Abstract
Previous meta-analyses have shown that the anti-diabetic agent metformin is associated with reduced cancer incidence and mortality. However, this effect has not been consistently demonstrated in animal models and recent epidemiological studies. We performed a meta-analysis with a focus on confounders and biases, including BMI, study type, and time related biases. We identified 71 articles published between January 1, 1966 to May 31, 2013 through Pubmed, ISI Web of Science (Science Citation Index Expanded), Embase, and the Cochrane library that were related to metformin and cancer incidence or mortality. Study characteristics and outcomes were abstracted for each study that met inclusion criteria. We included estimates from 47 independent studies and 65,540 cancer cases in diabetic patients. Overall cancer incidence was reduced by 31% (SRR=0.69, 95%CI: 0.52–0.90), although between-study heterogeneity was considerable (I2=88%). Cancer mortality was reduced by 34% (SRR=0.66, 95%CI: 0.54–0.81; I2=21%). BMI-adjusted studies and studies without time-related biases also showed significant reduction in cancer incidence (SRR=0.82, 95%CI: 0.70–0.96 with I2=76% and SRR=0.90; 95%CI: 0.89–0.91 with I2=56%, respectively), albeit with lesser magnitude (18% and 10% reduction, respectively). However, studies of cancer mortality and individual organ sites did not consistently show significant reductions across all types of analyses.
Although these associations may not be causal, our results show that metformin may reduce cancer incidence and mortality in diabetic patients. However the reduction seems to be of modest magnitude and not affecting all populations equally. Clinical trials are needed to determine if these observations apply to non-diabetic populations and to specific organ sites.
Keywords: cancer prevention, metformin, meta-analysis, Body Mass Index (BMI), time-related biases
Introduction
The recognition that hyper-insulinemic states such as metabolic syndrome or type II diabetes mellitus are associated with increased cancer risk has led to intensified interest in the potential of various anti-diabetic drugs to prevent cancer (1). Metformin, an oral, well tolerated biguanide that is used for first-line treatment of diabetes, has been shown to decrease the progression from prediabetes to overt diabetes (2–4). Its multiple actions at both cellular and organismal levels that contribute to anti-cancer effects include decreased insulin/insulin-like growth factor-1 (IGF-1) signaling, inhibition of the mammalian target of rapamycin (mTOR), inhibition of mitochondrial complex I in the electron transport chain, activation of AMP-activated kinase (AMPK), and reduction of endogeneous production of reactive oxygen species (ROS) and associated DNA damage (5).
The evidence for a cancer preventive effect for metformin, however, has not been consistently demonstrated in animal or human studies. Multiple studies examining the effect of metformin on tumor formation in rodent carcinogenesis models have shown results ranging from no effect to strong inhibition, albeit using doses that are not always achievable in humans (6–10). Epidemiologic studies comparing the incidence of cancer in diabetics using metformin with those using insulin or other anti-diabetic agents have shown somewhat variable results (11–15). Several authors performed meta-analyses to determine if a consistent effect on overall cancer incidence, cancer mortality or cancer incidence at specific target organs was evident (11–15). A shortcoming of these previous meta-analyses was the inability to identify subgroups that might benefit most (or suffer harm) from metformin. For instance, a recent clinical trial showed that metformin affected breast cancer proliferation differentially according to insulin resistance status and body mass index (BMI), with a trend toward inhibiting proliferation only in women with insulin resistance or high BMI (16). Furthermore, a number of the published studies suffered from time-related biases resulting in inappropriate comparison between metformin users versus non-users and potentially exaggerated metformin’s protective effects (17). Time-related bias in observational studies can produce illusory results in favor of metformin. They are most often a form of differential misclassification bias that can generally be avoided by appropriate accounting of follow-up time and exposure status in the design and analysis of such studies. There are different types of time-related biases. Immortal time bias refers to a period of cohort follow-up time during which a cancer event (that determines end of follow-up) cannot occur. Immortal time bias, for example, can arise when the period between cohort entry and date of first exposure to metformin, during which cancer has not occurred, is either misclassified or simply excluded and not accounted for in the analysis. This is frequently found in studies that compare ‘users’ against ‘non-users’. The use of a time-dependent approach takes into account this source of bias. In cohort studies where a first-line therapy with metformin is compared with second- or third-line therapies, patients are unlikely to be at the same stage of diabetes, which can induce confounding of the association with an outcome (e.g., cancer incidence) by disease duration. An outcome related to the first-line therapy may also be attributed to the second-line therapy if it occurs after a long period of exposure. Such a situation requires matching on disease duration and consideration of latency time windows in the analysis (17).
Therefore, we performed a systematic review and meta-analysis with emphasis on studies controlling for BMI, prospective studies, and studies without time-related biases.
Materials and Methods
Literature search
The aim of the study was to evaluate the association between metformin use and cancer incidence and mortality in diabetic patients. The meta-analysis was conducted in accordance with the guidelines for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and the PRISMA statement (18, 19). The search was carried out on observational studies and trials, without language restrictions. The literature from Jan 1, 1966 to May 31, 2013 was searched using the following databases: Pubmed, ISI Web of Science (Science Citation Index Expanded), Embase, and the Cochrane library. The following main keywords or corresponding MeSH terms were used: “Metformin”, “Biguanides”, or “Diabetes Mellitus, Type 2/therapy” and “cancer” or “neoplasms”. The search string used for Pubmed is the following: (Metformin AND cancer) OR (“Metformin”[Mesh] AND “Neoplasms”[Mesh] AND “epidemiologic studies”[Mesh]) NOT (“polycystic ovary syndrome” OR “Polycystic Ovary Syndrome”[Mesh]). A manual search was performed for references cited in the selected articles, and in selected reviews or books. This literature search was independently carried out by two academic investigators. Group discussion resolved any disagreement with article selection.
Methods of data extraction
Criteria for article inclusion in the analysis were: 1) independence from other studies in order to avoid giving double weight to estimates derived from the same study; when two or more studies were not independent, only the study with larger sample size was included; 2) sufficient information to allow adequate estimation of the hazard ratio (HR)/relative risk (RR)/odds ratios (OR), and 95% confidence intervals (i.e., crude data or adjusted estimates and standard errors, confidence intervals or p-values); 3) comparison of cancer incidence or mortality in diabetic patients (comparisons with non-diabetic populations were excluded).
We extracted fully adjusted risk estimates for ever use of metformin, alone or in combination with other anti-diabetic treatments, compared with anti-diabetic treatments other than metformin or no treatment, and we calculated the corresponding variance using the formula proposed by Greenland (20). Association between metformin and cancer incidence/mortality across selected studies was computed as a summary relative risk (SRR) with 95% confidence intervals (CIs).
Statistical analysis
Heterogeneity was evaluated using the I2 parameter, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than to chance. A threshold below 50% is generally considered acceptable (21). In order to account for possible sources of bias we considered the STROBE checklist proposed for observational epidemiological studies (22). Several sensitivity analyses were considered in this work, taking into account factors presented in the STROBE checklist that could introduce bias. Sub-group and sensitivity analyses and meta-regressions were carried out to investigate between-study heterogeneity and the influence of confounding factors, study design, interaction with other treatments, definitions of disease and population features on the risk estimates. A key factor considered was the adjustment for BMI, given its modifying effect on metformin activity on diabetes incidence (3) and breast cancer proliferation (16).
We also investigated heterogeneity due to study design since retrospective cohort studies could have important sources of bias. Sensitivity analyses were carried out to verify the effect of single studies and inclusion and exclusion criteria on the stability of the summary estimates, such as the use of insulin as treatment comparator. The SRR was estimated by pooling the study-specific estimates by random effects models fitted using SAS (Proc Mixed) with maximum likelihood estimates and CIs based on t-distribution (23), to be conservative.
In order to take into account time-related biases that can occur in observational studies (17), we carried out subgroup analyses including only studies that were designed or analyzed to avoid immortal time bias, time-window bias, and time-lagging issues. The summary estimates were based only on studies that specifically used time-dependent techniques needed to avoid immortal time bias and to treat exposures to the different antidiabetic agents as time-dependent variables.
To verify whether publication bias might affect the validity of the estimates, funnel plots were investigated considering regression of Ln(RR) on the sample size, weighted by the inverse of the pooled variance (24). All analyses were performed with SAS software version 8.02 and STATA software version 11.
Results
Meta-Analysis
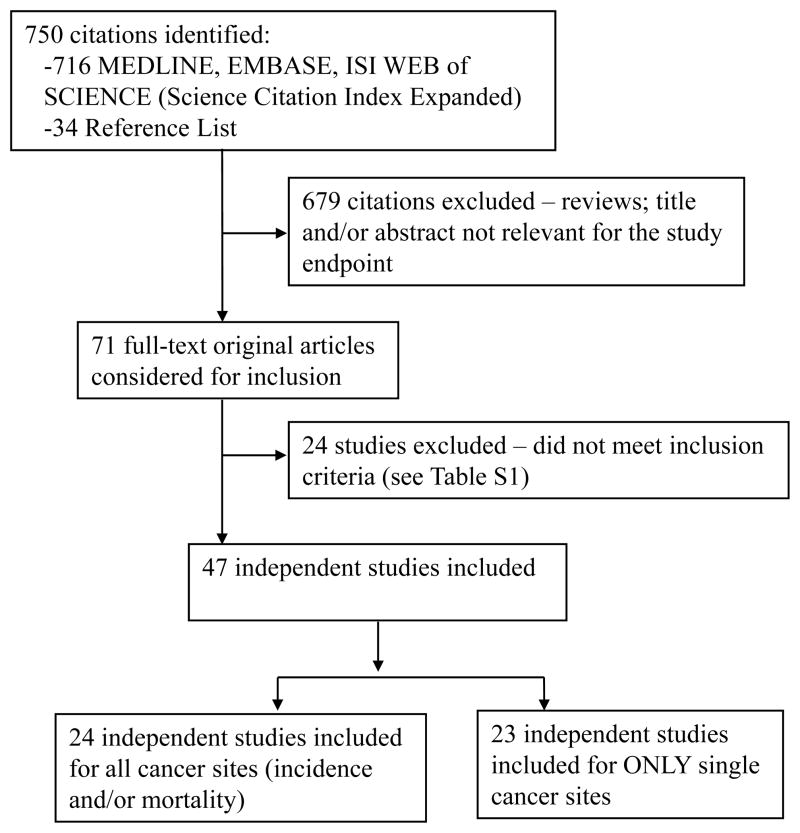
The flow diagram for study inclusion in the meta-analysis is shown in Figure 1. A total of 71 articles were retrieved and checked for relevance in terms of intervention, population studied and reporting of cancer incidence/mortality data. Twenty-four (25–48) articles were excluded (Supplemental Table 1). Since the UKPDS trials had partially overlapping patient populations, only the risk estimate for the metformin monotherapy trial was included (49).
Figure 1. Study flow diagram.
Of 750 citations identified, 47 independent studies were included in the analysis.
Overall we included estimates from 47 studies and 65,540 cancer cases: 19 studies (50–67) presented data on overall cancer incidence, 7 studies (38, 49, 54, 68–72) on overall cancer mortality, and 32 studies (45, 48, 50, 52–54, 56, 57, 59, 66, 73–96) reported estimates on single cancer sites. Table 1 shows the characteristics of these 47 studies. There were 11 prospective cohort studies, 16 case-control studies, 14 retrospective cohort studies and 6 clinical trials of diabetic patients randomized to metformin versus other treatment published between 1998 and 2013. Treatment comparators were sulfonylureas, insulin or other anti-diabetic treatments. If more than one estimate was presented, the estimate for metformin alone was preferred to metformin combined with other treatments and a comparator other than insulin was chosen.
Table 1.
Epidemiological studies of metformin and cancer risk.
| First Author, ref year (Country) | Study design | Endpoint | Sample size | Risk Estimates (95%CI) | Treatment comparison | Adjusting variables(other than age & sex) |
|---|---|---|---|---|---|---|
| UKPD Study Group 49, 1998 (UK) | RCT | mortality | Cases: 139 At risk: 753 |
Any site: 0.71 (0.29–1.76) † | diet alone (n=411) vs. intensive blood-glucose control policy with metformin (n=342). | n.a. |
| Schernthaner G 61,72 2004 QUARTET M (Europe) | RCT | incidence | Cases: 9 At risk: 1.194 |
Any site: 0.51 (0.14–1.90) | metformin monotherapy (n=597) vs. pioglitazone (n=597) | n.a. |
| Hanefeld M, 62,72 2004 QUARTET C (Europe and North America) | RCT | incidence | Cases: 9 At risk: 639 |
Any site: 1.99 (0.43–12.32) | metformin+sulfonylurea (n=320) vs. pioglitazone+ sulfonylurea (n=319) | n.a. |
| Yang YX 85, 2004 (UK) | General practice nested case-control in a retrospective cohort | incidence | Cases: 125 Controls: 1.195 |
Colon: 1.00 (0.60–1.70) | 3 or more years of metformin therapy vs. non-insulin users | Smoking, history of cholecystectomy, diabetes duration, BMI, sulphonylurea use, aspirin/NSAID use |
| Bowker SL 69, 2006 (Canada) | Population-based retrospective cohort | mortality | Cases: 407 At risk: 10.309 |
Any site: 0.77 (0.63–0.91) | metformin vs. sulfonylureas use | insulin use and CDS. |
| Monami M 55, 2009 (Italy) | Hospital-based retrospective case-control study | incidence | Cases: 195 Controls: 195 |
Any site: 0.28 (0.13–0.57) | exposure to metformin for more than 36 months vs. other hypoglycemic drugs users | duration of diabetes, BMI, HbA1c, comorbidity, smoking and alcohol abuse, concomitant hypoglycemic treatment |
| Oliveria SA 76, 2008 (USA) | Population-based retrospective cohort | incidence | Cases: 813 At risk: 191.223 |
Colon 0.67 (0.52–0.85) Bladder 0.99 (0.70–1.39) Liver 0.73 (0.34–1.56) Pancreas 1.26 (0.80–1.99) |
ever use of metformin monotherapy vs. never use | HBV and HCV infection, cirrhosis, alcoholism, polyps, obesity, ulcerative colitis, Crohn’s disease, cystic fibrosis, chronic pancreatitis, dermatomyositis, polymyositis, idiopathic DVT, partial gastrectomy, pelvic radiation, and schistosomiasis. |
| Currie CJ 59, 2009 (UK) | General practice retrospective cohort | incidence | Cases: 373 At risk: 7.897 |
Any site: 0.74 (0.65–0.84); Breast: 1.02 (0.71–1.45); Colon: 0.56 (0.40–0.76); Prostate: 0.93 (0.67–1.32); Pancreas: 0.20 (0.11–0.36) |
metformin monotherapy vs. sulfonylureas monotherapy | Smoking, comorbidity, HbA1c, diabetes duration, weight |
| Donadon V 80, 2009 (Italy) | Hospital-based retrospective case-control | incidence | Cases: 465 Controls: 490 |
Liver: 0.33 (0.10–0.70) | metformin users vs. nonusers | No adjusting variables were considered |
| Home PD 52, 2009, RECORD, (Europe) | RCT | incidence | Cases: 125 At risk: 2.225 |
Any site: 1.22 (0.86–1.74) Breast: 1.0 (0.32–3.10)* Prostate: 2.0 (0.68–5.82)* Pancreas: 5.99 (0.72–49.6)* Liver: 4.0 (0.18–88.7)* |
metformin (n=1122) vs. rosiglitazone (n=1103) | n.a. |
| Li D 86, 2009 (USA) | Hospital-based retrospective case-control | incidence | Cases: 255 Controls: 106 |
Pancreas: 0.38 (0.22–0.69) | metformin users vs. nonusers | race, smoking, alcohol, BMI, family history of cancer, duration of diabetes, and insulin use. |
| Libby G 54, 2009 (Scotland, UK) | Population-based retrospective cohort | incidence & mortality | Cases: 771 At risk: 8.170 |
Any site incidence: 0.63 (0.53–0.75); Breast: 0.60 (0.32–1.10); Colon: 0.60 (0.38–0.94); Lung: 0.70 (0.43–1.15) Any site mortality: 0.63 (0.49–0.81) |
metformin users vs. nonusers | Smoking, BMI, HbA1c, material deprivation, other drug use (sulphonylureas or insulin) |
| Wright JL 79, 2009 (USA) | Population-based retrospective case-control | incidence | Cases: 97 Controls: 101 |
Prostate: 0.56 (0.32–1.00) | metformin users vs. nonusers | BMI, statin and aspirin use, other diabetes treatment, PSA screening history, family history of PCa |
| Bodmer M 73, 2010 (UK) | General practice retrospective nested case-control | incidence | Cases: 17 Controls: 120 |
Breast: 0.44 (0.24–0.82) | users of 40+ prescriptions (>5 years) of metformin vs. nonusers# | General practice and calendar time by matching, other use of prandial glucose regulators, acarbose, estrogens, smoking, BMI, diabetes duration and HbA1c |
| Hassan MM 81, 2010 (USA) | Hospital-based retrospective case-control | incidence | Cases: 122 Controls: 86 |
Liver: 0.30 (0.20–0.60) | biguanide users vs. nonusers | Race, educational level, cigarette smoking, alcohol drinking, HCV, HBV, family history of cancer |
| Kahn SE 63, 2006 ADOPT, (USA) | RCT | incidence | Cases: 160 At risk: 4.351 |
Any site: ADOPT-G: 0.78 (0.53–1.14) ADOPT-R: 0.92 (0.63–1.35) Breast: 2.0 (0.60–6.62)* Colon: 1.75 (0.51–5.96)* Prostate: 1.0 (0.41–2.40)* Pancreas: 0.1 (0.005–1.84)* |
metformin (n=1454) vs. glibencamide (n=1441) vs. rosiglitazone (n=1456) | n.a. |
| Landman GWD 70, 2010 (Netherlands) | General practice prospective cohort | mortality | Cases: 122 At risk: 1.353 |
Any site: 0.43 (0.23–0.80) | metformin users vs. nonusers | Smoking, diabetes duration, HbA1c, serum creatinine, BMI, blood pressure, total cholesterol/HDL, albuminuria, insulin use, sulphonylurea use and macrovascular complications |
| Williams-Herman D,2010 64 (18 countries worldwide) | RCT | incidence | Cases: 18 At risk: 543 |
Any site: 0.61 (0.22–1.79) | Metformin (n=364) vs. Sitagliptin (n=179) | n.a. |
| Yang X 58, 2010 (China) | Hospital-based prospective cohort | incidence | Cases: 271 At risk: 6.103 |
Any site: 0.99 (0.70, 1.41) | sulphonylurea use + ever use of metformin vs. never use of metformin | BMI, smoking status, alcohol, HbA1c, SBP, LDL-C related risk, HDL and triglyceride, statins, RAS inhibitor usage, insulin usage. |
| Azoulay L 77, 2011 (Canada) | Population-based retrospective nested case-control | incidence | Cases: 739 Controls: 7.359 |
Prostate: 1.23 (0.99–1.52) | ever vs. never users of metformin# | HbA1c, alcohol use, obesity, smoking, lower urinary tract symptoms, previous cancer, previous use of NSAID, antihypertensive drugs, and statins, use of other antidiabetic agents |
| Baur DM 65, 2011 (Germany) | Hospital-based prospective cohort | incidence & mortality | Cases: 66 At risk: 1.308 |
Any site incidence: 0.66 (0.26–1.64) Any site mortality: 0.71 (0.2–2.59) |
metformin users vs. nonusers | Smoking, BMI, HbA1c |
| Bosco JLF 74, 2011 (Denmark) | Population-based retrospective nested case-control | incidence | Cases: 393 Controls: 3.930 |
Breast: 0.81 (0.63–0.96) | metformin for at least 1 yr vs. women not prescribed antidiabetic medication, or used metformin for at least 1 yr. | diabetes complications, clinical obesity year of birth, parity, postmenopausal hormone use. |
| Ferrara A 75, 2011 (USA) | Population-based prospective cohort | incidence | Cases: 9.082 At risk: 252.467 |
Breast: 0.90 (0.80–1.00); Colon: 1.00 (0.90–1.20); Prostate: 1.00 (0.90–1.10); Pancreas: 1.20 (1.00–1.50); Lung: 1.00 (0.80–1.10); NHL: 1.00 (0.80–1.20); Corpus Uteri: 0.90 (0.80–1.20); Kidney/renal pelvis: 1.30 (1.0–1.6); Rectum: 0.90 (0.70–1.20); Melanoma: 0.80 (0.60–1.10) |
ever use of pioglitazone and metformin vs. never use of metformin | year of cohort entry, race/ethnicity, income, smoking, glycemic control, diabetes duration, creatinine levels, congestive heart failure, use of other diabetes medications |
| Hense HW 51, 2011 (Germany) | Population-based prospective cohort | incidence | Cases: 1.364 At risk: 26.742 |
Any site: 0.95 (0.90–1.01) | metformin (only) users vs. nonusers | diabetes duration, BMI, insulin therapy |
| Lai SW 84, 2011 (Taiwan) | Population-based retrospective cohort | incidence | Cases: 129 At risk: 19.624 |
Lung: 0.55 (0.37–0.82) | metformin users vs. nonusers | pulmonary tuberculosis, chronic obstructive pulmonary disease, and propensity score (quintile). |
| Lee JH 53, 2011 (South Korea) | Population-based prospective cohort | incidence | Cases: 339 At risk: 15.717 |
Any site: 0.12 (0.08–0.19); Colon: 0.36 (0.13–0.98); Liver: 0.06 (0.02–0.16); Esophagus: 0.44 (0.07–2.61); Stomach: 1.41 (0.42–4.73) |
at least 2 prescription of metformin vs. any other oral antihyperglycemic medication | other oral antihyperglycemic medication, Charlson comorbidity index score, metformin dosage and duration |
| Mellbin LG 71, 2011 (Sweden) | Prospective cohort follow-up analysis from RCT | mortality | N=1073 N events=37 |
Any site: 0.25 (0.08–0.83) | patients using metformin vs. not using at discharge | smoking habits, previous myocardial infarction or previous congestive heart failure, creatinine at randomisation, percutaneous transluminal coronary angioplasty or coronary artery bypass grafting during the hospitalisation, and mean updated blood glucose |
| Morden NE 56, 2011 (USA) | General-practice retrospective cohort | incidence | Cases: 5.466 At risk: 81.681 |
Any site: 1.01 (0.94–1.08); Breast: 1.28 (1.05–1.57); Colon: 0.94 (0.72–1.22); Prostate: 0.97 (0.76–1.24); Pancreas: 1.25 (0.89–1.75) |
metformin vs. not in insulin treated patients | race, low-income subsidy status, comorbidities, tobacco exposure, Charlson, comorbidities excluding malignancy, diabetes, insulin dose quartiles |
| Bo S 68, 2012 (Italy) | Hospital-based retrospective cohort | mortality | Cases: 122 At risk: 3.703 |
Any site: 0.56 (0.34–0.94) | metformin use vs. diet control only | diabetes duration, HbA1c, smoking, BMI, presence of retinopathy, nephropathy, coronary or peripheral artery disease, other co-morbidities and the use of antihypertensive drugs and acetylsalicylic acid. |
| Bodmer M 83, 2012 (UK) | General-practice retrospective nested case-control | incidence | Cases: 920 Controls: 5.519 |
Colon: 1.43 (1.08–1.90) | metformin users (50+ prescriptions) vs. non users | Diabetes duration, BMI, smoking, prior use of aspirin, NSAID, statins, estrogen use (women), sulphonylureas and insulin use |
| Bodmer M 82, 2012 (UK) | General-practice retrospective case-control | incidence | Cases: 307 Controls: 1.347 |
Pancreas: 0.83 (0.57–1.21) | metformin users vs. nonusers | BMI, smoking, alcohol consumption, diabetes duration, other antidiabetics drugs |
| Bodmer M 88, 2012 (UK) | General-practice retrospective case-control | incidence | Cases: 1.029 Controls: 6.174 |
Lung: 1.09 (0.85–1.38) | metformin users (40+ prescriptions) vs. non users | BMI and smoking |
| Chlebowski RT 48,95, 2013 (USA) | Prospective Cohort (WHI program) | incidence | Cases: 233 At risk: 68.019 |
Breast: 0.65 (0.46–0.91) | metformin vs. other antidiabetic drugs | Family history, prior breast biopsy, age at menarche, menopause, parity, age at first live birth, breastfeeding, education, smoking, alcohol use, BMI, physical activity, duration of prior estrogen alone, estrogen +progesterone use, bilateral oophorectomy, weight loss |
| Hsieh MC 89, 2012 (Taiwan) | Population-based prospective cohort | incidence | Cases: 6.554 At risk: 61.777 |
Any site: 0.56 (0.44–0.71) Breast: 0.57(0.33–0.97) Colon: 0.54 (0.39–0.76) Prostate: 0.97 (0.60–1.55) Lung: 0.64 (0.45–0.90) Liver: 0.66 (0.49–0.91) Pancreas: 0.63 (0.28–1.42) Stomach: 0.63 (0.39–1.08) |
metformin vs. sulfonylurea | Only age and sex |
| Lehman DM 78, 2012 (USA) | Population-based Retrospective Cohort | incidence | Cases: 360 At risk: 5.042 |
Prostate: 2.15 (1.83–2.52) | metformin versus sulfonylurea only (restricted to non-statin users)# | HbA1c, diabetes duration, race/ethnicity, Charlson comorbidity score. |
| Liao KF 91, 2012 (Taiwan) | Population-based prospective cohort | incidence | Cases: 56 At risk: 49.803 |
Pancreas: 0.85 (0.39–1.89) | metformin users vs. nonusers | No adjusting variables were considered |
| Magliano DJ 66, 2012 (Australia) | Community-based longitudinal cohort | incidence | Cases: 309 At risk: 1.294 |
Any site: 0.88 (0.67–1.17); Prostate: 2.16 (1.19–3.9) |
metformin users vs. nonusers | No adjusting variables were considered |
| Mazzone PJ 94, 2012, (USA) | Hospital-based retrospective case-control | incidence | Cases: 507 Controls: 507 |
Lung: 0.48 (0.28–0.81) | metformin users vs. nonusers | medication use, BMI, HbA1C, smoking |
| Ngwana G 50, 2012 (Belgium) | General-practice retrospective cohort | incidence | Cases: 221 At risk: 4012 |
Any site: 0.20 (0.03–1.64); Breast: 0.46 (0.07–3.10); Colon: 0.11 (0.01–1.07); Prostate: 0.61 (0.31–1.19) |
metformin vs. other antidiabetic treatments and diet only | weight and initial HbA1c |
| Redaniel MT 90, 2012 (UK) | General practice retrospective cohort | incidence | Cases: 873 At risk: 52657 |
Breast: 1.02 (0.79–1.3) | metformin vs. sulfonylurea | period, region, BMI, year of diagnosis. |
| Ruiter R 57, 2012 (Netherlands) | Hospital-based prospective cohort | incidence | Cases: 3.552 At risk: 85.289 |
Any site: 0.90 (0.88–0.91); Breast: 0.95 (0.91–0.98); Colon: 0.91 (0.88–0.94); Prostate: 0.92 (0.88–0.94); Pancreas: 0.73 (0.66–0.80); Liver: 0.67 (0.53–0.86); Lung: 0.87 (0.84–0.91); Esophagus: 0.90 (0.82–0.97); Stomach: 0.83 (0.76–0.90) |
metformin vs. sulphonylurea derivatives# | Age at first oral glucose-lowering drug prescription, number of other drugs used in the year before the start of OGLD, number of hospitalizations in the year before the start of OGLD, calendar time |
| Becker C 96, 2013 (UK) | General practice retrospective case-control | incidence | Cases: 291 Controls: 1.746 |
Endometrial: 0.88 (0.58–1.32) | metformin users (25+ prescriptions) vs. no prior use | BMI, smoking, diabetes duration |
| Chaiteerakij R 93, 2013 (USA) | Hospital-based retrospective case-control | incidence | Cases: 105 Controls: 34 |
Liver: 0.4 (0.2–0.9) | metformin users vs. nonusers | ethnicity, and residential area, propensity scores for statin-use |
| Chen HP 92, 2013 (Taiwan) | Population-based Retrospective case-control | incidence | Cases: 22.047 Controls: 25.773 |
Liver: 0.79 (0.75–0.83) | metformin users vs. nonusers | Cirrhosis, HCV, DM duration, Comorbidities, other medications |
| Chung HE 67, 2013 (South Korea) | Population-based retrospective cohort | incidence | Cases: 73 At risk: 1.217 |
Any site: 0.57 (0.39–0.85) | metformin users vs. nonusers | Not specified |
| Currie CJ 60, 2013 (UK) | General practice retrospective cohort | incidence | Cases: 4.029 at risk: 84.622 |
Any site: 0.91 (0.83–1.00) | metformin vs. sulfonylurea | systolic blood pressure, HbA1c, total cholesterol, serum creatinine, BMI, smoking status, antihypertensive-lipid-lowering, antiplatelet therapy, duration of diabetes, prior history of cancer, LVD, microvascular disease, number of contacts with the general practitioner in the year prior to the index date, Charlson comorbidity index |
| Smiechowski et al. 87, 2013 (Canada) | Population-based prospective nested case-control | incidence | Cases: 808 Controls: 7764 |
Lung: 0.94 (0.76–1.17) | metformin users vs. nonusers | diabetes duration, HbA1c, obesity, smoking, excessive alcohol use, previous cancer, chronic obstructive pulmonary disease, asthma, nonsteroidal anti-inflammatory drugs, aspirin, statins, and other antidiabetic drugs |
%CI- Percent Confidence Interval, UKPDS- United Kingdom Prospective Diabetes Study, RCT- Randomized Controlled Trial, N- number, BMI- Body Mass Index, NSAID- Non-steroidal Anti-inflammatory drug, CDS- chronic disease score, HbA1c - haemoglobin A1c, HBV- Hepatitis B Virus, HCV- Hepatitis C Virus, DVT- Deep Vein Thrombosis, HDL- High Density Lipoprotein, PSA- Prostate specific antigen, PCa- Prostate Cancer, ADOPT- A Diabetes Outcome Progression Trial, RECORD- Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes, SBP- systolic blood pressure, LDL-C- low density lipoprotein cholesterol, RAS- renin–angiotensin system, OGLD- oral glucose lowering drugs;
risk estimate for users of metformin alone;
ADOPT-G and ADOPT-R, glibenclamide and rosiglitazone arms of ADOPT study. Risk estimates represent multiple comparisons from a single trial, and the analysis takes account of correlation between these comparisons; risk estimates for single cancer sites were calculated from crude data;
Excluded patients on monotherapy with insulin.
We also examined SRRs stratified by BMI adjustment and time related bias. For the latter analysis, 18 studies were judged to have avoided these biases (49, 51, 52, 55, 57, 61, 62, 64, 70, 71, 75, 77, 78, 80, 86, 87). However, the small number of studies may imply lack of robustness of the SRR estimates and where fewer than 3 studies were adjusted for BMI, the BMI-adjusted SRRs are not reported [31]. Estimates from randomized clinical trials were considered to be adjusted for BMI.
Overall Cancer Incidence and Mortality – Effects of BMI and Study Type
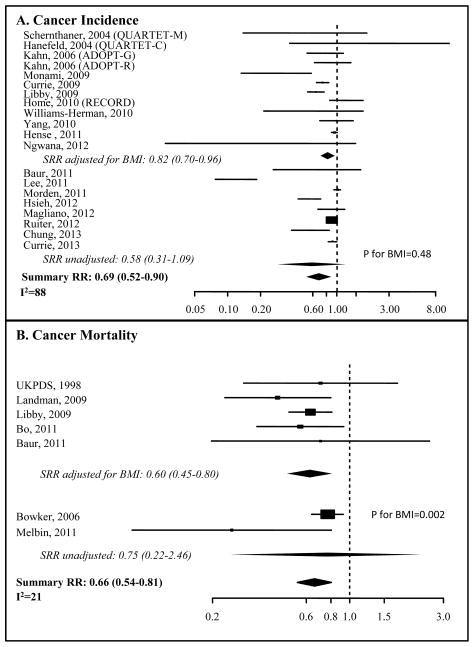
The SRRs for metformin and overall cancer incidence (50–59) and mortality (45, 50–54, 56–59, 73–84, 86, 87) are shown in Table 2 and Figure 2. A risk reduction of 31% (SRR=0.69, 95%CI: 0.52–0.90), with high heterogeneity (I2=88%), was estimated for overall cancer incidence in subjects taking metformin compared with other anti-diabetic compounds. There was a statistically significant, 34% reduction in cancer mortality (0.66, 95%CI: 0.54–0.81), with limited heterogeneity (I2=21%).
Table 2.
Summary Risk Estimates (SRRs) and 95% Confidence Intervals (95%CI) for all Endpoints
| Endpoints | Groups | SRR (95%CI) | I2 | n studies* |
|---|---|---|---|---|
| Cancer incidence | All studies | 0.69 (0.52, 0.90) | 88 | 19 |
| Adjusted for BMI | 0.82 (0.70, 0.96) | 76 | 11 | |
| Adjusted for time related bias | 0.90 (0.89, 0.91) | 56 | 8 | |
| Prospective studies | 0.71 (0.47, 1.07) | 89 | 12 | |
| Randomized Clinical Trials | 0.95 (0.69, 1.30) | 5 | 5 | |
| Cancer mortality | All studies | 0.66 (0.54, 0.81) | 21 | 7 |
| Adjusted for BMI | 0.60 (0.45, 0.80) | 0 | 5 | |
| Adjusted for time related bias | 0.45 (0.16, 1.26) | 0 | 3 | |
| Prospective studies | 0.48 (0.23, 0.97) | 0 | 4 | |
| Single cancer sites | ||||
| Breast | All studies | 0.88 (0.75, 1.03) | 60 | 13 |
| Adjusted for BMI | 0.82 (0.67, 1.00) | 48 | 7 | |
| Adjusted for time related bias | 0.94 (0.90, 0.99) | 32 | 6 | |
| Prospective studies | 0.94 (0.90, 0.99) | 44 | 7 | |
| Colon | All studies | 0.80 (0.64, 1.00) | 76 | 12 |
| Adjusted for BMI | 0.84 (0.50, 1.40) | 81 | 6 | |
| Adjusted for time related bias | 0.92 (0.85, 0.98) | 24 | 3 | |
| Prospective studies | 0.82 (0.57, 1.17) | 74 | 5 | |
| Prostate | All studies | 1.06 (0.80, 1.41) | 91 | 12 |
| Adjusted for BMI | 0.98 (0.68, 1.40) | 55 | 6 | |
| Adjusted for time related bias | 1.25 (0.87, 1.80) | 96 | 6 | |
| Prospective studies | 0.93 (0.89, 0.97) | 59 | 6 | |
| Pancreas | All studies | 0.75 (0.49, 1.15) | 84 | 11 |
| Adjusted for time related bias | 0.48 (0.16, 1.43) | 83 | 5 | |
| Time related unbiased | 0.77 (0.38, 1.55) | 40 | 5 | |
| Prospective studies | 0.89 (0.61, 1.29) | 80 | 6 | |
| Liver | All studies | 0.47 (0.28, 0.79) | 82 | 9 |
| Adjusted for time related bias | 0.65 (0.39, 1.08) | 38 | 3 | |
| Prospective studies | 0.78 (0.72, 0.85) | 52 | 5 | |
| Lung | All studies | 0.82 (0.67, 0.99) | 57 | 5 |
| Adjusted for smoking | 0.95 (0.82, 1.11) | 57 | 5 | |
| Adjusted for time related bias | 0.88 (0.81, 0.95) | 36 | 3 | |
| Prospective studies | 0.97 (0.69, 1.35) | 26 | 3 | |
n. estimates may not corresponds to n. of studies; BMI: Body Mass Index. SRR: Summary Relative risk
Figure 2. Forest Plot of the association between metformin and cancer incidence or cancer mortality.
Forest plots of risk estimates from observational studies and randomized controlled trials of metformin use and cancer incidence (A) or cancer mortality (B). Black boxes indicate hazard ratios (HRs), and horizontal lines represent 95% confidence intervals (CIs). Black diamonds represent the summary relative risk estimates. The vertical dotted line represents a risk estimate of 1.00.
A significant reduction in overall cancer incidence in metformin users was also found when the estimates were adjusted for BMI (SRR=0.82, 95%CI: 0.70–0.96; I2=76%), but not in BMI-unadjusted studies (SRR=0.58 with 95%CI: 0.31–1.09 and I2=94%; P=0.49 for the difference between estimates). However, no reduction was found when the analysis was restricted to prospective studies (SRR=0.71, 95%CI: 0.47–1.07, I2=89%) or randomized clinical trials (SRR=0.95, 95%CI: 0.69–1.30; I2=5%), although the latter studies included only 321 events. Meta-regression also indicates that publication year is not associated with risk estimates (P=0.59), nor was there an association with the use of insulin treatment as comparator (P=0.89).
The SRR for cancer mortality from BMI-adjusted results confirmed a significant reduction with metformin use (SRRs adjusted for BMI: 0.60, 95%CI: 0.45–0.80; I2=0), whereas the reduction from unadjusted estimates was not significant (SRR=0.75; 95%CI: 0.23–2.46; I2=71%). Analysis of prospective studies only showed a statistically significant reduction with metformin, in contrast to the effect seen on cancer incidence (SRR=0.48, 95%CI: 0.23–0.97; I2=0).
Organ Specific Analyses – Effects of BMI and Study Type on Cancer Incidence
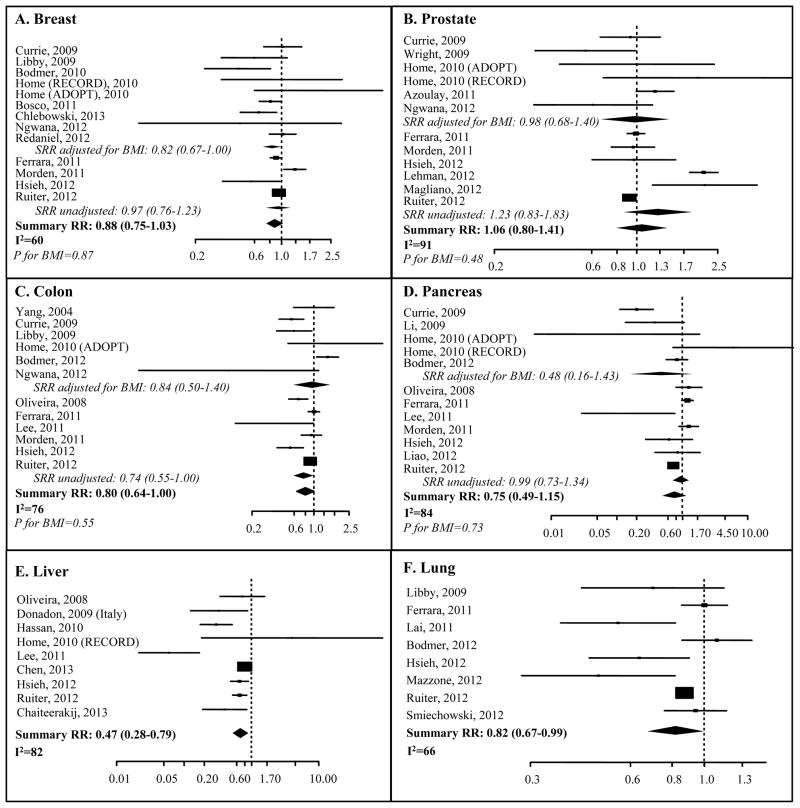
The SRR estimates for breast, prostate, colon, pancreas, liver and lung are illustrated in Figure 3 and Table 2. The risk reduction with metformin use in unadjusted analyses reached statistical significance only for liver (9 studies, SRR=0.47, 95%CI: 0.28–0.78; I2=82%) and lung cancer (8 studies, SRR=0.82, 95%CI: 0.67–0.99; I2=66%) (Table 2). Analysis of prospective studies confirmed this association for liver but not lung cancer. Too few liver or lung cancer studies were available to address the effect of BMI. Most notably, the summary estimate for lung cancer adjusted for smoking showed no significant association (SRR=0.95; 95%CI: 0.82–1.11; I2=58%).
Figure 3. Forest plots of the association between metformin use and individual site cancer incidence.
Forest plots of risk estimates from observational studies and randomized controlled trials of metformin use and breast cancer (A), prostate cancer (B), colon cancer (C), pancreas cancer (D), liver cancer (E), lung cancer (F). Black boxes indicate hazard ratios (HRs), and horizontal lines represent 95% confidence intervals (CIs). Black diamonds represent the summary relative risk estimates. The vertical dotted line represents a risk estimate of 1.00. P for BMI is the p-value for the interaction between BMI-adjusted analysis and unadjusted analysis.
The meta-analysis of the thirteen studies on breast cancer risk showed a non-significant trend (SRR=0.88, 95%CI: 0.75–1.03; I2=60%). However, BMI adjustment showed borderline significance in 5 studies (SRR=0.82, 95% CI, 0.67–1.00; I2=48%). Analysis of 7 prospective studies showed statistical significance (SRR=0.94, 95% CI: 0.90–0.99; I2=44). Metformin treatment and prostate cancer risk did not show any association in 12 studies (SRR=1.06, 95% CI: 0.80–1.41, I2=91%), even upon BMI adjustment. However, in the subgroup of 6 prospective studies, the reduction became significant (SRR=0.93; 95%CI: 0.89–0.97; I2=59%), albeit with low magnitude. For colon cancer, the SRR suggested borderline significant risk reduction (12 studies, 0.80, 95%CI: 0.64–1.00; I2=76%). The SRRs from subgroups of studies adjusted for BMI and with prospective designs did not suggest a significant reduction in cancer risk. No risk reduction was found for metformin use in pancreatic cancer (SRR=0.75, 95%CI: 0.49–1.15, I2=84%) even after BMI adjustment or when the analysis was limited to 6 prospective studies (SRR=0.89, 95% CI: 0.61–1.29, I2=80%).
We also evaluated the effect of the BMI adjustment within studies (not only between studies) when the data were available. For 12 observational studies we were able to extract risk estimates adjusted for BMI (or a proxy such as obesity) and crude estimates in order to measure the size of this confounding (supplemental table 2). Overall, the data show similar RR estimates between fully adjusted and crude RR estimates, suggesting limited confounding effect. Summary risk estimates for individual organs were obtained only for breast cancer, for which we had at least 4 studies. SRRs were very similar: 0.79 (0.54, 1.16) and 0.72 (0.48, 1.07) for adjusted and unadjusted estimates, respectively.
These analyses focused on patients with diabetes. In some studies the diagnosis of diabetes was not verified and the comparator was “anti-diabetic drug users” (57, 69, 73, 82, 83). A sensitivity analysis excluding those studies did not modify the results except for colorectal cancer, which became statistically significant [SRR=0.73 (95% CI, 0.58–0.92)] after excluding the paper by Ruiter et al. (83). When the potential bias due to insulin treatment as comparator was taken into account, the final conclusions did not change. No indication for publication bias was found for any of the summary estimates.
Analysis of Studies Without Time-related Biases
The SRRs for overall cancer incidence, organ specific cancer incidence, and overall cancer mortality obtained from analysis of studies that avoided time-related biases are shown in Table 2. The SRR for overall cancer incidence was statistically significant in 8 studies (SRR=0.90; 95%CI: 0.89–0.91; I2= 56%). The SRR for breast and colorectal cancer also became statistically significant: SRR=0.94 (95%CI: 0.90–0.99; I2= 32%) and SRR=0.92 (95%CI: 0.85–0.98; I2= 24%), respectively. On the other hand, the risk reduction for overall cancer mortality and liver cancer incidence lost statistical significance (SRR=0.45; 95%CI: 0.16–1.26 and SRR=0.65; 95%CI: 0.39–1.08, respectively). For lung cancer, the SRR suggested significant risk reduction, but adjustment for smoking eliminated the effect.
When only studies without time related biases and adjusted for BMI were analyzed, the SRR for overall cancer incidence and breast cancer lost significance: SRR=0.94 (95%CI: 0.88–1.01) and SRR=0.89 (95%CI: 0.56–1.41), respectively. These numbers, however, were small.
Discussion
Research on metformin use and cancer risk and mortality has expanded considerably over recent years, with conflicting data arising from different epidemiological, human, and animal carcinogenesis studies. Several previous meta-analyses have concluded that diabetic patients who use metformin have significantly lower risk of overall cancer incidence (30–40%), mortality, and site-specific cancer incidence than those who use other anti-diabetic medications (11–14). However, the studies included in these meta-analyses are susceptible to several confounders and biases. Here we focused for the first time on two critical issues with potential to skew the literature, the effect of BMI and time-related biases in observational studies. The main results from our study show that metformin use is associated with decreased overall cancer incidence even after adjustment for BMI or time-related biases, but the magnitude of this effect is considerably smaller than observed without such adjustments (10–18% versus 31%). Simultaneous adjustment for both BMI and time-related biases results in loss of statistical significance, albeit based on few studies. This is reminiscent of results from Thakkar et al., who showed a diminution in metformin’s effect when considering cohort studies (30%) versus case-control studies (10%) versus randomized controlled trials (no effect) (14). Examination of individual organ sites, which is limited by fewer available studies for analysis, shows nonsignificant associations or similarly smaller effects after adjustment. Taken together, these data underscore the importance of understanding the limitation in the current literature and suggest that if metformin use is associated with a reduced risk of cancer, the effect may be smaller than previously shown.
Obesity and its surrogate, high BMI, are intimately linked to increased risk of several cancer types (97, 98). Potential mechanisms include both direct and indirect effects of obesity on insulin, IGF-1, sex hormones, adipokines, and inflammation, many of which are directly impacted by metformin. In our analysis, BMI-adjusted studies showed statistically significant reduction in cancer incidence and mortality while unadjusted studies showed no effect. In 12 prospective studies where it was possible to compare BMI adjusted versus crude estimates within each study, similar RR estimates were noted, suggesting limited confounding effect of BMI. Likewise, summary risk estimates within 4 breast cancer studies were similar. BMI adjustment did not significantly affect the cancer risk estimates for individual organ sites, although the risk estimates for breast cancer became borderline significant. A direct correlation between BMI and inflammation, adipocyte size, and aromatase expression has been shown in breast tissue from women undergoing breast cancer surgery, pointing to inflammation as a potential biologic basis for the cancer-obesity connection (99). However, BMI and insulin resistance had a modifying effect on the metformin modulation of breast cancer cell proliferation in a pre-surgical trial (16). Furthermore, metformin is the drug of choice in obese diabetic patients since it reduces weight (3, 100), so its use is associated with obesity. Thus, modification of the cancer-obesity relationship by metformin is likely complex and requires extensive study.
A recent review by Suissa and Azoulay underscored the prevalence of time-related biases in observational studies, potentially leading to inflated estimates of the protective effect of metformin (17). These biases include immortal time bias (unexposed time is misclassified as drug-exposed time), time-window bias (differential exposure opportunity time windows between exposed and unexposed subjects), and time-lag bias (comparison of treatment given during different stages of the disease). Of note, exclusion of time-biased studies from our analysis resulted in statistically significant 10% risk reduction in overall cancer incidence, although the magnitude is substantially smaller than the previously reported 30–40%. In organ-specific analyses, reduction in colorectal cancer incidence became significant (8%), while liver cancer risk reduction became non-significant. Exclusion of time-biased studies in the analysis of cancer mortality resulted in loss of statistical significance.
The effect of metformin use on cancer mortality may result from different mechanisms than the effect on incidence. Retrospective analyses suggest that diabetics treated with metformin during chemotherapy have better survival than those treated with other anti-diabetic agents (28, 101). Interestingly, mouse xenograft models show that metformin targets breast cancer stem cells and synergizes with doxorubicin to prevent relapse (102). If metformin increases the effectiveness of chemotherapy, then its inclusion in chemotherapeutic regimens may exert a favorable impact on survival.
This study has several limitations. These include heterogeneity of study designs and treatment comparators. More than two-thirds of the studies had a retrospective design, which is prone to important sources of bias. However, our analyses of prospective studies generally found similar SRRs, although for breast, liver and prostate cancer, these results became statistically significant. A second limitation is the nature of the comparator group, which mainly included treatment with insulin and insulin secreatagogues. These classes of agents increase insulin levels and have been associated with increased cancer risk (14, 55, 69, 103). Thus the potential protective effect of metformin in an untreated or non-insulin using population cannot be precisely estimated. A third factor to consider is allocation bias, with metformin users being at different stage of diabetes than comparators, as discussed previously with regard to time-lag bias. Generally, treatment with metformin starts at a younger age, likely because of treatment guidelines (104) and in subjects with higher BMI, possibly because of its weight lowering effects (105). While the majority of studies adjusted for confounders such as age, we here presented the analyses adjusting for BMI and excluding time-biased studies. However, BMI is dynamic, and weight gain is an important risk factor for mortality of several cancers (106). Therefore, adjustment for a single BMI value might be inadequate to account for confounding by BMI dynamics over time. Finally, the effect of other confounders, both known (but not adjusted for) or heretofore unrecognized, should not be underestimated. This is best illustrated by lung cancer, where overall and time-unbiased analyses point to a protective effect, whereas adjustment for smoking, which is by far the most important cause of lung cancer, leads to loss of significance.
A critical question emerging from this meta-analysis of studies in diabetic patients is the generalizability to non-diabetic populations. Our data demonstrate a cancer preventive signal, albeit of lesser magnitude once the appropriate adjustments are made than previously reported. This signal now needs to be studied in controlled clinical trials focusing on carefully defined populations, such as the pre-diabetic population in the Diabetes Prevention Program trial (3, 4), for which long-term follow-up to ascertain the effects of metformin on cancer incidence is currently ongoing. However, it needs to be emphasized that existing data about metformin use in non-diabetic populations are severely limited. Clinical trials are needed to determine if the observations seen in diabetic populations can be expanded to pre-diabetic or non-diabetic populations and to whom they should be expanded for the best benefit/risk ratio. Although some of these early phase trials are ongoing, additional information is needed prior to making general recommendations or launching large-scale clinical efforts.
Supplementary Material
Acknowledgments
Funding/Support: The study was supported by grants from the Italian Association for Cancer Research AIRC (IG 12072), the Italian Ministry of Health (RF-2009-1532226), and the Italian League Against Cancer (14/08) to Andrea DeCensi. Andrea DeCensi’s work was partially performed during a sabbatical at the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health.
Footnotes
Conflicts of interest: None
References
- 1.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168:2070–80. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–43. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 6.Bojkova B, Orendas P, Garajova M, Kassayova M, Kutna V, Ahlersova E, et al. Metformin in chemically-induced mammary carcinogenesis in rats. Neoplasma. 2009;56:269–74. doi: 10.4149/neo_2009_03_269. [DOI] [PubMed] [Google Scholar]
- 7.Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–57. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662–71. doi: 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 9.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544–52. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale-Cross L, Molinolo AA, Martin D, Younis RH, Maruyama T, Patel V, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5:562–73. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 12.Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–22. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism. 2013;62:922–34. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 17.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–73. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup Df, BJAMSC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of Clinical Epidemiology. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of Clinical Epidemiology. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 24.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20:641–54. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 25.Berstein LM, Boyarkina MP, Teslenko SY. Familial diabetes is associated with reduced risk of cancer in diabetic patients: a possible role for metformin. Med Oncol. 2012;29:1308–13. doi: 10.1007/s12032-011-9840-0. [DOI] [PubMed] [Google Scholar]
- 26.Buchs AE, Silverman BG. Incidence of malignancies in patients with diabetes mellitus and correlation with treatment modalities in a large Israeli health maintenance organization: a historical cohort study. Metabolism. 2011;60:1379–85. doi: 10.1016/j.metabol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum. 2008;51:593–7. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- 28.He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–80. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752–9. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 30.Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, Melani C, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–31. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168:925–31. doi: 10.1093/aje/kwn190. [DOI] [PubMed] [Google Scholar]
- 32.Niraula S, Pond G, De Wit R, Eisenberger M, Tannock IF, Joshua AM. Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study. Can Urol Assoc J. 2011:1–8. doi: 10.5489/cuaj.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–8. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 34.Stefansdottir G, Zoungas S, Chalmers J, Kengne AP, Knol MJ, Leufkens HG, et al. Intensive glucose control and risk of cancer in patients with type 2 diabetes. Diabetologia. 2011;54:1608–14. doi: 10.1007/s00125-011-2104-x. [DOI] [PubMed] [Google Scholar]
- 35.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–15. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 36.van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. 2012;55:654–65. doi: 10.1007/s00125-011-2390-3. [DOI] [PubMed] [Google Scholar]
- 37.Yang X, So WY, Ma RC, Kong AP, Lee HM, Yu LW, et al. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care. 2011;34:375–80. doi: 10.2337/dc10-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hitron A, Adams V, Talbert J, Steinke D. The influence of antidiabetic medications on the development and progression of prostate cancer. Cancer Epidemiol. 2012;36:e243–50. doi: 10.1016/j.canep.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins-Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:4605–12. doi: 10.1210/jc.2012-3034. [DOI] [PubMed] [Google Scholar]
- 41.Qiu H, Rhoads GG, Berlin JA, Marcella SW, Demissie K. Initial metformin or sulphonylurea exposure and cancer occurrence among patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:349–57. doi: 10.1111/dom.12036. [DOI] [PubMed] [Google Scholar]
- 42.Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409–16. doi: 10.1530/EJE-12-0369. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012;44:1042–7. doi: 10.1016/j.dld.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Spratt DE, Zhang C, Zumsteg ZS, Pei X, Zhang Z, Zelefsky MJ. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol. 2013;63:709–16. doi: 10.1016/j.eururo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai SW, Chen PC, Liao KF, Muo CH, Lin CC, Sung FC. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, et al. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479–86. doi: 10.1111/j.1478-3231.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 47.Vu K, Busaidy N, Cabanillas ME, Konopleva M, Faderl S, Thomas DA, et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:355–62. doi: 10.1016/j.clml.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chlebowski RT, McTiernan A, Wactawski-Wende J, Manson JE, Aragaki AK, Rohan T, et al. Diabetes, metformin, and breast cancer in postmenopausal women. J Clin Oncol. 2012;30:2844–52. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 50.Ngwana G, Aerts M, Truyers C, Mathieu C, Bartholomeeusen S, Wami W, et al. Relation between diabetes, metformin treatment and the occurrence of malignancies in a Belgian primary care setting. Diabetes Res Clin Pract. 2012;97:331–6. doi: 10.1016/j.diabres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Hense HW, Kajuter H, Wellmann J, Batzler WU. Cancer incidence in type 2 diabetes patients - first results from a feasibility study of the D2C cohort. Diabetol Metab Syndr. 2011;3:15. doi: 10.1186/1758-5996-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53:1838–45. doi: 10.1007/s00125-010-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta Diabetol. 2009;46:279–84. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 56.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, Korc M. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. 2011;34:1965–71. doi: 10.2337/dc11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–24. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X, So WY, Ma RC, Yu LW, Ko GT, Kong AP, et al. Use of sulphonylurea and cancer in type 2 diabetes-The Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2010;90:343–51. doi: 10.1016/j.diabres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 60.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98:668–77. doi: 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89:6068–76. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 62.Hanefeld M, Brunetti P, Schernthaner GH, Matthews DR, Charbonnel BH. One-year glycemic control with a sulfonylurea plus pioglitazone versus a sulfonylurea plus metformin in patients with type 2 diabetes. Diabetes Care. 2004;27:141–7. doi: 10.2337/diacare.27.1.141. [DOI] [PubMed] [Google Scholar]
- 63.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 64.Williams-Herman D, Johnson J, Teng R, Golm G, Kaufman KD, Goldstein BJ, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:442–51. doi: 10.1111/j.1463-1326.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 65.Baur DM, Klotsche J, Hamnvik OP, Sievers C, Pieper L, Wittchen HU, et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort. Metabolism. 2011;60:1363–71. doi: 10.1016/j.metabol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Magliano DJ, Davis WA, Shaw JE, Bruce DG, Davis TM. Incidence and predictors of all-cause and site-specific cancer in type 2 diabetes: the Fremantle Diabetes Study. Eur J Endocrinol. 2012;167:589–99. doi: 10.1530/EJE-12-0053. [DOI] [PubMed] [Google Scholar]
- 67.Chung HH, Moon JS, Yoon JS, Lee HW, Won KC. The Relationship between Metformin and Cancer in Patients with Type 2 Diabetes. Diabetes Metab J. 2013;37:125–31. doi: 10.4093/dmj.2013.37.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bo S, Ciccone G, Rosato R, Villois P, Appendino G, Ghigo E, et al. Cancer mortality reduction and metformin: a retrospective cohort study in type 2 diabetic patients. Diabetes Obes Metab. 2012;14:23–9. doi: 10.1111/j.1463-1326.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 69.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;29:1990–1. doi: 10.2337/dc06-0997. [DOI] [PubMed] [Google Scholar]
- 70.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–6. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia. 2011;54:1308–17. doi: 10.1007/s00125-011-2084-x. [DOI] [PubMed] [Google Scholar]
- 72.Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 73.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–8. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosco JL, Antonsen S, Sorensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011;20:101–11. doi: 10.1158/1055-9965.EPI-10-0817. [DOI] [PubMed] [Google Scholar]
- 75.Ferrara A, Lewis JD, Quesenberry CP, Jr, Peng T, Strom BL, Van Den Eeden SK, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care. 2011;34:923–9. doi: 10.2337/dc10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliveria SA, Koro CE, Ulcickas Yood M, Sowell M. Cancer incidence among patients treated with antidiabetic pharmacotherapy. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2008;2:47–57. [Google Scholar]
- 77.Azoulay L, Dell’Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev. 2011;20:337–44. doi: 10.1158/1055-9965.EPI-10-0940. [DOI] [PubMed] [Google Scholar]
- 78.Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care. 2012;35:1002–7. doi: 10.2337/dc11-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–22. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506–11. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–46. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol. 2012;107:620–6. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 83.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of metformin is not associated with a decreased risk of colorectal cancer: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:280–6. doi: 10.1158/1055-9965.EPI-11-0992-T. [DOI] [PubMed] [Google Scholar]
- 84.Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012;13:143–8. doi: 10.1016/j.cllc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 85.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smiechowski BB, Azoulay L, Yin H, Pollak MN, Suissa S. The Use of Metformin and the Incidence of Lung Cancer in Patients With Type 2 Diabetes. Diabetes Care. 2012 doi: 10.2337/dc12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bodmer M, Becker C, Jick SS, Meier CR. Metformin does not alter the risk of lung cancer: a case-control analysis. Lung Cancer. 2012;78:133–7. doi: 10.1016/j.lungcan.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Experimental Diabetes Research. 2012;2012:413782. doi: 10.1155/2012/413782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Redaniel MT, Jeffreys M, May MT, Ben-Shlomo Y, Martin RM. Associations of type 2 diabetes and diabetes treatment with breast cancer risk and mortality: a population-based cohort study among British women. Cancer causes & control : CCC. 2012;23:1785–95. doi: 10.1007/s10552-012-0057-0. [DOI] [PubMed] [Google Scholar]
- 91.Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012;27:709–13. doi: 10.1111/j.1440-1746.2011.06938.x. [DOI] [PubMed] [Google Scholar]
- 92.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–15. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 93.Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, et al. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648–55. doi: 10.1002/hep.26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer. 2012;12:410. doi: 10.1186/1471-2407-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chlebowski RT, Aragaki AK, McTiernan A. Reply to S. Gandini et al. Journal of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.46.9130. [DOI] [PubMed] [Google Scholar]
- 96.Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of endometrial cancer: a case-control analysis. Gynecol Oncol. 2013;129:565–9. doi: 10.1016/j.ygyno.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 97.Goodwin PJ, Pritchard KI, Ennis M, Clemons M, Graham M, Fantus IG. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–5. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 98.Handelsman Y, Leroith D, Bloomgarden ZT, Dagogo-Jack S, Einhorn D, Garber AJ, et al. Diabetes and Cancer-An AACE/ACE Consensus Statement. Endocr Pract. 2013;19:675–93. doi: 10.4158/EP13248.CS. [DOI] [PubMed] [Google Scholar]
- 99.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005:CD002966. doi: 10.1002/14651858.CD002966.pub3. [DOI] [PubMed] [Google Scholar]
- 101.Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117:5103–11. doi: 10.1002/cncr.26151. [DOI] [PubMed] [Google Scholar]
- 102.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goodwin PJ, Thompson AM, Stambolic V. Diabetes, metformin, and breast cancer: lilac time? J Clin Oncol. 2012;30:2812–4. doi: 10.1200/JCO.2012.42.3319. [DOI] [PubMed] [Google Scholar]
- 104.International Diabetes Federation. Global Guideline for Type 2 Diabetes. 2005 [cited 2012; Available from: http://www.idf.org/global-guideline-type-2-diabetes-2005.
- 105.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.