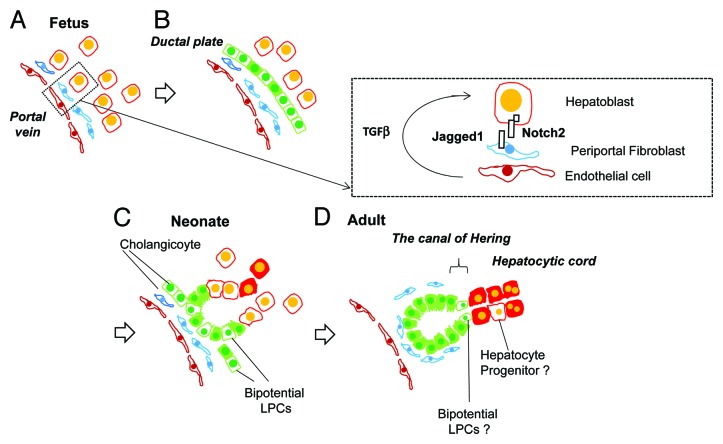
In early liver organogenesis, the foregut endoderm develops into the liver bud by FGFs and BMPs secreted from the cardiac mesoderm.11 “Hepatoblasts” forming the liver bud actively proliferate and differentiate to hepatocytes and cholangiocytes. Isolation of hepatoblasts from fetal livers based on specific surface antigens accelerated our understanding about liver organogenesis at the molecular level. We previously established a method to isolate hepatoblasts from E14.5 mouse livers by identifying delta-like 1 (Dlk-1) as a specific marker.12 At earlier stages, hepatoblasts are identified as Dlk-1+EpCAM+ cells.13 Expression of Dlk-1 on hepatoblasts is observed between E10 and 16 and quickly downregulated thereafter. With its strong expression on hepatoblasts, Dlk-1 antibodies can easily and quickly isolate the cells through magnetic cell sorting (>95% pure). Hepatoblasts are also isolated as CD45−TER119−cKit−cMet+CD49f+/low,14 E-cadherin+,15 or CD13+ cells.16 Purified hepatoblasts form colonies containing albumin+ hepatocytes and cytokeratin 19 (CK19)+ cholangiocytes in a clonal way and are engrafted in congeneric recipient livers as hepatocytes and cholangiocytes. In vivo, in mid gestation, hepatoblasts are committed to cholangiocytes in the periportal area and hepatocytes in the parenchymal region (Fig. 1A and B).17,18 A small number of hepatoblasts may remain in early postnatal mouse livers, which eventually differentiate into hepatocytes around weaning. We found that colony-forming cells abundantly exist in the parenchymal region of mouse livers by 4 wk after birth. In particular, we could isolate self-renewable progenitors from 1-wk-old (W) mouse livers though they were committed to the hepatocyte lineage (our unpublished data).
Figure 1. Liver development and transition of tissue localization of LPCs. (A) Hepatoblasts are fetal bipotential liver stem/progenitor cells (LPCs), which abundantly exist in fetal liver by mid gestation. Around E15, hepatoblasts near the portal vein are committed to cholangiocytes by the activation of Notch signaling pathways through direct interaction with Jagged-1+ portal fibroblasts as well as TGFβ by receiving the ligand secreted from endothelial cells and/or fibroblasts. (B) The ductal plates are the primitive structure of bile ducts. Cholangiocytes in this structure function as LPCs, which have the ability to differentiate into hepatocytes. (C) In late gestation and neonatal period, cholangiocytes establish tubular structures though part of the cells still exist in the ductal plate. During this period, many cholangiocytes maintain the ability to differentiate into hepatocytes and may function as LPCs. (D) During postnatal development, most of the cholangiocytes lost the ability to differentiate into hepatocytes. However, a small number of LPCs exist in normal adult liver. Although their tissue localization has not been definitely identified, LPCs may exist in or near the canal of Hering, the boundary between hepatic cord and bile ducts.
Developing liver (perinatal stage)
Hepatoblasts around the portal vein are committed to cholangiocytes by activation of Notch and TGFβ signals and form ductal plates, the primitive structure of bile ducts.19-22 Those “immature” cholangiocytes readily express cholangiocyte markers including Sry box containing gene 9 (Sox9), osteopontin (OPN), and EpCAM.23 When cholangiocytes in the ductal plate were labeled around E15 in Sox9-CreERT2 knock-in mice by peritoneal injection of tamoxifen to the pregnant mouse, they differentiated to ductular cholangiocytes, the canal of Hering, and periportal hepatocytes.24 This result indicates that cholangiocytes that form the ductal plate succeed to bidirectional differentiation potential from hepatoblasts. In contrast to the embryonic ductal plate, it is unknown whether late fetal and neonatal cholangiocytes function as LPCs. We isolated EpCAM+ cholangiocytes from livers between E17.5 and 8W and examined their clonal proliferation and differentiation potential using a colony assay. Between E17.5 and 2W after birth, about 1–2% of EpCAM+ cells clonally proliferated and showed bidirectional differentiation capability, demonstrated by the formation of colonies that contained albumin+ hepatocytes and CK19+ cholangiocytes. In particular, a portion of the 1W EpCAM+ cells showed self-renewable capability and successfully engrafted as hepatocytes and cholangiocytes in recipient livers of nude mice when transplanted through the spleen (our unpublished data). Although it is unknown whether neonatal cholangiocytes supply hepatocytes in vivo similar to fetal ones, cholangiocytes likely possess potential to function as LPCs at least by the neonatal period (Fig. 1C).
EpCAM+ cholangiocytes gradually lose the ability to form bipotential colonies in vitro beyond 2W. Similarly, the number of bipotential LPCs in the biliary tree enriched in the CD13+CD49f+CD133+ fraction gradually decrease during postnatal development.4 We found that neonatal, but not adult, EpCAM+ cells differentiated into hepatocytes in vitro in the presence of oncostatin M and Matrigel®.25 By comparing neonatal and adult cholangiocytes, we found that transcription factors related to cholangiocyte differentiation, including hairy enhancer of slit 1 (Hes1), Sox9, hes-related family bHLH transcription factor with YRPW motif 1 (Hey1), and grainyhead-like 2 (Grhl2), were expressed at higher levels in adult cholangiocytes than in neonatal ones. In particular, Grhl2, which we identified as a transcription factor promoting epithelial integrity of cholangiocytes,26 was further downregulated in neonatal cells during hepatocyte differentiation. Its expression was maintained consistently in adult cholangiocytes during the culture. We further found that Grhl2 inhibited expression of HNF4α and C/EBPα, both essential factors for hepatocyte differentiation. These results indicate that most of the EpCAM+ cells lose bipotency during differentiation into mature epithelial cells (Fig. 1C and D).25
Residential stem cell in an adult liver: heterogeneity of the biliary cells
As we mentioned above, the number of LPCs is very limited in mouse normal adult livers. Nevertheless, recent studies using fluorescence-activated cell sorting (FACS) to prospectively isolate cells positive for cholangiocyte markers3,4 and using genetic lineage tracing27,28 suggest that LPCs exist in normal adult liver. Such results raise two possibilities: 1) a specific cell population expressing cholangiocyte markers, whose tissue localization is distinctive to cholangiocytes, exists as LPCs and 2) cholangiocytes are a heterogeneous population and some cells possess LPC properties. We further discuss these possibilities in the next section.
The canal of Hering
The canal of Hering is a boundary structure connecting biliary tree and hepatic cords (Fig. 1D). Adult LPCs likely reside in or near this area because ductular structures expand around the boundary in severely injured livers. Efforts have been made to identify and enrich LPCs from adult livers as CD45−TER119−cKit−CD13+CD133+ 4 and EpCAM+ cells.3 The cells clonally proliferate and differentiate into hepatocytes or cholangiocytes depending on culture conditions. They are engrafted as MHs in recipient livers upon transplantation. When bile ducts and the canal of Hering were labeled with YFP or LacZ in Sox9-CreERT2:ROSA mice, LacZ-labeled hepatocytes appeared near the portal vein and expanded along the portal to centrilobular axis, which strongly supported “streaming model.”27 However, this result has been argued by the following reports. Malato et al. labeled hepatocytes by injecting adeno-associated virus 8 (AAV8)-Cre to ROSA-YFP mice.29 Because no YFP− MHs cells were found even at 24 wk after virus injection, they concluded that cellular turnover of MHs mostly relies on their self-duplication. When cholangiocytes and the canal of Hering were eternally labeled using OPN-CreERT2:ROSA-YFP, without any injury, YFP+ MHs did not emerge even at 5 mo after labeling.30 Although it is possible that Sox9 but not OPN is expressed in “residential LPCs,” it is still ambiguous whether LPCs positive for cholangiocyte markers continuously supply hepatocytes in normal cellular turnover.
Heterogeneity of cholangiocytes
Several groups have identified morphological and functional heterogeneity of intrahepatic bile ducts in rats and mice.31-33 Large and small ducts consist of relatively large and small cholangiocytes, respectively. Large cholangiocytes were considered to be more “mature” and “functional” with expression of transporters, such as cystic fibrosis transmembrane conductance regulator (CFTR) and anion exchanger 2 (AE2) and respond against secretin, whereas small cholangiocytes were relatively immature cholangiocytes. We recently found that EpCAM+ cholangiocytes were divided into two different cellular fractions after digesting liver tissue with conventional two-step collagenase perfusion. The biliary tree associated with connective tissue, which corresponds to Glisson’s capsule, was obtained as undigested tissue, from which ductular cholangiocytes were isolated as EpCAM+ cells after further collagenase/hyaluronidase treatment. We named them “biliary tree EpCAM+ cells (BT-EpCAM+ cells).” In addition, EpCAM+ cells were also isolated from the cell suspension liberated from liver tissue only by collagenase perfusion. Because the latter fraction contained “non-parenchymal cells (NPC)” cells such as stellate, Kupffer, and sinusoidal endothelial cells, we named them “NPC-EpCAM+ cells.” We considered that NPC-EpCAM+ cells may be isolated from small terminal ductules because they are unlikely to be strongly associated with connective tissue. Interestingly, in adult liver, colony-forming cells were found more frequently in the NPC-EpCAM+ fraction (our unpublished data). Although we have not examined such NPC-EpCAM+ cells and BT-EpCAM+ cells are identical to small and large cholangiocytes, our results support the idea that cholangiocytes are a heterogeneous cell population in terms of proliferation and differentiation potential.34
Peribiliary glands
Extrahepatic bile ducts (EHBDs) are composed of hepatic, cystic, pancreatic, and common ducts. In developmental periods, cells in EHBDs are competent to stimulation inducing a pancreatic fate. Notably, EHBDs were replaced with ectopic pancreatic tissues in Hes1-KO mice, indicating that the Notch pathway inhibits pancreatic fate for normal development of EHBDs.35 Recent reports focused on glandular structures (peribiliary glands; PBGs) along EHBDs and have suggested that PBGs contain multipotent stem cells.36 Surprisingly, these studies demonstrated that PBGs express markers related to early pancreatic differentiation, such as Pdx1, and genes related to pluripotency, such as Sox2 and Oct4. Human PBG cells differentiate into hepatocytes, cholangiocytes, and pancreatic cells including β-cells in defined culture conditions and successfully engraft as hepatic and pancreatic cells in immunodeficient mice.37 LPCs in PBGs expand in culture but have not been prospectively isolated from other EHBD cells. Although these cells may be an attractive source for cell or islet transplantation to relieve diabetes, their function in liver homeostasis is unclear. By identifying specific markers for progenitors in PBGs, physiological significance of putative LPCs that reside in PBGs can be further examined by prospective isolation and genetic lineage tracing.
Committed progenitors for hepatocytes
The limited contribution of residential LPCs in cellular turnover in normal livers indicates that self-duplication of MHs rather than differentiation of LPCs would be a major mechanism supplying new hepatocytes. However, it remains unknown whether hepatocytes are a homogenous cell population in terms of the ability to produce new hepatocytes. In rats and humans, committed progenitors for hepatocytes called small hepatocytes were found and their characteristics have been reported,38,39 though it remains unknown if similar cells exist in mouse livers. We isolated relatively small hepatocytes from mouse livers between 1W and 8W and examined their clonal proliferation and differentiation using a colony assay. Although the frequency of colony formation was remarkably reduced by weaning, some colonies emerged from hepatocytes isolated from aged livers, suggesting that a portion of the hepatocytes maintain strong proliferative capability (our unpublished data) (Fig. 1D). Although more experiments are required to conclude that the cells are similar to rat and human small hepatocytes, some of the mouse hepatocytes, which possess potential to continuously supply new hepatocytes, may work in turnover of MHs in normal liver.
Stem cells in injured livers
In contrast to normal cellular turnover and recovery from acute liver failures, proliferative capability of MHs and/or cholangiocytes is inhibited or impaired in chronically injured livers because of the continuous loss of cells and fibrotic environment. In such cases, DRs associated with expansion of small progenitor-like cells are induced in the periportal region.40 These progenitor-like cells present in DRs are called “oval cells,” which are named after their ovoid nuclei,41 or “atypical hepatocytes”42 or “hepatobiliary cells,” whose names are derived from co-expression of both hepatocyte and cholangiocyte markers.43 In the most studied experimental model inducing “oval cells,” rats are administered with 2-acethylaminofluorene (2-AAF) that suppresses hepatocyte proliferation, and then 70% PHx is performed.44 Histological analysis demonstrated that expanding ductular structures contained heterogeneous cell populations and therefore, may contain LPCs and transitional cells (intermediate cells) that are on the way to differentiate into hepatocytes or cholangiocytes.45,46 Pulse-labeling with 3H-thymidine and histological analysis demonstrated that oval cells differentiated into MHs through small basophilic hepatocytes.44 We previously demonstrated that the boundary of expanding ductular structures and hepatic cord contain oval cells that express Dlk-1, a marker of hepatoblasts and possibly a marker of intermediate cells differentiating into MHs.47 In the rat 2AAF/PHx model, several reports suggest that the origin of oval cells is cholangiocytes. Petersen et al. demonstrated that pretreatment of 4,4'-Methylenedianiline (4,4'-diaminodiphenylmethane: DAPM), a cholangiocyte specific toxin, blocked the appearance of oval cells.48 In addition, the paucity of oval cells in the 2AAF/allyl alcohol protocol indicated that the canal of Hering may be more important than the rest of biliary tree because it could be predominantly affected by the loss of periportal hepatocytes caused by allyl alcohol.49
The 2AAF/PHx protocol does not induce ductular reactions associated with LPC expansion in mice. Alternatively, administration of a chemical called dipin50 and feeding mice with 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-diet51 or choline-deficient diet with ethionine supplementation (CED)52 have been used to induce DRs. Recently, LPCs in the DDC-feeding model were isolated based on expression of surface antigens, including CD133,53 EpCAM,3 and CD24.54 In these studies, isolated LPCs clonally proliferated and differentiated into hepatocytes and cholangiocytes in vitro and in vivo. Genetic lineage tracings using two different lines of Sox9-CreERT2 knock-in mice showed that the biliary tree including the canal of Hering supply “ductular cells,” which eventually differentiate to MHs.27,28 However, these results were confounded by the following reports using OPN-CreERT230 and AAV8-Cre29 models, where expanding ductular cells did not supply MHs in DDC-model. On the other hand, when bile ducts and the canal of Hering were labeled using OPN-CreERT2:ROSA-YFP mice, YFP+ cells forming ductular structures differentiated into MHs in mice fed with CDE-diet but not in chronic CCl4-injured and DDC-injured livers.30 The authors suggested a possibility that the fate of LPCs in DDC and CDE models attribute to differential association with the ECM layer; LPCs are completely surrounded by ECM layers in DDC-injured livers but not in CDE-injured ones. Boulter et al. demonstrated that hepatocytic differentiation was promoted in the CDE model by Wnt3a secreted from macrophages that engulfed debris of damaged hepatocytes, whereas macrophages could not access LPCs in DDC-injured livers that are completely surrounded by a thick ECM layer.55 Alternatively, it can be assumed that accumulation of bile juice in the DDC-model may promote cholangiocyte differentiation of LPCs, whereas severe damage on MHs in CDE-injured livers may induce their hepatocytic differentiation.
As described above, LPCs are expanded in severely damaged livers. In contrast to the idea that residential LPCs expand or produce transit-amplifying cells, it is considered that LPCs are absent in normal situations; instead, the cells that are activated and contribute to liver regeneration are called “facultative stem cells.”56 Trop2+,3 Foxl1+,57 and Lgr5+ 58 cells that do not exist in normal livers are induced in DDC-injured livers. Among these cells, Foxl1+ and Lgr5+ cells differentiate into MHs and cholangiocytes in vitro and in vivo. It should be noted that Foxl1 and Trop2 are expressed in most of the expanding ductular cells, whereas Lgr5+ cells are limited in number. These results support the idea that a portion of the cholangiocytes or the canal of Hering is induced to become “facultative” LPCs expressing Trop2, Foxl1, or Lgr5 upon chronic liver injuries.
Lineage conversion
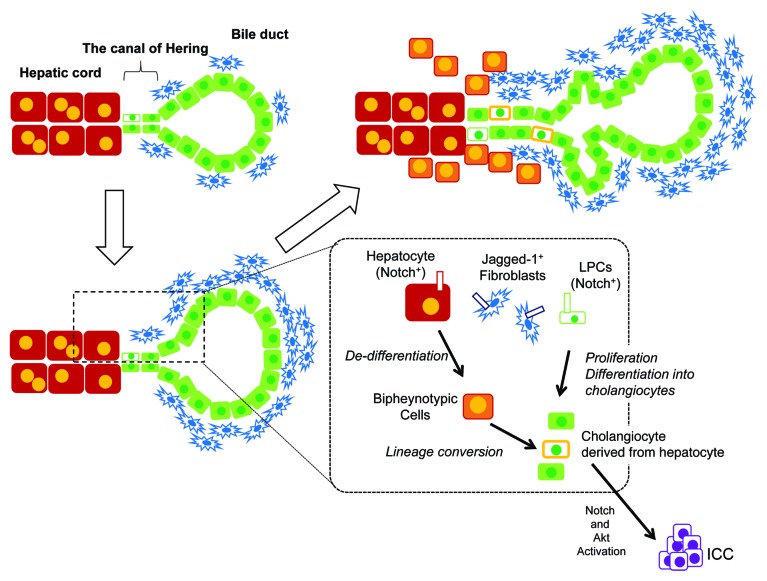
In addition to self-duplication and involvement of LPCs, the lineage conversion between MHs and cholangiocytes is the third possible way of supplying liver epithelial cells. Analyses of human cholestatic, biliary obstructive or biliary autoimmune diseases have shown that hepatocytes around the portal veins express genes that are normally specific to cholangiocytes.59 In addition, biliary transcription factors are expressed in human hepatocytes in chronic biliary diseases.60 Dipeptidyl peptidase IV (DPPIV)−-rats reconstituted with DPPIV+ hepatocytes, in which only donor-derived hepatocytes are DPPIV+, were exposed to DAPM followed by bile duct ligation (BDL). Remarkably, in this model, 45% of the new biliary ductules expressed DPPIV, indicating that they were derived from MHs.9 Nishikawa et al. showed that MHs convert to ductular cholangiocytes in collagen gel, and TNFα promoted the conversion.61 Recent works using genetic lineage tracing demonstrated that MHs convert to cholangiocytes in injured livers. Yanger et al. labeled MHs in AAV8-Cre:ROSA-YFP mice by tamoxifen injection before inducing liver injuries and found robust expression of cholangiocyte markers, such as Sox9 and OPN, in hepatocytes around the portal veins in DDC-, CDE-, and BDL-injured mice. They also found cholangiocytes derived from hepatocytes incorporated into ductular structures.10 Moreover, intrahepatic cholangiocarcinoma (ICC) was derived from MHs under simultaneous activation of Notch and Akt.62 Another group showed that thioacetamide-induced ICC originated from hepatocytes, depending on activation of the endogenous Notch-Hes1 pathway.63 Therefore, although it remains unclear if all or only part of MHs have the potential of converting into cholangiocytes, hepatocyte-to-cholangiocyte conversion is one of the intrinsic mechanisms for supplying cholangiocytes under chronic liver injury.
Nevertheless, the efficiency of the conversion needs to be elucidated. Yanger et al. provided a quantitative study demonstrating that approximately 4% of the ductular cells were derived from hepatocytes,10 whereas Malato et al. did not identify cholangiocytes derived from hepatocytes.29 We also found cholangiocyte-like cells derived from hepatocytes in Mx1-Cre:ROSA mice fed with the DDC-diet. In our experiments, approximately 2% of the CK19+ ductular cells were derived from hepatocytes (our unpublished data). These results indicate that most of the hepatocytes expressing some of the cholangiocyte markers stay in a “biphenotypic status” without completely converting to cholangiocytes. Therefore, the difference between complete and “partial” hepatocyte-to-cholangiocyte conversion needs to be assessed and is probably associated with protection of liver tissue from chronic injuries.
Yanger et al. further demonstrated that ectopic activation of the Notch signal induced hepatocyte-to-cholangiocyte conversion.10 They also provided evidence that this process was impaired in mice lacking RBPJκ, which is an essential factor of Notch signaling, suggesting that the Notch pathway is involved in hepatocyte-to-cholangiocyte lineage conversion. It is well known that the Notch signal induces hepatoblasts to differentiate into cholangiocytes in fetal livers. Given that MHs respond to Notch in a similar manner to hepatoblasts, MHs may possess great differentiation plasticity. However, it should be noted that direct interaction between ligand- and receptor-expressing cells is crucial to activate the Notch signaling pathway. Both in developing and injured livers, periportal fibroblasts are considered to be the main source of Jagged-1 (insets of Figs. 1 and 2). Therefore, the intrinsic Notch pathway may be activated in hepatocytes near the portal triads, in which MHs can convert to cholangiocytes. It should also be noted that many “biphenotypic cells” are sustained and do not completely convert into cholangiocytes, at least in DDC-injured livers (Fig. 2). Even in extended injury, numerous biphenotypic cells co-expressing Sox9 and HNF4α were observed in the area dissociating from the periportal mesenchymal layer. This raises the possibility that soluble factors are involved in this “partial lineage conversion” in addition to the Notch pathway. Interestingly, in the rat model with the combination of BDL and repeated DAPM administration, a significant number of hepatocytes converted into cholangiocytes, where expression of TGFβ was remarkably increased.64 In mice, at least in vitro, Sox9 expression increased by TGFβ,23,65 suggesting that TGFβ is a candidate to induce such a transition in chronically injured livers.
Figure 2. LPC expansion upon chronic liver injuries. When mice are fed with DDC-containing diet, ductular reaction associated with activation of LPCs is induced. In this model, residential LPCs or a portion of the cholangiocytes interact with expanding Jagged-1+ portal fibroblasts and thereby activate the Notch signaling pathway are activated in these cells. The Notch signal may induce proliferation of LPCs or their progeny and direct them to differentiate along the cholangiocyte lineage. At the same time, Jagged-1+ fibroblasts interact with hepatocytes near the portal vein and activate the Notch signal in these hepatocytes. They become “biphenotypic cells,” which express some cholangiocyte-specific markers such as Sox9 and OPN. A portion of the “biphenotypic cells” further convert into cholangiocytes, which are incorporated into expanding ductular structures. Upon further stimulation (e.g., inducing Akt activation), cholangiocytes derived from hepatocytes may turn into intrahepatic cholangiocarcinoma (ICC). However, most of the biphenotypic cells remain around the boundary between ductular structures and the parenchymal region.
Concluding Remarks
Adult residential LPCs have been enriched in cellular fractions positive for cholangiocyte markers. However, physiological roles of residential LPCs in normal liver are controversial. In contrast, LPCs supply MHs in livers suffering from continuous damage. A specific population of cholangiocyte marker+ cells that show LPC properties in vitro are likely activated and involved in supplying MHs and cholangiocytes in damaged livers. Alternatively, it is still possible that cholangiocytes, which are next to niche inducing “stemness,” turn to LPCs in injured livers. To identify which is the case, it is necessary to demonstrate heterogeneity of cholangiocyte marker+ cells within liver tissue.
LPCs are involved in tissue repair in chronically injured livers, though they supply different lineage cells depending on types of injury.30,55 They mainly supply cholangiocytes in DDC- and BDL-injured livers, whereas LPCs supply hepatocytes in CDE-injured livers. We recently found that the ratio of bipotential and cholangiocytic colonies derived from the EpCAM+ fraction was different among these three models. EpCAM+ cells derived from BDL-injured livers mostly formed cholangiocytic colonies, whereas those from CDE-livers formed a significant number of bipotential colonies (our unpublished data). Therefore, there is a possibility that each niche determines the differentiation preference of LPCs as well as their proliferative capability. If we distinctively identify signals inducing and expanding LPCs and those determining the preference of differentiation, it may be possible to isolate cholangiocyte marker+ LPCs, expand them, and then determine the direction of differentiation to acquire a sufficient number of functional hepatocytes that can be used for drug screening and cell transplantation.
Recent results demonstrating hepatocyte-to-cholangiocyte conversion shed light on the remarkable plasticity of MHs. Moreover, mechanical insights gave us crucial information; signals important for LPC activation and differentiation potentially affect the status of MHs. For example, the Notch pathway is involved in cholangiocyte differentiation of LPCs as well as hepatocyte-to-cholangiocyte conversion.10,55 Many soluble factors, including HGF, FGF7, and Wnt3a, which are involved in LPC responses,55,66,67 may be able to regulate the differentiation status of MHs. LPCs greatly contribute to expansion of ductular structures in BDL- and DDC-injured livers. However, in these livers, MHs are also damaged and need to be compensated. Because the direction of LPC differentiation is mostly limited to the cholangiocyte lineage in these injured livers, other compensatory mechanisms may work to supply new hepatocytes. It should be noted that hepatocytes with a biphenotypic status, which are characterized with an increase in expression of some cholangiocyte markers as well as a decrease in MH markers, are abundant in DDC- and BDL-injured livers. The biphenotypic cells can proliferate and efficiently re-acquire hepatocyte functions in vitro (our unpublished data). This raises the possibility that biphenotypic hepatocytes may be able to supply MHs more efficiently than LPCs. Interestingly, recent studies investigating regulatory mechanisms suggest a possibility that the Notch pathway simultaneously induces LPC expansion and hepatocyte-to-cholangiocyte conversion at least in DDC-injured livers (inset of Fig. 2).10,55 It is important to understand how different pathways of epithelial cell supply are coordinated for maintenance of liver functions and cellular homeostasis in response to many types of liver injuries. Such information is crucial to understand chronic liver diseases where the balance between LPC activation and MH plasticity may be deteriorated.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fuchs E. Finding one’s niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–60. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya A, Kakinuma S, Yamazaki Y, Nakauchi H. Enrichment and clonal culture of progenitor cells during mouse postnatal liver development in mice. Gastroenterology. 2009;137:1114–26, e1-14. doi: 10.1053/j.gastro.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 6.Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166–75. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–9. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2014;59:1617–26. doi: 10.1002/hep.26753. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–44. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–24. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 12.Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775–86. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Okabe M, Suzuki K, Kamiya Y, Tsukahara Y, Saito S, Miyajima A. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: drastic change of EpCAM expression during liver development. Mech Dev. 2009;126:665–76. doi: 10.1016/j.mod.2009.06.939. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173–84. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitou M, Sugiyama Y, Ishikawa K, Shiojiri N. Purification of fetal mouse hepatoblasts by magnetic beads coated with monoclonal anti-e-cadherin antibodies and their in vitro culture. Exp Cell Res. 2002;279:330–43. doi: 10.1006/excr.2002.5615. [DOI] [PubMed] [Google Scholar]
- 16.Okada K, Kamiya A, Ito K, Yanagida A, Ito H, Kondou H, Nishina H, Nakauchi H. Prospective isolation and characterization of bipotent progenitor cells in early mouse liver development. Stem Cells Dev. 2012;21:1124–33. doi: 10.1089/scd.2011.0229. [DOI] [PubMed] [Google Scholar]
- 17.Shiojiri N. Development and differentiation of bile ducts in the mammalian liver. Microsc Res Tech. 1997;39:328–35. doi: 10.1002/(SICI)1097-0029(19971115)39:4<328::AID-JEMT3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Lemaigre FP. Development of the biliary tract. Mech Dev. 2003;120:81–7. doi: 10.1016/S0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 19.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–74. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 20.Clotman F, Jacquemin P, Plumb-Rudewiez N, Pierreux CE, Van der Smissen P, Dietz HC, Courtoy PJ, Rousseau GG, Lemaigre FP. Control of liver cell fate decision by a gradient of TGF beta signaling modulated by Onecut transcription factors. Genes Dev. 2005;19:1849–54. doi: 10.1101/gad.340305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–16. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 23.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–33. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–8, e1-4. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanimizu N, Nakamura Y, Ichinohe N, Mizuguchi T, Hirata K, Mitaka T. Hepatic biliary epithelial cells acquire epithelial integrity but lose plasticity to differentiate into hepatocytes in vitro during development. J Cell Sci. 2013;126:5239–46. doi: 10.1242/jcs.133082. [DOI] [PubMed] [Google Scholar]
- 26.Senga K, Mostov KE, Mitaka T, Miyajima A, Tanimizu N. Grainyhead-like 2 regulates epithelial morphogenesis by establishing functional tight junctions through the organization of a molecular network among claudin3, claudin4, and Rab25. Mol Biol Cell. 2012;23:2845–55. doi: 10.1091/mbc.E12-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 28.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D, Willenbring H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121:4850–60. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–, e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–69. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–40. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 33.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, LeSage G, Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–59. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 34.Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–7. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 36.Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM, Alvaro D. The biliary tree--a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol. 2012;9:231–40. doi: 10.1038/nrgastro.2012.23. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Lanzoni G, Carpino G, Cui CB, Dominguez-Bendala J, Wauthier E, Cardinale V, Oikawa T, Pileggi A, Gerber D, et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells. 2013;31:1966–79. doi: 10.1002/stem.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitaka T, Mikami M, Sattler GL, Pitot HC, Mochizuki Y. Small cell colonies appear in the primary culture of adult rat hepatocytes in the presence of nicotinamide and epidermal growth factor. Hepatology. 1992;16:440–7. doi: 10.1002/hep.1840160224. [DOI] [PubMed] [Google Scholar]
- 39.Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111–25. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- 40.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract. 1995;191:513–24. doi: 10.1016/S0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 41.Tatematsu M, Ho RH, Kaku T, Ekem JK, Farber E. Studies on the proliferation and fate of oval cells in the liver of rats treated with 2-acetylaminofluorene and partial hepatectomy. Am J Pathol. 1984;114:418–30. [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–22. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khuu DN, Najimi M, Sokal EM. Epithelial cells with hepatobiliary phenotype: is it another stem cell candidate for healthy adult human liver? World J Gastroenterol. 2007;13:1554–60. doi: 10.3748/wjg.v13.i10.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–7. [PubMed] [Google Scholar]
- 45.Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–51. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- 46.Behari J, Yeh TH, Krauland L, Otruba W, Cieply B, Hauth B, Apte U, Wu T, Evans R, Monga SP. Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis. Am J Pathol. 2010;176:744–53. doi: 10.2353/ajpath.2010.090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanimizu N, Tsujimura T, Takahide K, Kodama T, Nakamura K, Miyajima A. Expression of Dlk/Pref-1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expr Patterns. 2004;5:209–18. doi: 10.1016/j.modgep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–8. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 50.Factor VM, Radaeva SA, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994;145:409–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–40. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- 52.Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–22. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964–78. doi: 10.1002/hep.22558. [DOI] [PubMed] [Google Scholar]
- 54.Qiu Q, Hernandez JC, Dean AM, Rao PH, Darlington GJ. CD24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011;20:2177–88. doi: 10.1089/scd.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanger K, Stanger BZ. Facultative stem cells in liver and pancreas: fact and fancy. Dev Dyn. 2011;240:521–9. doi: 10.1002/dvdy.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, et al. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–92. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. . Nature. 2013;494:247–50. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, Strain AJ. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–13. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, Yoshioka T, Enomoto K. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166:1077–88. doi: 10.1016/S0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–8. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Limaye PB, Bowen WC, Orr A, Apte UM, Michalopoulos GK. Expression of hepatocytic- and biliary-specific transcription factors in regenerating bile ducts during hepatocyte-to-biliary epithelial cell transdifferentiation. Comp Hepatol. 2010;9:9. doi: 10.1186/1476-5926-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanimizu N, Kikkawa Y, Mitaka T, Miyajima A. α1- and α5-containing laminins regulate the development of bile ducts via β1 integrin signals. J Biol Chem. 2012;287:28586–97. doi: 10.1074/jbc.M112.350488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, Takikawa Y, Miyajima A. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169–81. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–26. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]