Abstract
We hypothesized (1) preclinical biologic evidence exists for the role of androgens in ovarian cancer development and (2) flutamide treatment of women at high risk (HR) for ovarian cancer may identify meaningful tissue biomarkers of androgen action and of ovarian cancer initiation. We showed that androgen ablation of male mice led to a 24-fold decrease in tumor burden from serous ovarian cells. In a phase 2 study, we studied the effect of pre-operative flutamide treatment (125 mg/day × 6 weeks) in 12 women vs. 47 controls, 47% with BRCA mutation. We analyzed immunohistochemical scores of candidate proteins CSF-1, CSF-1R, and ErbB4in the epithelium and stroma of fallopian tube, ovary, and ovarian endosalpingiosis (ES). Flutamide decreased the levels, notably, of CSF-1 and ErbB4 in ovarian stroma (P≤ 0.0006) and ES (P≤ 0.01); ErbB4 in ovarian epithelium (P=0.006) and CSF-1R in ES (P=0.009). Our logistic regression model clearly distinguished the flutamide patients from controls (P ≤ 0.0001). Our analysis of the precision of this model of CSF-1 and ErbB4 expression in ovarian stroma achieved 100% sensitivity and 97% specificity (AUC=0.99). Thus, our data suggest that a short 6-week exposure of flutamide reversed elevated levels of CSF-1 and ErbB4 (both of which we had previously found correlated with HR status). CSF-1 and ErbB4 in ovarian stroma led to a model with high predictive value for flutamide sensitivity. The effect of flutamide on marker expression in ES, previously associated with BRCA carrier status, suggests that ES may be a latent precursor to pelvic serous cancers.
Keywords: Androgen, high risk, biomarkers, ovary, flutamide
Introduction
Strong evidence has accumulated that suggests an etiologic association between androgens and the development of ovarian cancer (OC). Studies of an epidemiologic, genetic, and biologic nature have formed the basis of the evidence, already largely summarized (1-3). The ovaries and fallopian tubes are androgen-sensitive organs; at menopause, this milieu may help promote transformation of the relevant epithelium. The epidemiologic evidence includes an association between oral contraceptive pills (known to suppress testosterone production as well as ovulation) and the suppression of OC risk, along with an association between polycystic ovarian syndrome (a hyperandrogenic milieu) and an increase in OC risk (1). Testosterone supplementation is associated with the development of OC (4, 5). Conflicting evidence exists regarding the possible association between serum androgen levels or androgen receptor (AR) polymorphisms and OC risk (6-10).
Many of the biologic studies of the role of androgens, and many of the related therapeutic trials, have involved OC cells, OC tissue, and/or OC patients. For instance, an AR coactivator was shown to enhance invasiveness of OC cells (11). And the role of several androgen-regulated proteins, such as HIF-1, HuR, EGFR, and uPA, in promoting OC have been defined (3, 12-14). AR, a marker of androgen sensitivity and the predominant sex steroid receptor expressed in OC (15, 16), decreases after chemotherapy exposure (17)—a finding that likely underlies the lack of efficacy of the antiandrogen flutamide in patients with refractory OC (18). Antiandrogens may have the most beneficial effect earlier in the process of transformation to OC.
Much less work has involved tissues from healthy women or from those at high risk (HR) for OC. But such studies have found that androgen-treated ovarian epithelial cells showed increased proliferation (19) and that androgen dysregulation in ovarian epithelial cells from women with BRCA gene mutations predicted poor survival from OC (12).
In our current study, we searched for androgen-sensitive tissue biomarkers of OC risk. We initially focused on a select group of biomarkers (CSF-1, CSF-1R, ErbB4), given our recent clinical study's findings of expression changes in those biomarkers in HR women, as compared with controls at low risk (LR) for OC (20). In that clinical study, we observed a significant association between the presence of CSF-1 and ErbB4 in the ovary and HR or BRCA carrier status. Specifically, we found that, in younger women, the presence of CSF-1 in ovarian epithelium and ErbB4 in ovarian stroma detected HR status with 73% sensitivity and 93% specificity, derived from analysis of our receiver operating characteristic (ROC) curve (20). Signaling of the macrophage colony-stimulating factor (CSF-1/CSF-1R) pathway is established as having a role in OC progression (21-24); our recent clinical study suggested a role in OC initiation (20). ErbB4 has been suggested as a marker that increases along the continuum from normal ovary to cancer (25); our recent clinical study found a potential role of nuclear ErbB4 in OC initiation (20). Nonetheless, little to no information exists on the effect of androgen on either ErbB4 or CSF-1 signaling.
For our current study, we hypothesized that (1) in vivo preclinical biologic evidence exists for the role of androgens in OC development and that (2) flutamide treatment of HR women may identify meaningful tissue biomarkers of androgen action and of OC initiation. Given the clear association between androgens and ovarian carcinogenesis in the literature, along with the preclinical data we found in the first part of our current study, we designed a phase 2 biomarker study of flutamide treatment in HR women who were undergoing risk-reducing salpingo-oophorectomy (RRSO). We then analyzed the effect of a 6-week course of flutamide on the inhibition of CSF-1, CSF-1R, and ErbB4 expression in the fallopian tubes and ovaries of HR women, as compared with a prospectively acquired cohort of controls.
Materials and Methods
Preclinical studies
To determine if androgens could stimulate in vitro parameters of transformation, we used human epithelial ovarian cells to study the effect of 5α-dihydrotestosterone (DHT) on invasion, chemoattractant-directed motility, and adhesiveness to an extracellular matrix barrier. These cells were also used for the in vivo studies of androgen stimulated tumorigenicity and tumor burden in intact and castrate male nude mice.
Cells
For our preclinical studies, we used cell lines derived from human ovarian epithelium. NOSE.1 cells are spontaneously immortalized ovarian surface epithelial cells, previously described (26). MCV152 and ML5TA cells, obtained from Louis Dubeau, were derived from SV40 large TAg transformed ovarian serous cystadenoma cells ML10 and ML5 (27), transfected with telomerase, hTERT. The cells, having the equivalent of a p53 mutation, have been used to study the effect of BRCA1 silencing on proliferation (28). These serous ovarian cells have thus been used as a model to study initiation of the high-grade pathway in ovarian carcinogenesis. We characterized these cells to contain AR, CSF-1, and CSF-1R. For comparison purposes, we used Hey cells, derived from a xenograft of a metastatic human serous OC (29): they express CSF-1R and a large amount of CSF-1.
In vitro assays
We performed all assays as previously described (23). The extracellular matrix barrier consisted of human laminin, type IV collagen, and gelatin. Before hormone treatments, the cells were placed in 1%NuSerum O/N. For statistical comparisons, we used the t test or analysis of variance (ANOVA), using SigmaStat v2.03 (SPSS Inc.).
CSF-1 sandwich ELISA
To measure secreted CSF-1 protein levels in the conditioned medium, we used CSF-1 sandwich ELISA as previously described (23); results are reported as pg CSF-1/ml ± SEM.
In vivo tumorigenicity and tumor burden
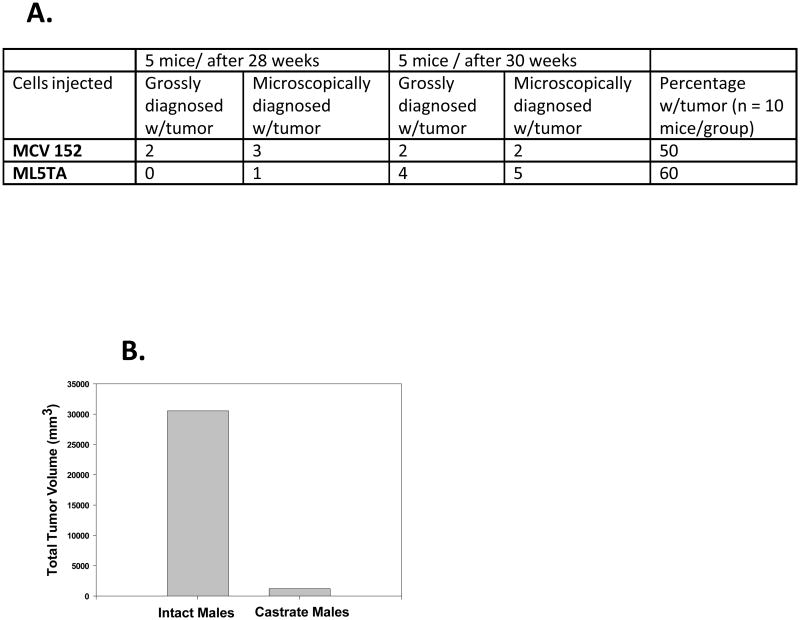
For all animal studies, we obtained approval from the appropriate Institutional Animal Care and Use Committee (IACUC) (Yale University or the University of Arizona). We first determined whether the MCV152 and ML5TA cells were tumorigenic in male nude mice. Ten male athymic NCr/nu mice were injected intraperitoneally (IP) with 1 × 106 cells each. Five mice from each group were initially sacrificed at 28 weeks, and the rest at 30 weeks. One of us, a gynecologic pathologist (WZ), performed both gross and microscopic examination.
Using those cells, we next studied the effect of androgen deprivation on the extent of tumor burden. Ten intact and 10 castrate male athymic NCr/nu mice were injected IP with 1 × 106 MCV152, or ML5TA, cells. The mice containing ML5TA cells were sacrificed at 43 weeks, and those with MCV152 were sacrificed at 47 weeks. For calculation of the total tumor volume per group, we measured tumor nodules bidirectionally. In a separate study, we measured levels of circulating testosterone, estradiol, and progesterone in the serum of intact and castrate male athymic NCr/nu mice. Testosterone levels were confirmed to be 10-fold higher in the intact mice (vs. the castrated mice), with no clear differences in estradiol or progesterone levels.
Phase 2 clinical trial
The University of Arizona Cancer Center multidisciplinary HR cancer genetics clinic opened in 2004, first focusing on women at HR for breast cancer and OC. Potential participants for this phase 2 trial were given the opportunity to participate either in the treatment arm or in the control arm, or to decline to participate. All participants completed a reproductive/hormone history questionnaire. Those in the treatment arm received 125 mg/day of flutamide orally for 6 weeks, until just before their surgery date. Experience has shown that HR women who are requesting RRSO will not wait longer than 6 weeks for the surgery. Indeed, many HR patients chose the control arm because they did not accept such a delay. This HR control cohort along with an additional LR control cohort in this trial was the subject of a previous report (20); the LR control cohort was not analyzed in our current study.
HR patients were eligible for the trial if they were ≥ 18 years of age, elected to undergo RRSO, and agreed to use a nonhormonal means of contraception before surgery. The criteria for HR are defined below. Additional inclusion criteria included adequate complete blood count, as well as adequate hepatic and renal function. Exclusion criteria included current liver disease, pregnancy or lactation, current use of hormone therapy, active treatment for cancer, or participation in another experimental drug study.
Flutamide, an agent that competes with testosterone for binding to AR, has been used most commonly in the treatment of prostate cancer. Generally, the dose used to treat prostate cancer patients is significantly higher than the 125 mg/day used in our current study. Most adverse events seen are related to liver toxicity. In women, lower doses of flutamide (≤ 250 mg/day) have successfully been used in the treatment of hirsutism; in one study (30), 9% of women had elevated aspartate transaminase (AST) and alanine transaminase (ALT) levels within the first 60 days of 250 mg/day therapy. After we completed our current study, a 10-year study was published of low (125 mg/daily) and ultralow (62.5 mg/daily) doses of flutamide on liver function in 203 young women with hyperandrogenism (31) showing that circulating AST/ALT levels increased in 9.4% during the first year of treatment, with 84% showing mild elevation only.
The University of Arizona institutional review board approved our current study, which we conducted in accordance with all applicable institutional and federal guidelines. During our study period (August 29, 2006, through May 20, 2011), we analyzed tissues from a total of 59 HR women. Included were 3 HR control patients who consented to donate samples to our tumor biorepository before undergoing RRSO. Of the 59 HR patients, 12 took the study drug for 6 weeks before undergoing RRSO, leaving 47 HR patients in the control group. We abstracted relevant clinical information from their medical records, such as menopausal status; body mass index; personal cancer history; genetic mutation status for the BRCA1, BRCA2, and Lynch syndrome mutations; and family history of cancer.
Criteria for HR
Women at HR for OC carried a BRCA1 or BRCA2 deleterious mutation (47.5%), a Lynch syndrome mutation (1 case), and/or defined by a family history of: ≥ 1 first-degree relative with epithelial OC (24%), ≥ 1 first-degree female relative with breast cancer when ≤ 40 years old (40.7%), > 1 first-degree female relative with breast cancer when ≤ 50 years old (30.5%), male relative with breast cancer (2 cases) and/or family history of breast cancer and OC (92%). The majority (57.6%) of the HR patients also had a personal history of breast cancer, with a median age at diagnosis of 43 years (range, 26 to 60 years). The BRCA mutation distribution favored BRCA2 over BRCA1, representative of our HR clinic population (32).
Pathologic analysis
All patients had undergone removal of at least 1 ovary and 1 fallopian tube. All fallopian tubes and ovaries chosen for our immunohistochemical analysis were morphologically unremarkable. In addition, 83% of the HR patients underwent a concomitant hysterectomy as part of their risk-reducing surgery.
The University of Arizona procedure for pathologic analysis of fallopian tubes and ovaries from HR women begins with a complete submission of the tissues, with sections of the tubal fimbria taken by optimizing the surface area of the tubal fimbria by the SEE-FIM protocol (33, 34). One of us (WZ) examined the tissues, paying attention to serous intraepithelial carcinoma and dysplasia within the fallopian tube (mainly tubal fimbria), as well as to endosalpingiosis within the ovary. P53 staining to search for p53 signatures was performed in the fallopian tube and in the ovary.
Immunohistochemical analysis
We mounted 5-μm sections of ovary and fallopian tube on slides and subjected them to deparaffinization, dehydration, quenching in methanol, rehydration, and antigen retrieval in 10-mM citrate buffer (pH 7.0) under high pressure and at high temperature. Staining for all antibodies (Abcam ab19391, for ErbB4; ab61137, for CSF-1R; and ab9693, for CSF-1) was first optimized on control tissues, on review by one of us (WZ). Slides were blocked with serum and stained with primary antibody, then incubated overnight. Negative controls included the absence of the primary antibody. A biotinylated secondary antibody was then added and incubated for an hour the next day. Afterwards, the slides were stained with an avidin-biotin enzyme complex (ABC Kit, Vector Labs) to increase the staining specificity. Slides were then stained with a solution of 3, 3′-diaminobenzidine (DAB) (Vector Labs), counterstained in hematoxylin, dehydrated, and permanently mounted. Slides were scored by two of us (NA and WZ), both blinded to the treatment group. The intensity of stain (0-3) and the percentage of area stained (0-100%)were multiplied to yield a product (total score), as previously described (20-22). In addition to cytoplasmic stains, nuclear stains were also scored when present, specifically in reference to the nuclear staining for ErbB4. Within the ovary and fallopian tube (ampulla and fimbria), we separately scored epithelium and stroma. Lastly, we specifically scored the biomarker stains in ovarian endosalpingiosis.
Statistical analysis
To summarize patients' demographic characteristics, we used descriptive statistics, depending on the underlying distribution. To compute correlations between quantitative variables, we used the Kendall's Τ rank correlation; between qualitative variables, the Fisher exact test. To compute differences between biomarkers for flutamide-treated women and controls, we used the Wilcoxon rank sum test.
To distinguish between the flutamide-treated women and controls, we developed logistic regression models using the above-mentioned biomarkers and patient characteristics. To check the assumptions of the models, we used univariate and multivariate modeling. The result of our final logistic model was used to create the ROC curve (to estimate the precision of the model) and also to compute the best estimates for sensitivity and specificity. The area under the curve (AUC) is a reliable estimate of the predictive power of the model. An AUC value of ≥ 0.8 represents good predictive power.
We defined the statistical significance as P < 0.05. All computations were performed using SAS 9.3 (Cary, NC).
Results
Preclinical in vitro studies
We found that DHT significantly stimulated motility, by 1.6-fold, of MCV152 cells toward fibronectin (Figure 1A, P = 0.001). In fact, with DHT stimulation, the degree of motility matched that of the Hey OC cells. These results were validated in both NOSE.1 cells (in which DHT significantly stimulated motility, by 1.6 to 2.2-fold, in different experiments), and in ML5TA cells (in which DHT stimulated a small, by 1.2-fold, but significant increase in motility).
Figure 1.
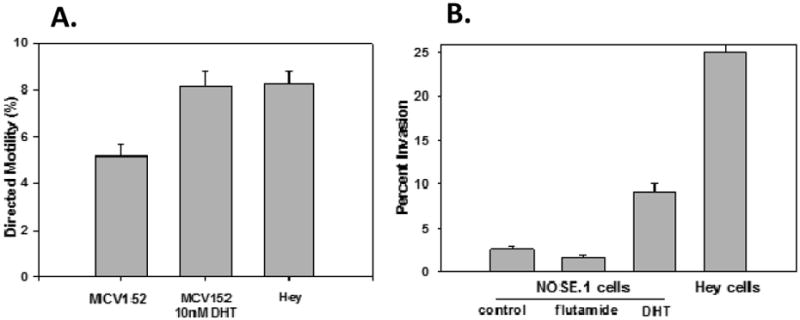
A) Androgen treatment increased the directed motility of MCV152 cells toward fibronectin. MCV152 cells with or without 10 nM 5α-dihydrotestosterone (DHT) for 66h, were studied for directed motility compared to Hey ovarian cancer cells. DHT treatment led to a significant increase in cellular motility compared to vehicle control treated cells (5.16 ± 0.53 vs. 8.16 ± 0.65, P= 0.001), and matched the motility of the invasive Hey cells (8.24 ± 0.59). Each bar represents the mean value of 4separate experiments. Error bars represent +/- standard error of the mean (SEM).
B) Androgen treatment increased, while the antiandrogen flutamide decreased, the invasiveness of NOSE.1 cells (P < 0.001). NOSE.1 cells were treated with vehicle (control), 5uM flutamide, or 10 nM DHT for 48h during the invasion assay. Treatment of NOSE.1 cells with DHT led to a significant increase in ability to invade through extracellular matrix by 3.6-fold compared to control (2.54 ± 0.32 vs. 9.20 ± 0.82). In contrast, flutamide treatment led to an inhibition in invasiveness (by 0.68-fold), vs. control. Hey ovarian cancer cells remained much more invasive (by 2.7-fold) than the DHT-treated NOSE.1 cells. Error bars represent +/- SEM.
DHT increased invasiveness of NOSE.1 cells by 3.6-fold (as compared with controls), as shown in a representative experiment (Figure 1B, P < 0.001). This significant finding was validated in MCV152 cells (1.54-fold). Figure 1B also shows that the antiandrogen flutamide inhibited invasiveness through an extracellular matrix barrier: a decrease of 0.68-fold. The invasive and virulent Hey cells still retained a high degree of invasiveness, higher by 2.7-fold as compared with the DHT-treated NOSE.1 cells.
Adhesiveness to extracellular matrix of MCV152 cells was not affected by DHT treatment. Treatment of MCV152 cells with DHT for 66 hours did not lead to any difference in adhesiveness, as compared with vehicle-treated cells (OD585: 0.431 ± 0.042 vs. 0.438 ± 0.037, respectively). Hey cells retained a superior ability to adhere to matrix components (OD585: 0.649 ± 0.011, P = 0.0023).
In light of our in vitro findings suggesting that DHT increased parameters of transformation, and the evidence in the literature supporting CSF-1 enhancement of tumorigenicity and metastasis in ovarian cancer (23, 24), we next studied the effect of 10nM DHT or etiocholanolone (controls) for 66 hours on secreted CSF-1 levels in the conditioned medium of NOSE.1 cells. DHT increased CSF-1 levels increased by 2.9 fold, from 1.4 ± 0.25 pg/ml to 4.1 ± 1.1 pg/ml; P = 0.037). Similarly, DHT increasedCSF-1 levels in MCV152 cells by 2.0 fold.
Preclinical in vivo studies
We next studied whether serous ovarian cells had the capacity to form tumors in male mice. A previous report had suggested that such cells may not be tumorigenic in female mice (27). It is understood that male mice have significantly higher androgens than do female mice, and thus these experimental results may not be directly translatable to women. Yet we reasoned that a clear effect on tumorigenicity should be able to be demonstrated in a model of high androgen exposure. Figure 2A shows that both MCV152 and ML5TA cells were tumorigenic up to 60% of the time in intact male nude mice. Tumor growth of ML5TA cells appeared to lag, as compared with MCV 152 cells (P = 0.057). However, by 30 weeks, both cell lines evinced a similar degree of tumorigenicity. Microscopic examination of tumors generated from both cell lines confirmed a moderately differentiated invasive cancer, similar to typical human serous OC (read by WZ).
Figure 2.
A) Ovarian serous epithelial cells are tumorigenic in male nude mice.
B) Extent of tumor burden in intact and castrate male nude mice, from ovarian serous epithelial cells. The total tumor volume was dramatically less, by 24-fold, when the cells were implanted in castrate male mice.
We then determined, in a separate experiment, the effect of androgen ablation on the extent of tumor burden from ML5TA and MCV152 cells in male nude mice. When we analyzed the ML5TA cells for the number of tumors per mouse, the castrate male mice averaged 1.1 tumors each, while the intact male mice averaged 3.2 tumors each. When we analyzed the total tumor volume as a measure of the extent of tumor burden (Figure 2B), the castrate male group had a volume of 1270 mm3, while the intact male group had a volume of 30521 mm3. This represents a dramatic 24-fold decrease in tumor burden with androgen ablation. The results with MCV152 cells paralleled those with ML5TA cells, with a 16.4-fold decrease in the total tumor volume between intact and castrate males.
We found a statistically significant difference between the groups in the total tumor volume per mouse when 1 clear outlier mouse was removed from the analysis (P = 0.01); otherwise the results were notable (P = 0.1). Thus, it appears that castrate male nude mice with approximately 10-fold less circulating androgens had a lag in tumor latency, along with fewer tumors and a reduced total tumor volume per mouse, as compared with intact male nude mice.
Phase 2 clinical trial
In our patients, we found that flutamide at 125 mg/day for 6 weeks was well tolerated, as expected. Two patients (16.7%) experienced grade 1 nausea or noticed a change in the color of their urine. But we observed no abnormalities in liver function test results.
We found no significant demographic differences between the 2 groups (flutamide vs. controls) except that a personal history of breast cancer was more common in the controls (P < 0.001) (Table 1).
Table 1. Patient characteristics.
| Flutamide (n = 12) | Control (n = 47) | ||
|---|---|---|---|
| n (%) | n (%) | P | |
| Age (years) | NS | ||
| Mean ± SD (range) | 45.2±8.4 (36-58) | 46.9±9.1 (27-66) | |
| Menopause | NS | ||
| Yes | 3 (25) | 20 (43) | |
| No | 9 (75) | 26 (57) | |
| BMI | NS | ||
| Mean ± SD (range) | 26.2±7.1 (19.3-45.0) | 28.9±7.4 (20.1-56.2) | |
| Number of Pregnancies | NS | ||
| 0 | 2 (16) | 6 (13) | |
| 1-3 | 9 (74) | 27 (59) | |
| ≥4 | 1 (8) | 13 (28) | |
| Hormone Replacement: | |||
| Estrogen only | NS | ||
| Ever | 1 (20) | 4 (11) | |
| Never | 4 (80) | 30 (88) | |
| Progesterone only | NS | ||
| Ever | 1 (20) | 0 (0) | |
| Never | 4 (80) | 34 (100) | |
| Combined | NS | ||
| Ever | 2 (40) | 2 (6) | |
| Never | 3 (60) | 30 (93) | |
| Oral Contraceptive Use | NS | ||
| Ever | 7 (58) | 33 (76) | |
| Never | 5 (42) | 10 (23) | |
| Endometriosis | NS | ||
| Yes | 1 (8) | 2 (4) | |
| No | 11 (92) | 45 (96) | |
| History of Breast Cancer | <0.001 | ||
| Yes | 1 (8) | 33 (70) | |
| No | 11 (92) | 14 (30) | |
| BRCA Positive | NS | ||
| Yes | 9 (75) | 19 (40) | |
| No | 2(17) | 19 (40) | |
| Not tested | 1(8) | 9(19) | |
| History of Tubal Ligation/Hysterectomy | NS | ||
| Yes | 4 (33) | 14 (31) | |
| No | 8 (67) | 31 (69) |
BMI, body mass index; NS, not significant; SD, standard deviation
The effect of flutamide on CSF-1 and ErbB4 was dramatic (Table 2). Its predominant effect was in the ovary, either in the stroma or in the epithelial cells comprising endosalpingiosis. In the ovarian stroma, flutamide significantly decreased expression of both CSF-1 (P = 0.0006) and ErbB4 (P < 0.0001), with no significant correlation between the 2 biomarkers. CSF-1 in ovarian stroma was correlated with CSF-1 in the fimbria epithelium in the flutamide-treated group only (P = 0.003). In the ovarian epithelium, flutamide also significantly decreased the expression all 3 biomarkers, but less strongly than observed in the ovarian stroma: CSF-1 (P = 0.01), ErbB4 (P = 0.006), and CSF-1R (P = 0.04). In ovarian endosalpingiosis, flutamide also significantly decreased the expression of all 3 biomarkers: CSF-1 (P = 0.01), ErbB4 (P = 0.005), and CSF-1R (P = 0.009). In general, the effect of flutamide on CSF-1R was less dramatic, except in endosalpingiosis (P = 0.009).Our analysis of the fallopian tube for expression of these biomarkers did not yield such significant findings. Representative examples of the effect of flutamide on immunohistochemical staining for those biomarkers in the relevant tissues are shown in Figure 3.
Table 2. Immunohistochemical scores.
| Adnexal Site | Biomarker | Flutamide (n = 12) vs. Control (n = 47) (P*) | BRCA Mutation Carriers Only: Flutamide (n = 9) vs. Control (n = 19) (P) |
|---|---|---|---|
|
| |||
| Ampulla Epithelium | CSF-1 | NS | 0.031 |
| ErbB4 | NS | NS | |
| CSF-1R | NS | NS | |
|
| |||
| Ampulla Stroma | CSF-1 | NS | NS |
| ErbB4 | NS | NS | |
| CSF-1R | NS | NS | |
|
| |||
| Fimbria Epithelium | CSF-1 | NS | NS |
| ErbB4 | NS | NS | |
| CSF-1R | NS | NS | |
|
| |||
| Fimbria Stroma | CSF-1 | 0.01 | NS |
| ErbB4 | NS | NS | |
| CSF-1R | NS | NS | |
|
| |||
| Ovarian Endosalpingiosis | CSF-1 | 0.012 | 0.051 |
| ErbB4 | 0.005 | 0.010 | |
| CSF-1R | 0.009 | 0.005 | |
|
| |||
| Ovarian Epithelium | CSF-1 | 0.010 | 0.010 |
| ErbB4 | 0.006 | 0.015 | |
| CSF-1R | 0.037 | NS | |
|
| |||
| Ovarian Stroma | CSF-1 | 0.0006 | 0.003 |
| ErbB4 | <0.0001 | 0.001 | |
| CSF-1R | 0.010 | NS | |
Statistical significance was defined as P < 0.05.
NS, not significant
Figure 3.
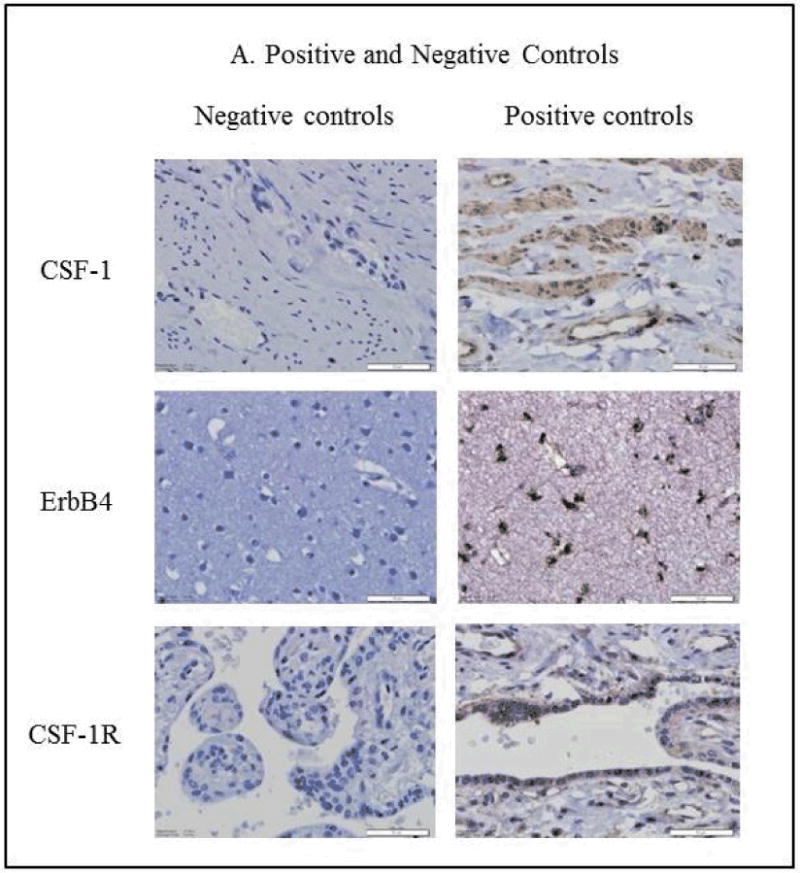
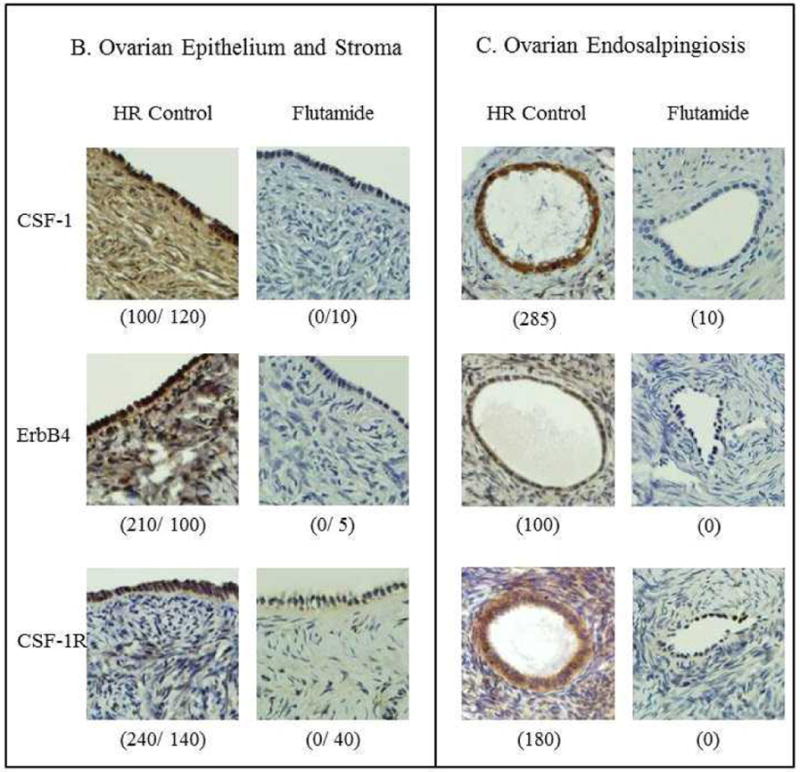
A) Shown are positive and negative immunohistochemical controls for CSF-1 (human cervical stromal cells), ErbB4 (cerebral cortex), and CSF-1R (placenta) expression.
Representative examples of CSF-1, ErbB4, and CSF-1R expression in HR control and flutamide groups are shown in B) ovarian stroma and epithelium, with total scores for staining in parentheses (epithelium/stroma) and in C) ovarian endosalpingiosis, with total scores for staining in parentheses.
When we restricted the biomarker analysis of the effect of flutamide to women who carry a BRCA mutation, our important findings remained applicable in this subset of HR patients. The effect of flutamide on CSF-1 and ErbB4 staining is still highly significant in the ovarian stroma; as is its effect on all 3 biomarkers in ovarian endosalpingiosis (Table 2).
Of the 59 patients in our HR cohort, 45 (76.3%) had ovarian endosalpingiosis. We described in our previous report (20) an association between endosalpingiosis and both HR and BRCA carrier status. In our current study, we found that the epithelial cells comprising ovarian endosalpingiosis were particularly sensitive to the effect of flutamide. Expression of all 3 biomarkers studied underwent significant downregulation after flutamide treatment of only 6 weeks (Figure 3). We also observed in our previous report (20) that nuclear ErbB4 staining, which may represent a component of ErbB4 signaling, was associated with both HR and BRCA carrier status. We had observed nuclear ErbB4 staining mainly in the epithelial cells of the fimbria and of ovarian endosalpingiosis, but in our current study's HR cohort, nuclear ErbB4 was not sensitive to flutamide.
Our logistic regression model clearly distinguished between flutamide-treated women and controls (P < 0.0001). The most powerful variables on univariate analysis proved to be ErbB4 in ovarian stroma, with 82% sensitivity and 89% specificity (AUC = 0.94) and CSF-1 in ovarian stroma, with 82% sensitivity and 78% specificity (AUC = 0.84). When we restricted our analysis to BRCA carriers, ErbB4 and CSF-1 in ovarian stroma retained their strong predictive value (AUC = 0.95 and AUC = 0.85, respectively). In HR patients, a combined model of CSF-1 and ErbB4 expression in ovarian stroma achieved an impressive 100% sensitivity and 97% specificity (AUC = 0.99) (Figure 4).
Figure 4.
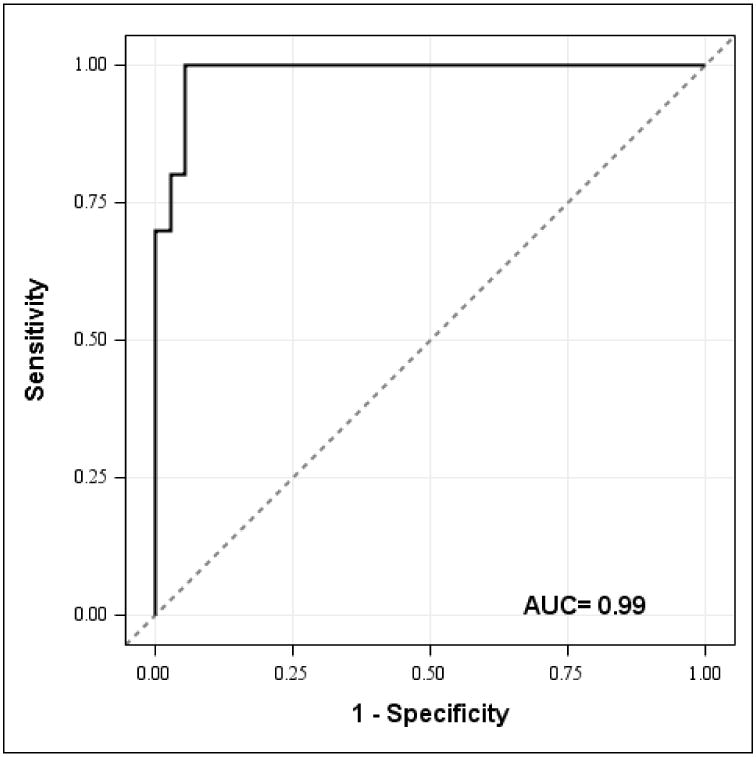
This receiver operating characteristic (ROC) curve resulting from our logistic regression model of CSF-1 and ErbB4 expression in ovarian stroma emphasizes the clear effect of flutamide in patients. The dashed diagonal line represents no logistic model effect. The ROC curve plots sensitivity which represents the true positive rate of all flutamide-treated women vs. (1-specificity) the rate of false positives of all flutamide-treated women at various thresholds of the logistic regression model. The area under the curve (AUC) is an indicator of the precision of the model. The point closest to the upper left corner defines the optimal cut point for the prediction. The model of CSF-1 and ErbB4 expression in the ovarian stroma as represented by the ROC curve demonstrates a sensitivity of 100% and a specificity of 97% (C = 0.99) when differentiating between flutamide and control patients.
Our pathologic analysis found that none of the fallopian tubes had serous intraepithelial carcinoma. We observed the P53 signature in 10.5% of the HR control patients with BRCA mutations, and only in the fallopian tube fimbria. We did not see a P53 signature in the flutamide-treated tissues. Because of the significance of CSF-1 expression in the ovarian stroma, CD34 co-staining was studied in 3 patients. We found that the stromal fibroblasts co-stain for both CD34 and CSF-1, while endothelial cells stain for CD34, and focally for CSF-1.
Discussion
The effect of a short course of the antiandrogen flutamide on the selected tissue biomarkers in these HR patients was dramatic. Our ROC analysis most clearly differentiated, with 100% sensitivity and 97% specificity, between adnexal tissues that were versus were not sensitive to the effect of flutamide. Both our preclinical in vitro and in vivo biologic data support the contribution of androgens in the environment on transformation of ovarian serous epithelial cells. Our collective data warrants a follow-up study to determine whether flutamide, or another antiandrogen, can be used as an effective chemopreventive agent for pelvic serous carcinoma.
An important criterion for chemoprevention is the clinical tolerability of the agent. We used a well-studied and relatively weak antiandrogen, but one whose toxicity at the dose given was predicted to be very low. We showed that flutamide in HR women over a short duration was tolerated extremely well. Based on subsequent long-term follow-up data from the hyperandrogenism literature (31), it appears that flutamide can be tolerated at this low dose for several years in most women. Its use for only 6 weeks still allowed us to identify potential tissue biomarkers of androgen action. Newer, more potent anti-AR agents are also available for future study. In ovarian cancer, one such agent (bicalutamide) has been evaluated as a type of maintenance therapy (35), but not as a chemopreventive agent.
We previously found that CSF-1 in ovarian epithelium, and ErbB4 in ovarian stroma, were significantly associated with HR status, when compared to a LR control cohort (20). Our current study now adds the finding that flutamide has a type of field effect on those 2 tissue biomarkers—an effect that is not restricted to ovarian epithelium, stroma, or endosalpingiosis. We noted a significant effect of flutamide on downregulation of CSF-1 and ErbB4 in ovarian epithelium, and of ErbB4 in ovarian stroma, an effect that we construe as a reversal of expression of the biomarkers associated with HR or BRCA carrier status.
However, we also observed the emphasis of flutamide on altering the expression of CSF-1 and ErbB4 in the ovarian stromal (versus epithelial) compartment, a highly significant finding that was the basis of our impressive ROC results. We interpret these flutamide-induced changes (which occurred widely within different regions of the ovary, as well as specifically in the stroma) to suggest a microenvironment effect on the adnexae. We predict that apparent downregulation of global androgen action may be inhibitory for development of pelvic serous carcinomas. Each of the 3 genes appear to be regulated by different mechanisms, and their downregulation by flutamide is unlikely to be primarily a direct antiandrogen effect, although we did observe a modest effect, by 2- to 3-fold, of DHT on stimulation of secreted CSF-1 levels in vitro.
In our previous report, we found an association between ovarian endosalpingiosis and both HR and BRCA carrier status (20). Also in that previous report, we noted an association, particularly in ovarian endosalpingiosis, between biomarker changes (ErbB4 and CSF-1R) and, specifically, BRCA carrier status (20). In our current study, we observed dramatic inhibition by flutamide of expression of all 3 biomarkers (CSF-1, CSF-1R, and ErbB4) in the epithelial cells comprising ovarian endosalpingiosis. Ovarian endosalpingiosis has been suggested to be a precursor to low-grade pelvic serous carcinomas (36, 37). HR and BRCA status, however, is most clearly associated with high-grade pelvic serous carcinomas. The effect of flutamide on biomarker expression in endosalpingiosis in our HR patients strengthens the likelihood that ovarian endosalpingiosis may be a latent precursor to pelvic serous cancers. Our data also add food for thought when considering the possibility that low-grade serous cancers may be related to HR and BRCA status.
In summary, we observed a dramatic effect of the antiandrogen flutamide on select biomarker expression in the adnexae of HR women. CSF-1 and ErbB4 expression in the ovarian stroma was highly sensitive and specific for flutamide sensitivity. Flutamide also reversed biomarker changes (ErbB4 in ovarian stroma and CSF-1 in ovarian epithelium) that we previously found to be most associated with HR status (20). Future studies of this cohort should include a global approach to biomarker identification, which will undoubtedly lead to identification of many more androgen-sensitive biomarkers in the epithelium and stroma of the fallopian tube and ovary, as well as in ovarian endosalpingiosis. In particular, our findings strongly warrant a follow-up study in HR women to determine whether flutamide (or another antiandrogen) can be used as an effective chemopreventive agent for pelvic serous carcinoma.
Acknowledgments
We greatly appreciate the contributions of Janice L. Cohen, Katie Lux, Nancy L. Folk, Nathalie Bonafe, Eugene P. Toy, Ina Menzl, Cynthia L. David, and HoHyung Woo.
Grant Support: This research was supported by National Institutes of Health (NIH) grant CA60665 (to S. Chambers), by NIH grant HL007479 sponsored Medical Student Research Program (to C. Gruessner), by University of Arizona Cancer Center Support grant NIH CA023074 (to S. Chambers), a grant from the Ovarian Cancer Research Fund (to S. Chambers), and a Better than Ever Women's Cancers Research grant (to S. Chambers). The work was also supported by the University of Arizona Cancer Center Clinical Research and Experimental Mouse Shared Services.
Footnotes
Conflicts of Interest: None of the authors have any conflicts to disclose.
The clinical trial (NCT00699907) was registered at the http://ClinicalTrials.gov website.
References
- 1.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–8. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 2.Papadatos-Pastos D, Dedes KJ, de Bono JS, Kaye SB. Revisiting the role of antiandrogen strategies in ovarian cancer. Oncologist. 2011;16:1413–21. doi: 10.1634/theoncologist.2011-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AJ, Karlan BY. Androgens and epithelial ovarian cancer: What's the connection? Cancer Biol Ther. 2008;7:1712–6. doi: 10.4161/cbt.7.11.7054. [DOI] [PubMed] [Google Scholar]
- 4.Hage JJ, Dekker JJ, Karim RB, Verheijen RH, Bloemena E. Ovarian cancer in female-to-male transsexuals: report of two cases. Gynecol Oncol. 2000;76:413–5. doi: 10.1006/gyno.1999.5720. [DOI] [PubMed] [Google Scholar]
- 5.Olsen CM, Green AC, Nagle CM, Jordan SJ, Whiteman DC, Bain CJ, et al. Australian Cancer Study Group (Ovarian Cancer) and the Australian Ovarian Cancer Study Group. Epithelial ovarian cancer: testing the ‘androgens hypothesis’. Endocr Relat Cancer. 2008;15:1061–8. doi: 10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]
- 6.Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA. 1995;274:1926–30. [PubMed] [Google Scholar]
- 7.Tworoger SS, Lee IM, Buring JE, Hankinson SE. Plasma androgen concentrations and risk of incident ovarian cancer. Am J Epidemiol. 2008;167:211–8. doi: 10.1093/aje/kwm278. [DOI] [PubMed] [Google Scholar]
- 8.Li AJ, Lerner DL, Gapuzan ME, Karlan BY. AIB1 polymorphisms predict aggressive ovarian cancer phenotype. Cancer Epidemiol Biomarkers Prev. 2005;14:2919–22. doi: 10.1158/1055-9965.EPI-05-0540. [DOI] [PubMed] [Google Scholar]
- 9.Kim SC, Ju W, Mahavni V, Geisler JP, Buller RE. CAG repeat length in exon 1 of the androgen receptor gene is related to age of diagnosis but not germ line BRCA1 mutation status in ovarian cancer. Int J Gynecol Cancer. 2006;16:190–4. doi: 10.1111/j.1525-1438.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 10.Schildkraut JM, Murphy SK, Palmieri RT, Iversen E, Moorman PG, Huang Z, et al. Trinucleotide repeat polymorphisms in the androgen receptor gene and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:473–80. doi: 10.1158/1055-9965.EPI-06-0868. [DOI] [PubMed] [Google Scholar]
- 11.Ligr M, Patwa RR, Daniels G, Pan L, Wu X, Li Y, et al. Expression and function of androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer. PLoS One. 2011;6:e26250. doi: 10.1371/journal.pone.0026250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motamed-Khorasani A, Jurisica I, Letarte M, Shaw PA, Parkes RK, Zhang X, et al. Differentially androgen-modulated genes in ovarian epithelial cells from BRCA mutation carriers and control patients predict ovarian cancer survival and disease progression. Oncogene. 2007;26:198–214. doi: 10.1038/sj.onc.1209773. [DOI] [PubMed] [Google Scholar]
- 13.Yi X, Zhou Y, Zheng W, Chambers SK. HuR expression in the nucleus correlates with high histological grade and poor disease-free survival in ovarian cancer. Aust NZ J Obstet Gynaecol. 2009;49:93–8. doi: 10.1111/j.1479-828X.2008.00937.x. [DOI] [PubMed] [Google Scholar]
- 14.Chambers SK, Ivins CM, Carcangiu ML. Urokinase-type plasminogen activator in epithelial ovarian cancer: a poor prognostic factor, associated with advanced stage. Int J Gynecol Cancer. 1998;8:242–250. doi: 10.1002/(sici)1097-0215(19981023)79:5<449::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Cardillo MR, Petrangeli E, Aliotta N, Salvatori L, Ravenna L, Chang C, et al. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J Exp Clin Cancer Res. 1998;17:231–7. [PubMed] [Google Scholar]
- 16.Chadha S, Rao BR, Slotman BJ, van Vroonhoven CC, van der Kwast TH. An immunohistochemical evaluation of androgen and progesterone receptors in ovarian tumors. Hum Pathol. 1993;24:90–5. doi: 10.1016/0046-8177(93)90067-q. [DOI] [PubMed] [Google Scholar]
- 17.Elattar A, Warburton KG, Mukhopadhyay A, Freer RM, Shaheen F, Cross P, et al. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol Oncol. 2012;124:142–7. doi: 10.1016/j.ygyno.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Tumolo S, Rao BR, van der Burg ME, Guastalla JP, Renard J, Vermorken JB. Phase II trial of flutamide in advanced ovarian cancer: an EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer. 1994;30:911–4. doi: 10.1016/0959-8049(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an androgen responsive tissue. Br J Cancer. 2002;86:879–85. doi: 10.1038/sj.bjc.6600154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruessner C, Gruessner A, Glaser K, AbuShahin N, Laughren C, Zheng W, et al. Biomarkers and endosalpingiosis in the ovarian and tubal microenvironment of women at high-risk for pelvic serous carcinoma. Am J Cancer Res. 2014;4:61–72. [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers SK, Kacinski BM, Ivins CM, Carcangiu ML. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res. 1997;3:999–1007. [PubMed] [Google Scholar]
- 22.Toy EP, Chambers JT, Kacinski BM, Flick MB, Chambers SK. The activated macrophage colonystimulating factor (CSF-1) receptor as a predictor of poor outcome in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;80:194–200. doi: 10.1006/gyno.2000.6070. [DOI] [PubMed] [Google Scholar]
- 23.Toy EP, Azodi M, Folk NL, Zito CM, Zeiss CJ, Chambers SK. Enhanced ovarian cancer tumorigenesis and metastasis by the macrophage colony stimulating factor (CSF-1) Neoplasia. 2009;11:136–44. doi: 10.1593/neo.81150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429–40. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pejovic T, Pande NT, Mori M, Mhawech-Fauceglia P, Harrington C, Mongoue-Tchokote S, et al. Expression profiling of the ovarian surface kinome reveals candidate genes for early neoplastic changes. Transl Oncol. 2009;2:341–9. doi: 10.1593/tlo.09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonafe N, Gilmore-Hebert M, Folk NL, Azodi M, Zhou Y, Chambers SK. Glyceraldehyde-3-phosphate dehydrogenase binds to the AU-rich 3′ UTR of CSF-1 mRNA in human ovarian cancer cells: possible role in CSF-1 post-transcriptional regulation and tumor phenotype. Cancer Res. 2005;65:3762–71. doi: 10.1158/0008-5472.CAN-04-3954. [DOI] [PubMed] [Google Scholar]
- 27.Luo MP, Gomperts B, Imren S, DeClerck YA, Ito M, Velicescu M, et al. Establishment of long-term in vitro cultures of human ovarian cystadenomas and LMP tumors and examination of their spectrum of expression of matrix-degrading proteinases. Gynecol Oncol. 1997;67:277–84. doi: 10.1006/gyno.1997.4880. [DOI] [PubMed] [Google Scholar]
- 28.Yu VM, Marion CM, Austria TM, Yeh J, Schönthal AH, Dubeau L. Role of BRCA1 in controlling mitotic arrest in ovarian cystadenoma cells. Int J Cancer. 2012;130:2495–504. doi: 10.1002/ijc.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–76. [PubMed] [Google Scholar]
- 30.Venturoli S, Marescalchi O, Colombo FM, Macrelli S, Ravaioli B, Bagnoli A, et al. A prospective randomized trial comparing low-dose flutamide, finasteride, ketoconazole, and cyproterone acetateestrogen regimens in the treatment of hirsutism. J Clin Endocrinol Metab. 1999;84:1304–10. doi: 10.1210/jcem.84.4.5591. [DOI] [PubMed] [Google Scholar]
- 31.Bruni V, Peruzzi E, Dei M, Nannini S, Seravalli V, Sisti G, et al. Hepatotoxicity with low- and ultralowdose flutamide: a surveillance study on 203 hyperandrogenic young females. Fertil Steril. 2012;98:1047–52. doi: 10.1016/j.fertnstert.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Nelson-Moseke AC, Jeter JM, Cui H, Roe DJ, Chambers SK, Laukaitis CM. An unusual BRCA mutation distribution in a high risk cancer genetics clinic. Fam Cancer. 2013;12:83–7. doi: 10.1007/s10689-012-9581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng W, Fadare O. Fallopian tube as main source for ovarian and pelvic (non-endometrial) serous carcinomas. Int J Clin Exp Pathol. 2012;5:182–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 35.Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2458–66. doi: 10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–99. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 37.Kurman RJ, Vang R, Junge J, Hannibal CG, Kjaer SK, Shih IeM. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–14. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]