Abstract
Liver bioengineering has been a field of intense research and popular excitement in the past decades. It experiences great interest since the introduction of whole liver acellular scaffolds generated by perfusion decellularization1–3. Nevertheless, the different strategies developed so far have failed to generate hepatic tissue in vitro bioequivalent to native liver tissue. Even notable novel strategies that rely on iPSC-derived liver progenitor cells potential to self-organize in association with endothelial cells in hepatic organoids are lacking critical components of the native tissue (e.g., bile ducts, functional vascular network, hepatic microarchitecture, etc)4. Hence, it is vital to understand the strengths and short comes of our current strategies in this quest to re-create liver organogenesis in vitro. To shed some light into these issues, this review describes the different actors that play crucial roles in liver organogenesis and highlights the steps still missing to successfully generate whole livers and hepatic organoids in vitro for multiple applications.
Keywords: liver organogenesis, liver bioengineering, organ bioengineering, hepatoblasts, bile duct morphogenesis, liver stem cell differentiation, liver decellularization
The Liver Extracellular Matrix
Although the ECM is only a minor constituent of the liver, it has an essential role by providing a structural framework to the liver cells. It also has a crucial role in facilitating cell attachment and migration, and controlling differentiation, repair and development.5 The complex structure that the ECM forms in the liver can be roughly divided in two major areas: the periportal region and the pericentral region, defined by the vascular domains of the portal triad and the central vein. From one area toward the other, there is a gradient of matrix molecules that can be divided in 3 different zones.6 This specific organization supports and preserves the metabolic zonation observed in liver hepatocytes, with hepatocytes from different zones having different sizes, enzymes and major functions.7 These matrix composition differences is mostly evident in the Space of Disse (perisinusoidal space, which is the location in the liver between a hepatocyte and a sinusoid), which has been also shown to undergo changes during liver ontogenesis.8 It lacks typical basement membrane proteins like laminin, entactin and perlecan but contains collagen IV. There is abundance of fibronectin, discontinuous deposits of collagen III and continuous network of collagen I.9 There is a also a gradient in the ECM composition in the space of Disse in the adult liver, where zone 1 (periportal region) displays fetal and neonatal ECM characteristics and zone 3 (pericentral region) displays adult characteristics.10
The stem cell niche found in Canals of Hering has three resident stem/progenitor populations: hHSCs, angioblasts and hepatic stellate cell precursors. The microenvironment of this niche is comprised of soluble paracrine signals and an extracellular matrix composed of HAs, an integrin α6β4 binding form of laminin, collagen type III and minimally sulfated CS-PGs. This niche is devoid of collagen type I or IV or HS-PGs. As the stem cells transition to hepatoblast stage and subsequently into successive lineage stages, changes are observed in the soluble paracrine signals and matrix composition. These changes in paracrine signals and matrix components dictate the stepwise differentiation of the stem cells to adult fates across the hepatic maturational gradient.11
In the adult liver (Fig. 1), the portal triad region, which includes hepatic artery, portal vein and bile ducts, has an organized basement membrane consisting of collagen IV, laminin, entactin and perlecan.8 Liver progenitor cells demonstrate distinct responses (expansion, differentiation) when grown on zone specific ECM matrices in 2D culture system. Zone 1 matrix molecules like laminin, Collagen III and IV induce clonogenic expansion of hFLPCs, while zone 3 matrix molecule collagen I induces growth arrest and differentiation, and fibronectin inhibits cell attachment.7
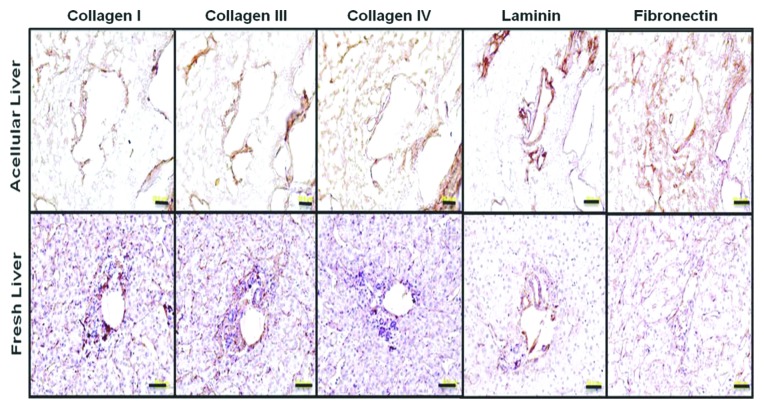
Figure 1. ECM protein localization on native human liver scaffold and native human liver. Immunostaining for collagen I, III, IV, fibronectin, and laminin (as indicated) show similar ECM component distribution in prepared acellular liver bioscaffold and native human liver sections. Scale 50μm (From Hepatology with permission from Wiley and Sons).
Liver epithelial cells interact with the surrounding ECM via integrin receptors found on their surface. These receptors, integrins, are transmembrane receptors that are responsible for diverse cell-cell contacts and cell-ECM interactions. When activated, integrins trigger signal transduction with information about the chemical composition and mechanical status of the ECM, producing transcriptional activation of different genes that regulate cell cycle, cell shape and motility.12 Hence, the integrin repertoire present in a particular cell population impacts the way these cells relate with their cellular neighbors and how they perceive and interact with their surrounding environment (cellular niche). The connection between the cell and the ECM also enables the cell to endure pulling mechanical forces without being detached of the ECM. They also play a critical role during ontogeny, where morphogenetic cellular movement and migration enable the formation of new tissues and organs.13 In this regard, hepatocytes and cholangiocytes have been shown to express markedly different combinations of integrins, which correlates with the differences in ECM composition surrounding both cell types and their specific location in the liver acinar units.14,15 Cholangiocytes express a variety of integrin receptors, including α2β1 (collagens, laminins), α3β1 (laminin 5), α5β1 (fibronectin and proteinases), α6β1 (laminins), α9β1 (VEGF-A, C, D, NGF), αVβ1 (vitronectin and fibrinogen), and α6β4 (laminins) dimers,16 while hepatocytes express only α1β1 (collagens and laminins), α5β1 (fibronectin and proteinases), and α9β1 (VEGF-A, C, D, NGF) dimers.14,17,18
The majority of signaling molecules implicated in ECM-integrin interactions, like Rho GTPases, Raf, Ras, FAK and MAPK/ERK, are ubiquitous mediators of signal transduction.19 FAK is thought to be an important mediator of downstream signaling responsible for regulation of cell growth following integrin dependent cell adhesion to ECM.20 The ECM gradient in the different compartments of the liver and changes in integrin receptor subunit expression observed during the differentiation of progenitor cells, combined with the striking differences observed in the attachment efficiencies, growth rate, morphology and differentiation of these cells on different matrix components in culture, proves that progenitor cell interaction with the ECM is vital for their maturation in the developing liver. However, the specific mechanisms and the pathways involved in this process remains to be elucidated.
Liver Development and the Stem Cell Niche
Liver development is a stepwise process that includes distinct biological events. The first step is the commitment of the foregut endoderm to the hepatic lineage. This specification process is regulated by transcription factors FoxA2 and GATA4 binding to their target sequences and facilitating the binding of C/EBPβ and nuclear factor 1 to activate transcription of albumin.21,22 These early hepatic progenitor cells (also referred to as hepatoblasts) express albumin and AFP, and have the potential to differentiate into hepatocytes and bile duct epithelial cells (cholangiocytes).23 The progenitor cells proliferate throughout the fetal development and achieve functional maturation at various stages along with differentiating into either hepatocytes or cholangiocytes. Detailed molecular mechanisms involved in the liver development have been described recently.21,22,24-26For many years, hHBs were considered to be the stem cell population of the liver, but recent studies have reported isolation of a stem cell population from fetal and post natal livers that are considered to be the precursor to the hepatoblasts. These hHSCs have a distinct antigenic profile from hepatoblasts. hHSCs express NCAM, EpCAM, SOX9, CK 8/18/19, sonic and indian hedgehog. They lack AFP, albumin, ICAM-1 and early cytochrome P450 enzymes, which are expressed in hepatoblasts. The AFP-/EpCAM+ population of hHSCs has been obtained from fetal, neonatal and adult livers of all ages. As mentioned earlier, hHSCs are considered to be the precursors to the hepatoblasts. The hepatoblasts are thought to be the transient amplifying progenitor cells, which are found throughout the parenchyma of fetal and neonatal livers. Maturation lineage studies demonstrated that hepatoblasts could become committed to either the hepatic or biliary lineages during the late developmental stages of the liver. These committed progenitors express either hepatic or biliary markers and do not express stem cell specific markers like NCAM and hedgehog proteins. The committed progenitors undergo terminal differentiation to form mature hepatocytes and cholangiocytes. This process of stepwise differentiation of hepatic stem cells is accompanied by changes in gene expression and thus leading to their lineage specification into mature phenotypes.27
The stem cell microenvironment (niche) plays an important role in regulating stem cell specification and differentiation into mature cell types. Paracrine signaling between the mesenchymal and epithelial cells and interactions with the ECM are the major components of the stem cell niche that modulates cell behavior.28 Various soluble paracrine signals released from non-epithelial cells (endothelial, mesenchymal stem cells, portal fibroblasts, hematopoietic stem cells, stellate cells) have been shown to be critical for progenitor cell maintenance and differentiation into hepatocytes and cholangiocytes.23,25,28-30 Bile ducts arise from the hepatoblasts lining the portal mesenchyme around the portal vein by undergoing a complex process of bile duct morphogenesis. The paracrine signaling gradient observed between the portal mesenchyme and the parenchymal space is implicated in regulating the biliary lineage specification of the hepatoblasts, as the hepatoblasts present in the parenchymal space do not undergo biliary differentiation.25,31,32 Two specific signaling pathways, TGFβ and Notch, have been suggested to be important for hepatoblast differentiation into cholangiocytes.33 Jagged 1, a Notch 2 ligand expressed by myofibroblasts, activates Notch 2 in the progenitor cells and induces expression of HNF1β, required for cholangiocyte differentiation, while suppressing HNF1α, HNF4α, and C/EBPα, which are required for hepatocyte differentiation.29,30 Portal mesenchyme secretes TGFβ, thus creating a gradient from periportal to parenchymal region, with highest activity observed near the periportal mesenchyme, where the biliary ducts develop.34 Several growth factors and soluble molecules like HGF, OSM and glucocorticoids have also been shown to regulate hepatoblast differentiation toward hepatocytes.21,23,25 Both stellate cells and endothelial cells have been shown to produce HGF. HGF acts via c-met receptor and stimulates expression of transcription factors C/EBPα and HNF4α, both required for hepatocyte differentiation.23,35 OSM is an interleukin 6-related cytokine produced from the developing hematopoietic cell. OSM induces hepatic differentiation by promoting HNF4α expression and this process is suppressed by TNFα.36 At the latest stages of development, hepatoblasts located in the parenchyma give rise to hepatocytes, while the hepatoblasts located near the portal mesenchyme differentiate into cholangiocytes. Such lineage specification of hepatoblasts, based on their location within the liver and the presence of gradient in ECM composition, suggests an important role for the ECM in the lineage specification.
The Liver Stem Cells and Matrix Mechanobiology
In addition to responding to biochemical signals, cell behavior is highly influenced by its mechanical environment, including the topography and stiffness of the surrounding tissue, fluid shear stress, and interstitial fluid pressure. The capability of a cell to respond to mechanical cues can lead to an array of cellular processes, including differentiation, injury response, motility and morphological changes.37-39 The ability of cells to sense and respond to the mechanical stiffness of its substrate, in the form of cell-matrix and cell-cell interactions is an important component of cellular mechanosensitivity. The material properties of tissue are determined by the chemical structure of the tissue as well as organization of those components. Collagen, fibronectin, and proteoglycans all contribute to mechanical strength and behavior and modifications of any of these components lead to alterations in the mechanical properties of the tissue.40 In most cases, the stiffness of a tissue or substrate is defined in terms of Elastic or Young’s modulus, a constant describing the materials ability to resist deformation, or the ratio of stress to strain. However, soft tissues, including the liver, are inherently more complex than the linear elastic modulus describes, displaying nonlinear elasticity (nonlinear stress strain relationship) and viscoelasticity (consisting of fluid and solid components).41 The effects of these more complicated material behaviors on cell mechanosensitivity are largely unknown.
Cell-matrix interactions occur at points known as focal adhesion complexes, which consist of integrins that connect the ECM to the actin-myosin cytoskeleton of the cell. Contraction of this actin-myosin cytoskeleton allows the cell to survey its mechanical environment through movement of the integrins, which pull on the ECM and then transmit that force back to the cytoskeleton. For example, if the ECM became stiffer, it would be more difficult for the cytoskeleton to contract, resulting in accumulation of more integrins, enlarged focal adhesions, and further development of the cytoskeleton.38 This is usually observed in soft tissue, when its ECM becomes stiffer due to chronic inflammatory processes or tissue regeneration is resolved with fibrosis.42 By contrast, soft substrates promote morphological changes characteristic of apoptosis, including cell rounding, nucleus condensation, and loss of focal adhesions.43
Integrin movement is related to downstream signaling pathways, with Rho guanosine triphosphatases (Rho GTPase) and the contraction of the actin-myosin cytoskeleton being the main mediators of this process.40 While less understood than cell-matrix interactions, cell-cell junctions are equally important in cell mechanosensitivity. Cell-cell interactions are thought to occur via cadherins that form a bridge between the cytoskeleton of two neighboring cells.44
The optimal stiffness for culturing and expanding any given cell type corresponds to the in vivo elastic modulus of its corresponding tissue. Variations in the stiffness of the tissue, a process that occurs in certain disease states, may lead to alterations in the normal behavior of a particular cell.42 In liver cirrhosis and fibrosis, liver tissue can increase by an order of magnitude,45 triggering an array of responses by various liver cells. When cultured on stiff substrates, hepatocytes begin to dedifferentiate and become proliferative, which is in stark contrast to their normally quiescent state.46-48 Portal fibroblasts differentiate into myofibroblasts when supplemented with TGF-β and cultured on a stiff substrate, an important process in the early stages of biliary fibrosis.49 HStCs, the major ECM producers in the liver, become increasingly transdifferentiated toward fibrogenic myofibroblasts as the stiffness of the underlying matrix is increased.50 Disruption of HStC integrins, through disruption of α5β1 and αvβ3 and by culturing the cells on poly-l-lysine enabling non-integrin dependant cell adhesion, promotes growth cycle arrest and maintains cells in a non-myofibroblastic state.51 Additionally, Rho GTPases, mediators of the integrin-sensing signaling pathways, are necessary for HStC transdifferentiation.52 The transdifferentiation process of HStCs to myofibroblasts is the primary mechanism of liver fibrosis and recent research suggests that the mechanosensitivity of HStCs plays a vital role in this process.
A groundbreaking paper by Engler et al.37 demonstrated that stem cell differentiation could be directed toward different lineages by altering the mechanical properties of the substrate to mimic specific tissue types. MSCs cultured on soft substrates mimicking brain differentiated toward neurogenic phenotypes, MSCs cultured on matrix mimicking muscle were myogenic, and the stiffest matrix mimicking collagenous bone produced osteogenic cells. These findings are incredibly important for the field of stem cell therapy, in directing differentiation of stem cells for clinical therapies. Following this work, Lozoya et al.53 researched a similar question pertaining to liver cells: can mimicking the physiological mechanical properties of liver tissue regulate liver stem and progenitor cells populations? As described above, the human liver contains a population of liver stem and progenitors cells that reside in a defined stem cell niche. The ECM components of the liver stem cell niche are different from the rest of the liver. Due to this altered composition of ECM components, one can infer that the mechanical properties, or stiffness, of that region may also be unlike the rest of the liver. In the work completed by Lozoya et al., hHSCs were cultured in 3D microenvironments constituted by HA hydrogels that mimicked the Canals of Hering. Six different hydrogel formulations, each with their own distinctive set of material properties, were seeded with hHSCs and various markers were used to determine the degree of differentiation of the stem cell colonies following one week of culture. The major indicator of hHSC mechanosensitivity came from analysis of the stem cell marker CDH1, also known as E-cadherin. CDH1 is a cell surface protein that establishes cell-cell adhesions, assesses the mechanical stiffness of neighboring cells, and triggers downstream signaling pathways involved in mechanosensitivity.44 The protein expression levels of CDH1 exhibited a dependence on stiffness, with the highest levels of expression occurring when the cells were cultured on the HAs hydrogel with a shear modulus of 200 Pa. CDH1 expression on the apical side of the 200 Pa HA hydrogel seeded cells demonstrates that these exposed cells may coordinate mechanical signals to adjacent cells through CDH1. This result demonstrates a stiffness-dependent behavior of the hHSCs, suggesting that culturing the cells in their preferable mechanical environment allows them to organize themselves in the same manner observed in the stem cell niche. However, there is still a lack of convincing evidence that substrate stiffness can direct differentiation of hHSCs toward mature liver cells, which would have huge implications for liver regeneration in medicine as well as understanding the involvement of hHSCs in liver disease.
In addition to responding to the mechanical properties of the substrate, liver stem and progenitor cells and human embryonic stem cells have been shown to differentiate into mature liver cells when exposed to shear stress in perfusion bioreactor cultures.54,55 In these studies, both fetal hepatoblasts and hESC were able to secrete or express higher levels of albumin and other functional markers as well as higher cytochrome P450 3A4/3A7 ratios. Viability was also higher inside of the bioreactors, showing that either by improved mass transfer (O2, nutrients, etc) or mechanical stimulation, the bioreactor presents superior conditions for liver cell survival and differentiation compared with conventional culture.54,55 Furthermore, hepatocytes cultured in a perfused Transwell device with controlled hemodynamics mimicking sinusoidal circulation exhibited polarized morphology, retention of differentiation markers (E-cadherin and HNF4α), the canalicular transporter [multidrug-resistant protein-2 (Mrp-2)], and significantly higher levels of liver function compared with non-flow cultures over a 2-wk period.56 Hepatocytes have also been shown to respond to a third type of mechanical force, parenchymal (interstitial) fluid pressure.57 In this regard, Hsu et al. have shown that when using a liver-assist device with a vascular network that supports a hepatic parenchymal compartment through a nanoporous membrane, the survival of seeded liver cells was highly dependent on parenchymal chamber pressures (with the lowest generated parenchymal pressure supporting excellent cell survival and function).57
Overall, the mechanosensitivity of liver cells has important implications for regeneration in both tissue engineering and disease. In regenerative medicine, the mechanical as well as biochemical environment must be properly tuned to direct stem and progenitor cell differentiation as well as maintaining differentiated cells. Despite the multiple studies mentioned above, the importance of mechanical signals in regeneration processes are just beginning to be realized and much research is yet to be done.
Native Liver ECM as a Scaffold for Liver Bioengineering
With the development of organ decellularization and whole liver scaffold generation, one other potential application emerged, the use of liver matrices for the bioengineering of human livers (Fig. 2). This was reported for the first time in June 200558 and since then perfusion decellularization as been applied to heart, lungs, pancreas, intestine and kidney generating decellularized organ scaffolds for organ bioengineering.1,2,59-62 These bioscaffolds preserve their tissue microarchitecture and an intact vascular network that can be readily used as a route for recellularization by perfusion of different cell populations with defined culture media. This organ engineering approach has several advantages over the injection of cell suspensions into solid organs. The matrices provide sufficient volume for the transplantation of an adequate cell mass up to whole-organ equivalents,45 without oxygen and nutrient limitations, since continuous perfusion of oxygenated culture media is provided.

Figure 2. Pig liver scaffold preparation. Porcine livers can also be harvested and efficiently decellularized with an identical technique as in human cadaveric donor retrieval, using cold perfusion with preserving solution via portal vein and hepatic artery. After back-table preparation, these organs were connected to a pressure controlled perfusion system, continuously infusing a decellularization solution for 24 h based on SDS. This particular system included a remote controlled pump with a pressure sensor all connected to a computer hosting the controlling software (Velasco et al. unpublished data).
Using the organ scaffold technology, several laboratories have recently bioengineered livers using human or animal cells.2,3,62-65 These bioengineered livers exhibit some of the functions of a native liver (albumin and urea secretion, drug metabolism, etc) and an endothelialized vascular network critical for blood vessel patency after transplantation. However, to date, no bioengineered liver has been able to sustain blood flow after transplantation for more than a few hours, due to undesired blood clotting. There are multiple potential reasons for these failures, but poor cellular re-vascularization of the scaffold’s vascular network is probably the most relevant one, with collagens fibers from the vascular basement membrane exposed to blood flow and activating the clotting cascade. The chemical and mechanical changes that occur once blood starts perfusing the bioengineered liver is also worth noting, because blood behaves as a non-Newtonian fluid, while most culture media behaves like water, a Newtonian fluid. This change in the fluid mechanical behavior might also have an impact in the scaffold’s vessel patency post-transplantation. Finally, despite the causes that lead to blood clotting upon transplantation, there is an urgent need to increase the efficiency of organ scaffold’s re-vascularization, to avoid blood clotting after in vivo anastomosis and make transplantation of bioengineered organs possible.
Hence, it is important to describe the methods that have been employed to date and analyze them at the light of the knowledge presented here on liver organogenesis, ECM and mechanobiology. Optimal conditions still need to be elucidated and may vary depending on the organ and cells.
Type of cells
To build a bioengineered liver it is necessary to re-construct the organ’s parenchyma, vasculature and underlying support/connective tissue structures. This is obviously a daunting task, highlighted by the limitations of the use of just a few hepatic cell populations, the norm in all of the re-cellularization protocols published so far. Furthermore, autologous vs. allogeneic cells and progenitor cell sources vs. adult cell populations is still a debate that lingers and that will impact the success of any liver bioengineering strategy.
Autologous cells limit the risk of transmissible agents, are less likely to be rejected and thus decrease or eliminate the need for harsh immunosuppressive drugs; however, they cannot be easily harvested or the numbers are often insufficient. Allogeneic cells can be isolated in larger quantities and from optimal patients and can be expanded and characterized in advance before their use. Nevertheless, its disadvantages are the increased risk of agent transmission and potential adverse immune reactions.66
Stem/progenitor cells (hESC, iPSC, fetal liver cells, adult-derived stem cells) can proliferate to large number of cells and differentiate to multiple cell lineages. However, hESC and iPSC still present some safety risks and an almost absolute absence of clinical research. iPSC, generated by the reprogramming of skin fibroblasts or other patient’s cells with a cocktail of pluripotency transcription factors, can be generated from an autologous source, minimizing the immunological issues.66 Moreover, hESC and iPSC have already been successfully differentiated into functional hepatocytes in vitro and effectively transplanted into animal models of liver disease.67-70 Fetal cells have less safety risks, but while retaining their proliferative capacity, they are committed to a particular organ/tissue fate.71 Adult-derived stem cells are even more restricted in their proliferation and differentiation; adult mature cells are already differentiated and have very limited proliferation ability.72 Regardless of the cell types used, the available technology to generate billions of cells in vitro (large scale suspension cultures66) in order to re-cellularize a human size liver scaffold is still very limited and confined to only two cellular types: hESC and iPSC. Hence, major advances are still required in the cell expansion enabling technologies and biology to definitively scale-up the capability of bioengineering human size livers.
Furthermore, except for one author,2 most authors used adult cells to repopulate decellularized livers.3,63,64 Although the repopulated cells may be functional in both cases, to obtain a fully functional organ all different cell types (Kupffer cells, stellate cells, pericytes, etc) need to be present in the bioengineered liver. The critical roles that these cells play in hepatic tissue biology and physiology make them, in our opinion, indispensible in order to achieve full function and more importantly, hepatic cell survival and successful tissue organogenesis in vitro.
Seeding methods
As mentioned before, cell behavior is highly influenced by its mechanical environment including the topography and stiffness of the surrounding tissue, fluid shear stress and interstitial fluid pressure. Therefore, how and where cells are infused seems to be of crucial importance. Three different cell-seeding methods have been reported: direct parenchymal injection, multistep infusion and continuous perfusion. During the direct parenchymal injection it is easy to control the seeding site and to avoid parenchymal cells in large vascular spaces. However, multiple injections are necessary and high cell density at injection sites may lead to aggregate formation and necrotic cores. Cell seeding by continuous perfusion is quite efficient at delivering large quantities of cells into the whole scaffold, but prolonged perfusion times may potentially damage them due to continuous exposure to elevated shear during the seeding. Shear stress resilient stem/progenitor cells are the cells of choice to be used with this method.2 Adult cells seeded by multistep cell infusion followed by continuous perfusion of media has resulted in a superior cell engraftment, greater ability to produce albumin, similar amount of albumin immunostaining to normal livers, inducible hepatic CYP1A1/1A-mediated EROD activity and superior ammonia clearance.63
To date, most authors have used venous access due to the larger size compared with arteries, and most studies used portal vein instead of vena cava, owing to the physiological anterograde flow. However, there are no studies comparing venous vs. arterial access. Nevertheless, regarding anterograde vs. retrograde perfusion flow, Baptista et al. have showed that the direction of flow has a direct impact on the areas of the acinar units that are primarily reached by the perfused cells.2
Cell number and concentration
In studies of hepatocyte transplantation in animal model of hepatic failure clinical improvement has been seen using less than 10% of the host’s liver mass. Assuming that the minimum cell mass necessary to support a patient with acute liver failure is approximately 5–10% of the total liver weight sets the requirement to approximately 10 billion hepatocytes for humans and approximately 50–100 million for the rat model. Most studies used ~50 million hepatocytes to recellularize rat liver scaffolds.2,3,63 Immunostaining of the recellularized scaffolds was similar to normal livers. In decellularized pig livers, 109 hepatocytes were infused.64
Another important variable that may critically influence the recellularization process is cell concentration. High cell concentrations of cells could lead to extensive cell death or occlusion of vessels. However, low cell density can be also deleterious to tissue formation and cell viability, since hepatocytes and their progenitors are unable to properly survive and function at low cellular densities.73 Hence, we believe that further studies are needed to determine the optimal cell density to seed in these whole organ scaffold bioreactors.
Bioreactor culture systems and pump flow rates/pressures
In terms of cell culture, the seeded scaffold may be considered as a complex cell culture system due to its architectural complexity and larger size. Due to its thickness and density, simple diffusion of oxygen and nutrients may be insufficient to maintain large numbers of cells alive. Thus, a continuous perfusion system is needed to supply oxygen and nutrients to cells located in all different areas of the scaffold. The continuous perfusion of cell culture media through the vascular network is achieved with a perfusion bioreactor. The goal is to create the most physiological environment in which cells can reach an optimal expansion, maturation and engraftment. Hence, in the liver, the perfect physiologic conditions would be the ones that mimic the hepatic dual circulatory system: the portal vein and the hepatic artery. Pressure in the portal vein is low (4–10mmHg), with a low pO2 (30–40mmHg) and provides around 75% of the total blood supply. Large amounts of substances and nutrients absorbed in the intestine reach the liver through the portal system. On the contrary, pressure in the hepatic artery is high (around 120mmHg), with a high pO2 (90–100mmHg) but provides only 25% of the total blood supply. Thus, very complex bioreactor systems with 2 independent circuits will be necessary to accurately replicate the in vivo mechanical and physiological environment, but unfortunately, this has not been developed to date. Further studies will be needed to completely address this topic.
At the core of any bioreactor perfusion system is a mechanical pump to drive the media through the graft placed in an appropriate vessel. In this point, the type of flow produced should mimic the physiologic flow found in the vascular structure being perfused. Hence, in the case of the liver continuous flow should be used through the portal vein, while pulsatile flow is required in the hepatic artery to keep physiologic conditions. These different types of flow can be generated by different kinds of pump (e.g., peristaltic, gear, propeller, diaphragm, etc) and in some cases, interconverted (e.g., the use of pulse dampeners converts pulsatile flow in continuous flow). Nevertheless, it seems reasonable that near physiological flow types, rates and pressures should be used in the bioreactors to generate the most conducive mechanical environment, closely emulating physiologic blood flow. However, parenchymal cells are typically protected from shear stress by the endothelium66; thus, in the absence of this protective layer, flow below in vivo values may be necessary. Moreover, flow within a decellularized organ may easily become turbulent and even with low flow rates some cells may be subjected to intolerable shear stress. For hepatocytes, a shear stress of 0.23 dynes/cm2 has been noted to correspond to high viability. In bioartificial livers (viable hepatocytes housed within man-made synthetic devices), fluid flow, shear stress and its distribution may be relatively easy to study and control with computational fluid dynamics.74,75 However, conditions in a natural scaffold are not that controllable. To date, there are no studies elucidating the best perfusion and pressures rates in recellularized grafts. If significant cell death is observed early during culture, shear is a very likely cause. In most studies, sub-physiological flow rates have been used to perfuse the graft (0.5ml/min2, 2ml/min63 and 15ml/min3) (Table 1). Some authors used higher flow rates when seeding cells and then changed to lower rates during the perfusion of culture media (3ml/min to 0.5ml/min).2 Regardless of the flow rates used further studies are necessary to determine the best parameters of fluid pressure to enhance liver organogenesis and tissue maturation in vitro.
Table 1. Studies in the literature.
| Author | Type Cells | Infusion Method | Via | Number Cells | Flow Rate | Time |
|---|---|---|---|---|---|---|
| Baptista 2010 |
hUVEC hFLC MS1 |
Continuous | PV IVC, PV, IVC+PV |
30x106 70 x106 100 x106 |
3ml/min → 0.5ml/min 5ml/min |
7d 3d |
| Uygun 2010 |
Rat MH Endothelial cells |
Multistep | PV | 200 x106 | 15ml/min | 5d 5d |
| Soto 2011 |
Rat MH | Direct injection Continuous Multistep |
PV | 10–50 x106 | 2ml/min | 7d |
| Yagi 2013 |
Pig MH | Multistep | PV | 100 x106 | 4ml/min | 7d |
Methods adopted by several authors in liver bioengineering. From endothelial or human fetal liver cells to mature hepatocytes, several authors have been able to re-cellularize with success liver acellular scaffolds using different methods. Abbreviations: hUVEC, human umbilical vein endothelial cell; PV, portal vein; hFLC, human fetal liver cell; IVC, inferior vena cava; MH, mature hepatocyte.
Culture media perfusion and oxygen control
The perfusion media used in engineered whole livers is based on the culture media used in cell culture of the constituent cell types. During seeding, the most common media used are based on William’s E or EMEM (Eagle’s minimal essential medium) with varying percentages of fetal bovine serum (FBS) to enhance and facilitate cell attachment.3,63,64 Many other elements are added to the basic medium in order to improve cell behavior and induce growth (insulin, dexamethasone, growth factors, etc). Once seeding is complete, FBS is usually removed and chemically defined serum free media is used for better control of the constituents and of cell function and behavior. However, the presence of multiple cell types with particular requirements implies further development of a “universal culture medium,” indeed similar in many ways to an “artificial blood” that runs through the bioreactor. Table 2 summarizes some of the different culture media developed to date for the recellularization of acellular scaffolds.
Table 2. – Culture media used by different authors.
| Author | Media |
|---|---|
| Baptista 20101 |
RPMI 1640, FBS, dexamethasone, penicillin-streptomycin, prolactin, glucagon, niacinamide, lipoic acid, triiodothyronine, hEGF, hHDL, hHGF, hGH, insulin, transferrin |
| Uygun 20103 |
William’s E, FBS, insulin, EGF, glucagon, hydrocortisone, penicillin-streptomycin |
| Soto 201163 |
EMEM, EGF, HGF, dexamethasone, insulin, human transferring, selenous acid supplement, penicillin-streptomycin |
| Yagi 201363 |
DMEM, EGF, hidrocortisone, insulin, glucagon, penicillin-streptomycin |
Different combinations of culture media, drugs, growth factors and hormones are used to perfuse recellularized scaffols.
The oxygenation of the culture media by an oxygenator connected to an atmospheric gas mixture with 95% O2 and 5% CO2 was used by some authors, achieving partial oxygen tension of approximately 300mmHg3. However, most of the published works lack an automated control of the gases present in the culture media or any oxygen carrier, something deemed essential for cell survival and long-term cellular function.
Duration of bioreactor pre-conditioning
Finally, one of the last main points that impacts liver bioengineering is the duration of bioreactor pre-conditioning. If the main goal is to achieve a complete recellularization of the seeded scaffold, several weeks may be necessary to promote efficient cell engraftment and rearrangement (endothelization of decellularized blood vessels has been shown to take 2 wk in vitro).76 However, if stem cells are used, more than one month may be needed to completely differentiate them into mature cells.
During the recellularization process in the bioreactor, it is also possible to determine multiple parameters of organ function and its suitability for transplant by the measurement of several cellular products and metabolites, such as urea and albumin production, or drug metabolism, since the use of histological analyses might potentially damage the bioengineered organ. There are also several groups using perfusion systems in marginal organs that allow a continuous evaluation of organ function.77 These systems are very similar to perfusion bioreators, and the conclusions obtained by their experience might be translated to bioengineered organs to determine their readiness for transplantation. These perfusion machines also allow the quantification of viability markers.75 The most used are the traditional enzymatic markers of hepatic damage such as aspartate transaminase (AST) and alanine transaminase (ALT), but there are also some less known markers, such as liver fatty acid-binding protein (L-FABP), glutamate dehydrogenase (GLDH), α-glutathione-S-transferase (α-GST), HA, CK18 and β-galactosidase that can provide additional information on graft viability.
In most studies, seeded scaffolds were kept in the bioreactors constantly perfused with culture media from 5 to 7 d.2,63 Primary hepatocytes are known to require between 7 to 10 d in culture before their metabolic activity and gene transcription levels stabilize.78 During this period of time, mature hepatocytes seeded into the scaffold showed metabolic activity with urea and albumin production2,3 and ammonia clearance,2,63 drug metabolism3 such as inducible hepatic CYP1A1/1A-mediated EROD activity.63 Regarding cell localization, after 4h of perfusion hepatocytes were placed in and around vessels and after 1 or 2 d throughout the matrix.3 When human fetal liver cells were used, hepatocytic and biliary lineages were shown by immunofluorescence staining after 7 d2. However, it is important to highlight that the reported hepatic function in these previous studies is still much lower than the physiologic requirements necessary for a liver post-transplantation. Hence, major functional improvements are still necessary to make bioengineered livers a viable alternative source for liver transplantation.
Future perspectives and clinical applicability
It is not easy to predict how clinical translation of liver bioengineering will be established. Nevertheless, the main goal of this technology remains as its guiding wire, to provide new livers into the “donor” pool that can be used in real transplants to substitute non-functioning livers of patients on the transplant waiting list. To achieve this goal several challenges need to be met. As mentioned before, the origin of the organs for decellularization, their sterilization, cell source, bioreactor technologies, etc are all important components of this huge challenge.
The immunogenicity of decellularized native scaffolds is a major issue that needs to be adequately assessed for clinical translation. The acellular matrix conserves the protein structure, and the decellularization can leave residual antigen epitopes that can activate an immunologic response from the host patient. All those residual antigens must be object of further studies to determine which defense mechanisms might be activated by the scaffold.79,80 Currently, animal organs seem to be an extraordinary source of human size scaffolds, but their use also implies some potential issues. Example of this are the species-specific antigens that need to be accurately identified and must be removed. Decellularized porcine heart valves have demonstrated the potential to initiate macrophage and lymphocyte activation and immunoglobulin deposition, depending on the methods used in their preparation.81 In addition, the possibility of transmission of zoonosis has to be absolutely excluded.
The first generation of structured organoids, though not fit for transplantation, provides a unique opportunity to study some very important issues such as the organogenesis reminiscent of in vivo tissue development, to carry on assays for drug screening, and to recapitulate genetic diseases for pathobiological study. All of these lead to a concept similar of human on-a-chip or organ on-a-chip, but at a larger scale.82 The introduction of mini-organoids in bioreactors is also an interesting field of investigation of in vitro reactions of living tissues. Nevertheless, the final goal of this area of research is the generation of human scale bioengineered livers suitable for transplantation into patients in need, representing a truly novel source of organs for transplantation that directly addresses the present scarcity of these.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Baptista PM, Orlando G, Mirmalek-Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6526–9. doi: 10.1109/IEMBS.2009.5333145. [DOI] [PubMed] [Google Scholar]
- 2.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 3.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010 doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–10. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 6.Susick R, Moss N, Kubota H, Lecluyse E, Hamilton G, Luntz T, Ludlow J, Fair J, Gerber D, Bergstrand K, et al. Hepatic Progenitors and Strategies for Liver Cell Therapies. Ann N Y Acad Sci. 2001;944:398–419. doi: 10.1111/j.1749-6632.2001.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 7.McClelland R, Wauthier E, Uronis J, Reid L. Gradients in the liver's extracellular matrix chemistry from periportal to pericentral zones: influence on human hepatic progenitors. Tissue Eng Part A. 2008;14:59–70. doi: 10.1089/ten.a.2007.0058. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Arch A Pathol Anat Histopathol. 1993;423:77–84. doi: 10.1007/BF01606580. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1–11. doi: 10.1007/BF01606425. [DOI] [PubMed] [Google Scholar]
- 10.Reid LM, Fiorino AS, Sigal SH, Brill S, Holst PA. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992;15:1198–203. doi: 10.1002/hep.1840150635. [DOI] [PubMed] [Google Scholar]
- 11.Lozoya OA, Wauthier E, Turner RA, Barbier C, Prestwich GD, Guilak F, Superfine R, Lubkin SR, Reid LM. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials. 2011;32:7389–402. doi: 10.1016/j.biomaterials.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volpes R, van den Oord JJ, Desmet VJ. Integrins as differential cell lineage markers of primary liver tumors. Am J Pathol. 1993;142:1483–92. [PMC free article] [PubMed] [Google Scholar]
- 15.Couvelard A, Bringuier AF, Dauge MC, Nejjari M, Darai E, Benifla JL, Feldmann G, Henin D, Scoazec JY. Expression of integrins during liver organogenesis in humans. Hepatology. 1998;27:839–47. doi: 10.1002/hep.510270328. [DOI] [PubMed] [Google Scholar]
- 16.Volpes R, van den Oord JJ, Desmet VJ. Distribution of the VLA family of integrins in normal and pathological human liver tissue. Gastroenterology. 1991;101:200–6. doi: 10.1016/0016-5085(91)90478-4. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SK, Vlahakis NE. Integrin alpha9beta1: Unique signaling pathways reveal diverse biological roles. Cell Adhes Migr. 2010;4:194–8. doi: 10.4161/cam.4.2.10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–52. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–8. doi: 10.1042/0264-6021:3390481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaigre FP. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Tanimizu N, Miyajima A. Molecular mechanism of liver development and regeneration. Int Rev Cytol. 2007;259:1–48. doi: 10.1016/S0074-7696(06)59001-1. [DOI] [PubMed] [Google Scholar]
- 23.Kinoshita T, Miyajima A. Cytokine regulation of liver development. Biochim Biophys Acta. 2002;1592:303–12. doi: 10.1016/S0167-4889(02)00323-3. [DOI] [PubMed] [Google Scholar]
- 24.Zorn AM. Liver development. StemBook. 2008 doi: 10.3824/stembook.1.25.1. [DOI] [Google Scholar]
- 25.McLin VA, Zorn AM. Molecular control of liver development. Clin Liver Dis. 2006;10:1–25. doi: 10.1016/j.cld.2005.10.002. [v.] [DOI] [PubMed] [Google Scholar]
- 26.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. •••;18:175–89. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. •••;53:1035–45. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Yao HL, Cui CB, Wauthier E, Barbier C, Costello MJ, Moss N, Yamauchi M, Sricholpech M, Gerber D, et al. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology. •••;52:1443–54. doi: 10.1002/hep.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. •••;18:572–9. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci. 2004;117:3165–74. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 31.Lemaigre FP. Development of the biliary tract. Mech Dev. 2003;120:81–7. doi: 10.1016/S0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 32.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka M, Itoh T, Tanimizu N, Miyajima A. Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem. •••;149:231–9. doi: 10.1093/jb/mvr001. [DOI] [PubMed] [Google Scholar]
- 34.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–33. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–3. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 36.Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T, et al. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–36. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;•••:126. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;•••:124. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;•••:310. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 40.Wells R. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 41.Suh J, DiSilvestro M. Biphasic poroviscoelastic behavior of hydrated biological soft tissue. Journal of Applied Mechanics-Transactions of the Asme. 1999;66:528–35. doi: 10.1115/1.2791079. [DOI] [Google Scholar]
- 42.Lee DA, Knight MM, Campbell JJ, Bader DL. Stem cell mechanobiology. J Cell Biochem. 2011;112:1–9. doi: 10.1002/jcb.22758. [DOI] [PubMed] [Google Scholar]
- 43.Kocgozlu L, Lavalle P, Koenig G, Senger B, Haikel Y, Schaaf P, Voegel JC, Tenenbaum H, Vautier D. Selective and uncoupled role of substrate elasticity in the regulation of replication and transcription in epithelial cells. J Cell Sci. 2010;123:29–39. doi: 10.1242/jcs.053520. [DOI] [PubMed] [Google Scholar]
- 44.Smutny M, Yap AS. Neighborly relations: cadherins and mechanotransduction. J Cell Biol. 2010;•••:189. doi: 10.1083/jcb.201005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georges PC, Hui J-J, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;•••:293. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 46.Hansen LK, Wilhelm J, Fassett JT. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006;72:72. doi: 10.1016/S0070-2153(05)72004-4. [DOI] [PubMed] [Google Scholar]
- 47.Fassett J, Tobolt D, Hansen LK. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006;•••:17. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semler EJ, Ranucci CS, Moghe PV. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol Bioeng. 2000;•••:69. doi: 10.1002/1097-0290(20000820)69:4<359::aid-bit2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;•••:46. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 50.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;•••:39. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 51.Iwamoto H, Sakai H, Nawata H. Inhibition of integrin signaling with Arg-Gly-Asp motifs in rat hepatic stellate cells. J Hepatol. 1998;•••:29. doi: 10.1016/s0168-8278(98)80256-0. [DOI] [PubMed] [Google Scholar]
- 52.Yee HF. Rho directs activation-associated changes in rat hepatic stellate cell morphology via regulation of the actin cytoskeleton. Hepatology. 1998;•••:28. doi: 10.1002/hep.510280336. [DOI] [PubMed] [Google Scholar]
- 53.Lozoya O, Wauthier E, Turner R, Barbier C, Prestwich G, Guilak F, Superfine R, Lubkin S, Reid L. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials. 2011;32:7389–402. doi: 10.1016/j.biomaterials.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmelzer E, Triolo F, Turner ME, Thompson RL, Zeilinger K, Reid LM, Gridelli B, Gerlach JC. Three-Dimensional Perfusion Bioreactor Culture Supports Differentiation of Human Fetal Liver Cells. Tissue Eng Part A. 2010;•••:16. doi: 10.1089/ten.TEA.2009.0569. [DOI] [PubMed] [Google Scholar]
- 55.Miki T, Ring A, Gerlach J. Hepatic Differentiation of Human Embryonic Stem Cells Is Promoted by Three-Dimensional Dynamic Perfusion Culture Conditions. Tissue Eng Part C Methods. 2011;•••:17. doi: 10.1089/ten.TEC.2010.0437. [DOI] [PubMed] [Google Scholar]
- 56.Dash A, Simmers MB, Deering TG, Berry DJ, Feaver RE, Hastings NE, Pruett TL, LeCluyse EL, Blackman BR, Wamhoff BR. Hemodynamic flow improves rat hepatocyte morphology, function, and metabolic activity in vitro. Am J Physiol Cell Physiol. 2013;304:C1053–63. doi: 10.1152/ajpcell.00331.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu WM, Carraro A, Kulig KM, Miller ML, Kaazempur-Mofrad M, Weinberg E, Entabi F, Albadawi H, Watkins MT, Borenstein JT, et al. Liver-assist device with a microfluidics-based vascular bed in an animal model. Ann Surg. 2010;252:351–7. doi: 10.1097/SLA.0b013e3181e982ba. [DOI] [PubMed] [Google Scholar]
- 58.Baptista PMSM, Atala A, Soker S. A Novel Whole Organ Bioscaffold System for Tissue Engineering and Regenerative Medicine Applications. 3rd International Society for Stem Cell Research International Meeting. San Francisco, CA, USA, 2005. [Google Scholar]
- 59.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 60.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 61.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis. 2010;6:134–6. doi: 10.4161/org.6.2.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17:677–86. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, Itano O, Kawachi S, Tanabe M, Coudriet GM, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22:231–42. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res. 2012;173:e11–25. doi: 10.1016/j.jss.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 66.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 68.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24:1412–9. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 69.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–42. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51:1810–9. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782:61–74. doi: 10.1016/j.bbadis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253–60. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 73.Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003;9:757–66. doi: 10.1089/107632703768247430. [DOI] [PubMed] [Google Scholar]
- 74.Consolo F, Fiore GB, Truscello S, Caronna M, Morbiducci U, Montevecchi FM, Redaelli A. A computational model for the optimization of transport phenomena in a rotating hollow-fiber bioreactor for artificial liver. Tissue Eng Part C Methods. 2009;15:41–55. doi: 10.1089/ten.tec.2008.0213. [DOI] [PubMed] [Google Scholar]
- 75.Mareels G, Poyck PP, Eloot S, Chamuleau RA, Verdonck PR. Three-dimensional numerical modeling and computational fluid dynamics simulations to analyze and improve oxygen availability in the AMC bioartificial liver. Ann Biomed Eng. 2006;34:1729–44. doi: 10.1007/s10439-006-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deutsch M, Meinhart J, Vesely M, Fischlein T, Groscurth P, von Oppell U, Zilla P. In vitro endothelialization of expanded polytetrafluoroethylene grafts: a clinical case report after 41 months of implantation. J Vasc Surg. 1997;25:757–63. doi: 10.1016/S0741-5214(97)70307-0. [DOI] [PubMed] [Google Scholar]
- 77.Balfoussia D, Yerrakalva D, Hamaoui K, Papalois V. Advances in machine perfusion graft viability assessment in kidney, liver, pancreas, lung, and heart transplant. Experimental and clinical transplantation. 2012;10:87–100. doi: 10.6002/ect.2011.0167. [DOI] [PubMed] [Google Scholar]
- 78.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–6. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 79.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raeder RH, Badylak SF, Sheehan C, Kallakury B, Metzger DW. Natural anti-galactose alpha1,3 galactose antibodies delay, but do not prevent the acceptance of extracellular matrix xenografts. Transpl Immunol. 2002;10:15–24. doi: 10.1016/S0966-3274(01)00044-2. [DOI] [PubMed] [Google Scholar]
- 81.Bayrak A, Tyralla M, Ladhoff J, Schleicher M, Stock UA, Volk HD, Seifert M. Human immune responses to porcine xenogeneic matrices and their extracellular matrix constituents in vitro. Biomaterials. 2010;31:3793–803. doi: 10.1016/j.biomaterials.2010.01.120. [DOI] [PubMed] [Google Scholar]
- 82.Luni C, Serena E, Elvassore N. Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol. 2014;25:45–50. doi: 10.1016/j.copbio.2013.08.015. [DOI] [PubMed] [Google Scholar]