Abstract
Extracorporeal photopheresis (ECP) and the purine analog pentostatin exert potent immunomodulatory effects. We evaluated the use of these treatment modalities to prevent GVHD in a canine model of unrelated dog leukocyte antigen mismatched hematopoietic cell transplantation (HCT), after conditioning with 920 cGy total body irradiation (TBI). We have shown previously in this model that 36/40 dogs given methotrexate (MTX) alone as postgrafting immunosuppression engrafted and that 25 of 40 dogs had severe GVHD and median survival of 21 days. In the current study, 9 dogs received conditioning with 920 cGy TBI and postgrafting MTX either with ECP on days −2 to −1 alone (n=5) or ECP on days −6 and −5 combined with 2 doses of pentostatin (days −4 to −3) (n=4). Seven of 9 dogs achieved engraftment. Six dogs developed severe acute GVHD (4 in the group with ECP alone and 2 with Pentostatin and ECP). We failed to demonstrate a positive impact of ECP and pentostatin for the prevention of GVHD compared to historical control dogs.
Keywords: graft versus host disease, extracorporeal photopheresis, hematopoietic cell transplantation
INTRODUCTION
The degree of major and minor histocompatibility antigen mismatch between donor and recipient in hematopoietic cell transplantation (HCT) is the most important risk factor for graft-versus-host-disease (GVHD).1 Immunocompetent donor T-cells play an essential role in the pathogenesis of GVHD. GVHD can be prevented by graft T-cell depletion but at the expense of a higher risk of graft rejection and relapse.2 Despite the development of effective immunosuppressive drugs and their successful use in HLA-matched related and unrelated HCT, GVHD remains the major cause for morbidity and mortality in allogeneic HCT.1 Clinical trials of HLA-mismatched allogeneic HCT are still complicated by an unacceptable high risk of GVHD and rejection,3,4 particularly if a nonmyeloablative conditioning is used.5 New strategies for conditioning and postgrafting immunosuppression to reduce the intensity and severity of GVHD are therefore warranted.
Extracorporeal photopheresis (ECP) was initially used to successfully treat patients with cutaneous T-cell lymphoma 6. The immunosuppressive effects of ECP have also been used in patients with autoimmune disorders, solid organ rejection and GVHD.7–12 Pentostatin is a purine analog which induces T-cell apoptosis through adenosine deaminase inhibition. Used as part of the conditioning regimen in HCT pentostatin can produce prolonged host T-cell depletion preventing graft rejection and GVHD.13–15,15
Miller et al. developed a conditioning regimen combining ECP, pentostatin and 600 cGy total body irradiation (TBI) for human leukocyte antigen (HLA)-identical and non-identical (5/6 HLA match) allogeneic HCT. Using cyclosporine (CSP) and methotrexate (MTX) as postgrafting immunosuppression they report a low incidence of acute (grade II to IV of 9%) and chronic GVHD (43%). These rates seem low compared to the reported incidence to be expected with a similar regimen without ECP and pentostatin.16,17 Both pentostatin and ECP result in T-cell and host DC depletion and a shift of the remaining DC and T-cell population to a tolerogenic DC2 and T-regulatory population which may lead to the observed low incidence of GVHD.
In order to elucidate the potential role of ECP or pentostatin either individually or in combination on reducing the incidence of GVHD we report on our results using a well-established dog model of dog leukocyte antigen (DLA)-nonidentical marrow grafts.
MATERIALS AND METHODS
Dogs and DLA typing
Litters of beagles, harriers, Walker hounds, and crossbred dogs were used in this study as described previously.18 Dogs weighed from 13.5 to 23 (median, 14.4) kg and were 18 to 31 (median, 27) months old. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The study was performed in accordance with the principles outlined in the Guide for Laboratory Animal of Sciences, National Research Council. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care. In group A, donors and recipients were unrelated and were obtained from different breeding colonies or were of different pedigrees for at least five generations. DLA-nonidentical littermates were selected on the basis of identity for highly polymorphic MHC class I and class II microsatellite markers and identity for DLA-DRB1 alleles as determined by direct sequencing.19–21
Marrow transplantation, and supportive care
DLA-nonidentical marrow grafts
All recipient dogs were conditioned for transplantation by 920 cGy TBI at 7 cGy/minute using a linear accelerator (Varian CLINAC 4, Palo Alto, CA).22 Dogs in group A1 received ECP administered on days −2 and −1 with TBI on day 0 and dogs in group A2 received ECP on days −6 and −5, intravenous (IV) infusion of pentostatin at a dose of 4mg/m2 on days −4 and −3, and TBI on day 0 (Table 1). Donor marrow cells from DLA-nonidentical donors were aspirated under general anesthesia through needles inserted into humeri and femora and stored in heparinized tissue culture medium at 4°C for no more than 6 hours.22 Within 4 hours of TBI, harvested marrow cells were infused IV into recipients at a median dose of 2.9 (range, 1.9 to 6.1) ×108 total nucleated cells (TNC)/kg. The day of marrow grafting was designated as day 0. In addition to marrow graft, recipients were given IV infusions of peripheral blood buffy coat cells obtained by leukapheresis from the marrow donor on days 1 and 2, at a median dose of 2.3 (range, 1.2 to 6.9) ×108 TNC/kg to ensure consistent hematopoietic engraftment. MTX, at a dose of 0.4 mg/kg intravenously was used as postgrafting immunosuppression and administered on days +1, +3, +6 and +11, then weekly thereafter until day 102.
TABLE 1.
DLA-nonidentical marrow transplantation after ECP ± pentostatin and 920cGy with postgrafting MTX†
| Recipient Dog # | Buffy Coat TNCs × 108/kg | Marrow TNCs × 108/kg | Neutrophil Increasec | % Donor MNC Chimerism (Max-final) | Rejection | GVHDd | Survival (days) | Cause of Death | |
|---|---|---|---|---|---|---|---|---|---|
| Group A1a | E981 | 4.3 | 1.9 | Yes | 100-100 | No | S/G/L | 17 | GVHDe |
| G120* | 3.9 | 2.2 | Yes | 36 | No | S/G/L | 16 | GVHDe | |
| G113 | 6.9 | 3.4 | Yes | 100-0 | Yes | L | 50 | End of study | |
| G066 | 6.8 | 6.1 | Yes | 100-100 | No | S/G/L | 19 | GVHDe | |
| G170 | 2.3 | 2.8 | No | 67–100 | No | G/L | 20 | GVHDe | |
| Group A2b | G077 | 2.1 | 3 | No | 41–73 | No | N | 9 | Herpes infectione |
| G088 | 2.2 | 3.1 | Yes | 95–100 | No | S/G/L | 22 | GVHDe | |
| G095 | 1.8 | 2.7 | No | 71-0 | Yes | N | 22 | End of Study | |
| G227 | 1.2 | 2.9 | No | 74 | No | S/G/L | 10 | GVHDe |
Dogs given marrow grafts and additional donor PBMC from DLA-nonidentical littermates after conditioning with 920 cGy TBI and post grafting immunosuppression with methotrexate
DLA-nonidentical dogs given marrow grafts, extracorporeal photopheresis (ECP) product, pentostatin, and donor buffy coat cells after conditioning with 920 cGy total body irradiation (TBI) and postgrafting immunosuppression with methotrexate (MTX).
Dogs in group A1 received ECP product on days −2 and −1; 920 cGy TBI on day 0; donor buffy coat cells on days 1 and 2; MTX (0.4 mg/kg i.v. on days 1,3, 6, 11, and then weekly thereafter until day 102.
Dogs in group A2 received ECP product on days −6 and −5; 4 mg/m2 pentostatin i.v. on days −4, and −3; 920 cGy TBI on day 0; buffy coat and MTX regimen as in group A1.
Absolute neutrophil count increase greater than 500/μL.
S=skin; G=gut; L=liver.
Dogs were euthanized because of poor condition.
No DNA sample was obtained on day 7 after HCT.
All dogs received standard supportive care and clinical monitoring as described before.18
Hematopoietic engraftment was assessed by sustained increases in granulocyte and platelet counts after the post irradiation nadir, by documentation of donor originated cells with microsatellite marker studies in specimens from peripheral blood and marrow, by histological features of the marrow from biopsy or autopsy specimens, and by clinical and histopathologic findings of GVHD.
Clinical signs of GVHD included severe diarrhea due to gut involvement (G), conjunctival or skin erythema (S), and elevations of liver enzymes and bilirubin (L). Acute GVHD was defined as disease manifested before day 100, and chronic GVHD was defined as disease present after day 100. Dogs either died of or were euthanized because of complications of the study, or they were euthanized after completion of the study and complete autopsies, including histological examinations, were performed to assess marrow engraftment, GVHD, hematopoietic recovery, and possible toxic effects.
Extracorporeal photopheresis (ECP)
ECP of the animals was performed using the XTS® Photopheresis System (Therakos, Exton, PA, USA) according to standard procedures in the manufacturer’s guidelines and as described previously.18
Mixed leukocyte cultures (MLC) and natural killer (NK) cell cytotoxicity assay23,24
Mixed leukocyte cultures were used to assess the dogs’ cellular immune function before and after ECP as described previously.18 To evaluate NK cell activity before and after ECP, chromium release assays were performed as described previously.18
Chimerism analysis25
Donor and host cell chimerism were evaluated using a polymerase chain reaction (PCR) based assay of polymorphic (CA)n dinucleotide repeats with primers specific for informative microsatellite markers. Genomic DNA of the cells of interest was extracted, and PCR was performed under conditions described previously.26(Ref. Bethge et al., BMT 2011) The technique used enables to detect between 2.5% to 97.5% donor cell chimerism.27
Flow cytometry and monoclonal antibodies (mAbs)
Flow cytometry using a FACScan Flow Cytometer (Becton Dickinson, San Jose, CA) was used to quantify the leukocyte subsets. MAbs against canine CD3 (CA17.6B3, IgG2b), CD4 (CA13.1.E4, IgG1), CD8 (CA9.JD3, IgG2a),28 and TCRαβ (CA15.9D5, IgG1) were used. The anti-CD3, CD4, and CD8 mAbs were kindly provided by Dr. Peter Moore (School of Veterinary Medicine, University of California, Davis). Additionally we used antibodies against canine CD44 (S5, IgG1)29 and canine myeloid cells (DM5, IgG1).30 As isotype control, we used mAb 31A (IgG1) directed at the mouse Thy-1 receptor which does not cross-react with canine cells.31 All mAbs were produced and purified at the Biologics Production Facilities of the Fred Hutchinson Cancer Research Center (Seattle, WA). In addition, the commercially available antibodies Goat F(ab′)2 anti-Mouse Ig’s Fluorescein conjugate (Biosource Camarillo, CA) and anti-human CD14 (DAKO Corporation, Carpenteria, CA) crossreacting with canine CD1432 were used. The mAbs were fluorescein conjugated according to standard protocols.
Detection of apoptosis by Annexin V (Ax)/PI staining
Apoptosis of cells exposed to ECP was assessed by flow cytometry with the use of Annexin V binding, which allows detection of phosphatidylserine on the cell surface of apoptotic cells.33 Briefly, after overnight incubation at 37 °C in 5% humidified atmosphere, cells were harvested, lysed, washed with PBS and incubated with Annexin V-FITC (Pharmingen, San Diego, CA) and propidium iodide (PI) according to the manufacturer’s manual. Cells were analyzed by flow cytometry by means of CellQuest Analysis software (Becton Dickinson, Mountain View, CA). A minimum of 10 000 events were counted per sample. Cells positive for Annexin V but negative for PI are in early apoptosis, cells double positive for Annexin V and PI are in late apoptosis. Results are reported as a percentage of annexin V-FITC positive cells.
Statistical methods
The observed rates of GVHD and graft rejection among all dogs treated were compared in a descriptive manner to historical rates. Apoptosis parameters were assessed using the one-sample t-test, where post-ECP values were compared to pre-ECP values in order to test the null hypothesis that the mean difference (post-ECP minus pre-ECP) is equal to zero.
RESULTS
ECP products
In 9 dogs a total of 18 ECP procedures were performed. A median of 243 ml (range 223–309) of ECP product was collected on each day with a median white blood count of 13970/μl (range 6600 – 20,800), representing a median of 3.5 ×109 (range 1.4 ×109 – 5.1 ×109)total nucleated cells. Data on PBMCs treated which each cycle of ECP was available in 13/18 ECP procedures performed in the dogs. The percentage of PBMCs treated with each cycle of ECP was a median of 18% (range 11–34%). The percentages of different cell populations in the dogs’ peripheral blood and ECP products were similar. There was no difference between percentages of CD4+, CD8+, CD3+, TCRαβ, granulocytes and CD14+ cells populations in the dogs’ peripheral blood and ECP. All dogs tolerated the extracorporeal volume automatically determined by ECP machine. No immediate toxic effects were observed during either ECP procedure.
DLA-nonidentical marrow grafts
Nine dogs were studied. Five in group A1 (ECP alone) and four in group A2 (ECP + pentostatin). Table 1 summarizes the results of allogeneic marrow transplants in groups A1 and A2.
Group A1 (ECP alone) (n=5)
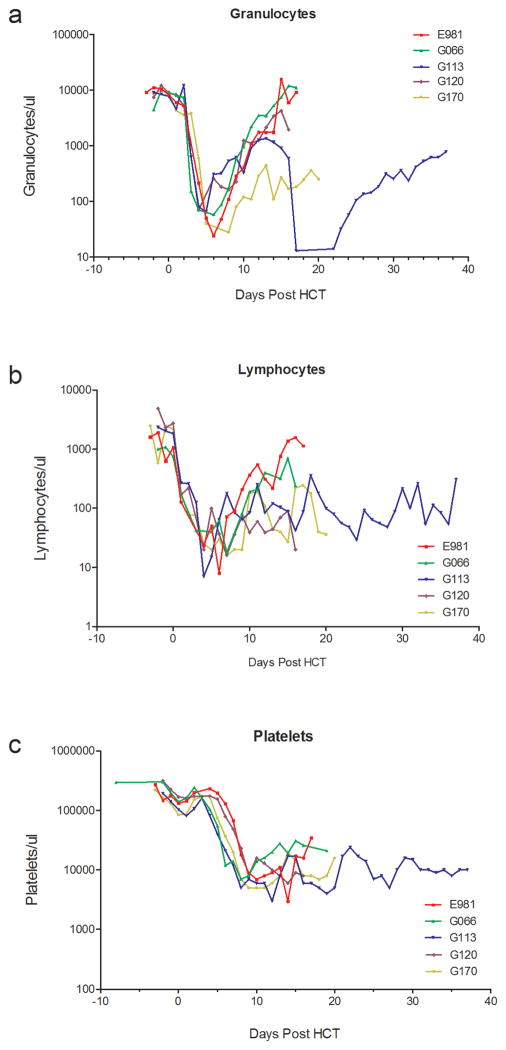
All dogs developed neutropenia (<500 granulocytes/μL) with nadirs of 7 to 16 granulocytes/μL between days 4 to 7 after HCT. Thrombocytopenia with <20,000/μL platelets occurred from day 6 onward with nadirs of 3000 to 5000 platelets/μL. No platelet transfusions were required apart from a single platelet transfusion in dog E981. Subsequent platelet recovery occurred slowly and recovery to a completely normal platelet counts had not occurred until the end of study. Four dogs had complete granulocyte and lymphocyte recoveries and one dog (G 170) had a partial recovery (Figure 1). Three dogs achieved full donor hematopoietic chimerism starting 7 days after HCT. Dog G113 rejected his graft on day 21 with subsequent complete autologous hematopoietic recovery and was released for adoption (Figure 3). All dogs (n=4) with sustained engraftment developed severe acute GVHD and were euthanized due to poor clinical condition after a median of 19 (range 16–20) days after HCT (Table 1).
Figure 1.
Peripheral blood granulocyte, lymphocyte, and platelet counts of DLA-nonidentical dog marrow transplantation given ECP product conditioned with 920 cGy TBI and postgrafting MTX
Figure 3.
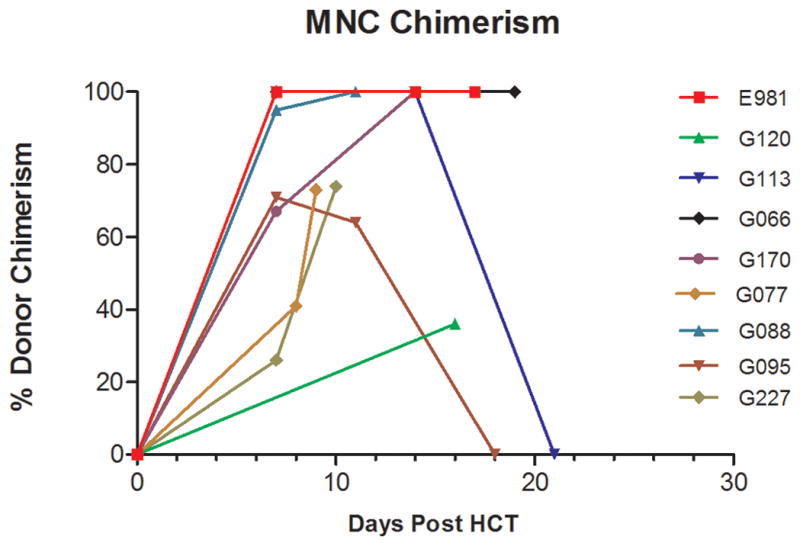
Percentage donor chimerism among PBMC in dogs given 920 cGy TBI and DLA-nonidentical marrow grafts. Dogs in group 1 (A1) received ECP on days −2 and −1 before transplant and dogs in group 2 (n=5) (A2) received ECP on days −6 and −5 and pentostatin (4mg/m2) on days −4 and −3 before transplant (n=4)
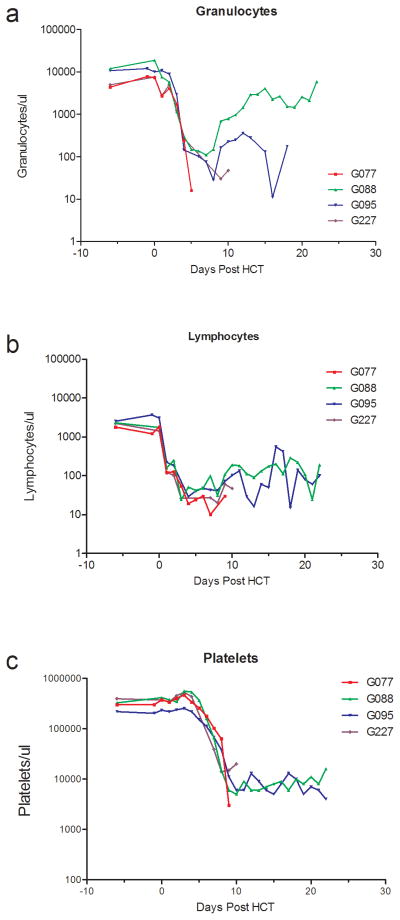
Group A2 (ECP+Pentostatin) (n=4)
All dogs developed granulocytopenia with <500 granulocytes/μL from day 4. Thrombocytopenia (<20,000/μL platelets) occurred from day 7 after HCT with nadirs of 3000 to 14000 platelets/μL. Two dogs received single and one dog received three platelet transfusions. Lymphocyte nadirs of 10 lymphocytes/μL occurred between days 3 and 8. One dog showed complete granulocyte and lymphocyte recovery, one dog showed a partial recovery and two dogs had no recovery. Platelet recovery occurred slowly and no dog recovered to normal platelet counts (Figure 2). One dog died on day 9 after HCT from canine herpes virus before achievement of hematological recovery. The dog had 73 % donor PBMC chimerism on day 9. One dog had an initial engraftment of 71 % on day 7, rejected the graft on day 18 and was euthanized on day 22 due to poor clinical condition. The other 2 dogs achieved 100 % and 91 % donor PBMC chimerism (Figure 3). Both developed severe acute GVHD and were euthanized due to poor clinical condition on day 22 and day 10 after HCT (Table 1).
Figure 2.
Peripheral blood granulocyte, lymphocyte, and platelet counts of DLA-nonidentical dog marrow transplantation given ECP product, pentostatin, conditioned with 920 cGy TBI and postgrafting MTX
In vitro characteristics of ECP PBMC
For all in vitro studies described below, PBMCs from the recipients were collected and analyzed from 4 different time points during ECP treatment: 1) peripheral blood pre ECP treatment (Pre ECP blood); 2) peripheral blood immediately after ECP product infusion (Post ECP blood); 3) Buffy coat (BC) cells from the ECP product before UVA light activation (Pre ECP BC); and 4) BC cells from the ECP product immediately after UVA light activation (Post ECP BC).
Alloreactivity
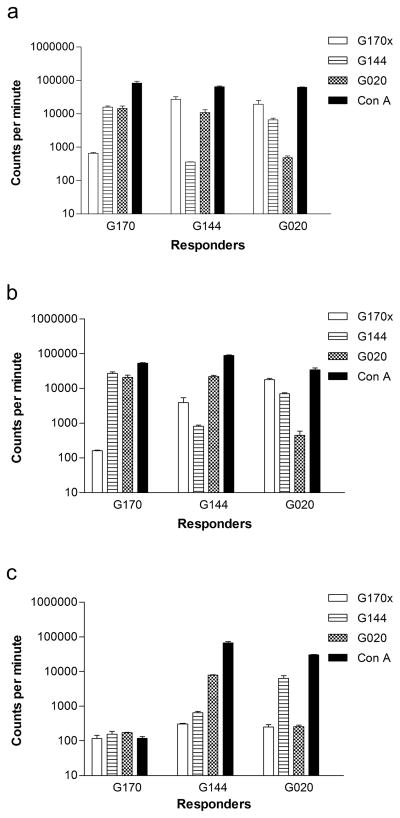
MLCs were performed to study the effects of UVA on PBMC alloreactivity. In 7 of dogs tested PBMCs from the ECP product after UVA light activation (Post-ECP BC) showed a median decrease of 97% (range 69–99) in alloreactivity against DLA-mismatched unrelated stimulator cells when compared to PBMCs from the ECP product before UVA light activation (Pre-ECP BC). In contrast there was no decrease in alloreactivity against DLA-mismatched unrelated stimulator cells of the Post-ECP blood when compared to the Pre-ECP BC. (Figure 4)
Figure 4.
Mixed leukocyte culture (MLC) assay of a nonidentical dog marrow transplantation before and after infusion of the extracorporeal photopheresis (ECP) product. (A) MLR comparing responses of G170 PBMCs (Recipient), G144 PBMCs (Donor), and G020 PBMCs (unrelated dog) before ECP. (B) MLR showing response of G170 PBMCs obtained before photopheresis to G144 and G020. (C) MLR showing response of G170 ECP product to buffy coat cells obtained after UVA light activation to G144 and G020
NK cell function
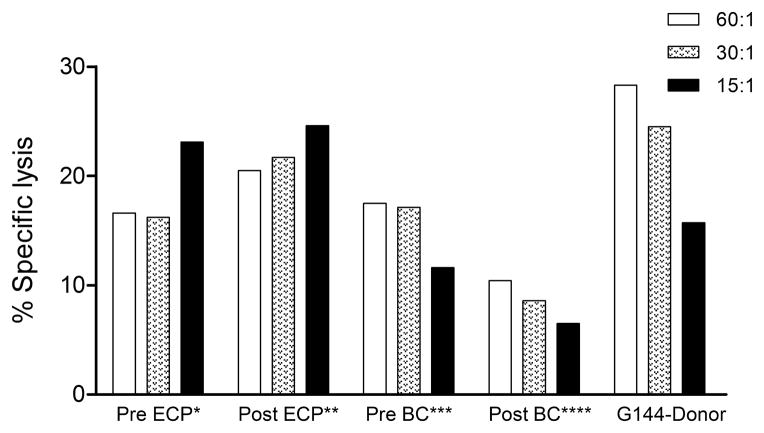
In 3 dogs tested PBMC from ECP product after UVA light activation (Post-ECP BC) showed a median decrease of 52% (range, 41–79%) in NK-function when compared with PBMCs from the ECP product before UVA light activation Pre-ECP BC). In contrast, there was no decrease in NK function of the PBMCs from the dog after ECP product infusion (Post-ECP blood) when compared to the ECP product before UVA light activation (Pre-ECP BC). (Figure 5)
Figure 5. NK-cell function.
NK assay of various G170 cell populations:
*Pre ECP = PBMCs obtained before photopheresis; **Post ECP = PBMCs obtained after infusion of ECP product; ***Pre BC = buffy coat cells obtained before UVA light activation; ****Post BC = buffy coat cells obtained after UVA light activation
Apoptosis
Levels of apoptosis induction before and after ECP were compared in 9 dogs studied. The mean +/− SEM percentage of lymphocytes demonstrating early and late apoptosis and flow cytometry data are shown in Figure 6.
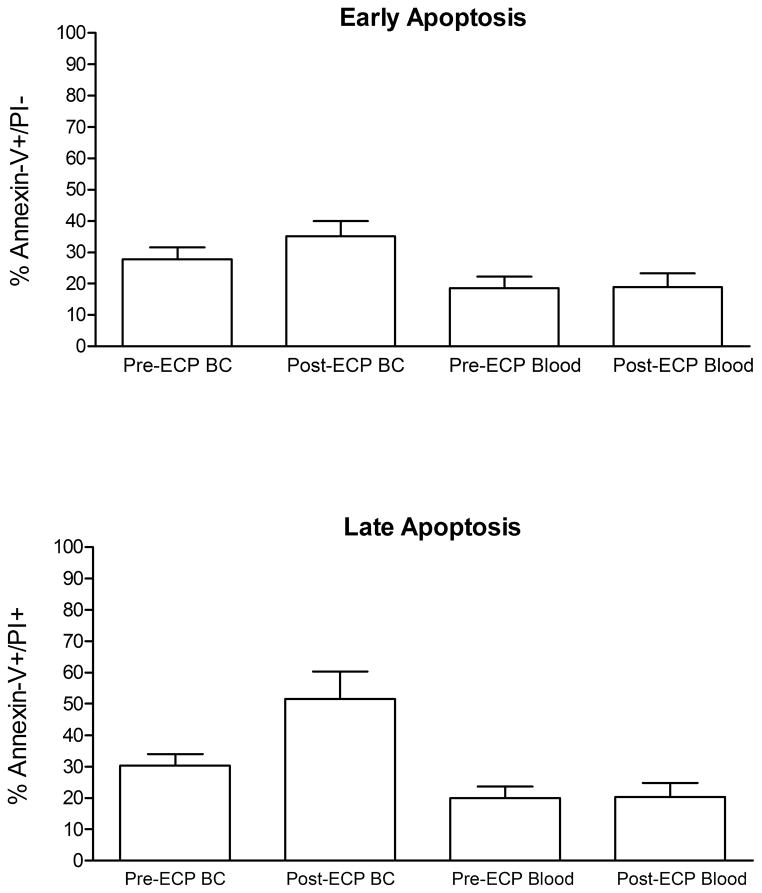
Figure 6. Apoptotic effect of extracorporeal photopheresis (ECP) on marrow transplantation.
Whole blood and buffy coat cells obtained before and after photopheresis were cultured overnight at 37°C, 5% humidified atmosphere, lyzed, washed, stained with Annexin-V-FITC, and propodium iodide (Pi), and analyzed with FACScan. (A) The mean ± SEM percentage of lymphocytes demonstrating early apoptosis (Annexin-V+/PI−). (B) The mean ± SEM percentage of lymphocytes demonstrating late apoptosis (Annexin-V+/PI+)
At early apoptosis, the difference between percent Annexin-V FITC+/PI− cells in blood drawn after ECP (post-ECP blood) and before ECP (pre-ECP blood) ranged from −11.0 to 9.5, with a mean difference of −0.2 +/− 6.6 (p=0.93). The difference between percent Annexin-V FITC+/PI− of BC cells after ECP (post-ECP BC) and before ECP (pre-ECP BC) ranged from −3.5 to 21.3, with a mean difference of 7.4 +/− 9.3, p=0.04.
As a measure of late apoptosis, the difference between percent Annexin-V FITC+/PI+ cells in post-ECP blood and pre-ECP blood ranged from −12.6 to 10.4, with a mean difference of 0.1 +/− 7.1 (p=0.96). The difference between percent Annexin-V FITC+/PI+ of post-ECP BC and pre-ECP BC ranged from −4.4 to 56.6, with a mean difference of 21.3 +/− 22.1 (p=0.02).
DISCUSSION
Both pentostatin and ECP result in T-cell and host DC depletion and a shift of the remaining DC and T-cell population to a tolerogenic DC2 and T-regulatory population leading to the low rate of GVHD observed by Miller et al with a regimen combining ECP, pentostatin and 600 cGy TBI for HLA-identical and non-identical (5/6) allogeneic HCT.13
In order to assess the role of ECP and pentostatin as additional immunosuppression for prophylaxis of GVHD we used our well established dog model. We have shown previously that dogs (n=10) that received allogeneic DLA-nonidentical marrow grafts after 920 cGy TBI, in association with donor PBMC infusion and postgrafting immunosuppression with MTX and CSP achieved 100% engraftment, and 50% developed acute GVHD with a median survival of 112 days.34,35 Compared to these results, in the present study 7 out of 9 dogs achieved sustained engraftment. All 6 engrafting dogs developed severe acute GVHD (4 in the group with ECP alone and 2 in the group with Pentostatin and ECP and one died with canine herpes virus infection). This observed rate in the current study is higher than results seen historically, and therefore, there was no evidence to suggest that combining MTX with the use of ECP alone or in combination with pentostatin is more effective than combining MTX with CSP to prevent GVHD in DLA-nonidentical canine transplantation. It seems that the high degree of histocompatibility cannot be overcome by combination of ECP and pentostatin.
The negative results of our studies on the potential role of ECP and pentostatin for GVHD prophylaxis might be explained by the pathophysiologic knowledge about the immunosuppressive effect of ECP and pentostatin. ECP has been successfully used to treat patients with acute and chronic GVHD in HCT.12,16,36–38 The mechanism of action of ECP in GVHD is fundamentally based in DC integrity and T-cell function.8,39 In vitro studies have shown that ECP treatment leads to a shift from an activated monocytoid dendritic cell (DC)/T-cell state to a tolerogenic state.39 In addition, an inverted CD4-to-CD8 and overall increase in the number of CD3−/CD56+ natural killer (NK) cells, and an attenuation of the capacity of dendritic cells to stimulate the proliferation of autologous or allogeneic T-cells in mixed lymphocyte assays was observed.39,40 ECP treatment was also associated with a shift in the cytokine profile of circulating T cells from a predominantly inflammatory or Th1 (interleukin-2 [IL-2], interferon-γ) profile to a Th2 (IL-4, IL-10) profile. It was subsequently demonstrated that ECP induced a population of CD4+ CD25+ T cells, which functioned as suppressors in mixed lymphocyte reactions, consistent with regulatory T cells.41 The evolving model for the mechanism of action of ECP is that it is the ingestion of apoptotic T cells, which initiates the process of activation and cytokine secretion by antigen-presenting cells, which subsequently leads to generation of tolerogenic DCs and subsequently a regulatory T-cell response.42 Pentostatin might further increase T-cell apoptosis through adenosine deaminase inhibition. We, as others, demonstrated that ECP induces apoptosis of T lymphocytes.43,44 Since the immunomodulation of DC depends on the presence of these apoptotic cells, we indirectly showed that ECP was effective in this animal model. The absence of apoptotic cells in peripheral blood of dogs immediately after ECP can be explained by reticuloendothelial system uptake or by a possible dilution factor as the estimated amount of PBMC collected in our dogs was a median of only 18% of total circulating PBMC.44 Although both NK and lymphocyte functions were decreased in ECP product, no difference was shown if PBMCs were collected from the dogs before and after ECP procedure. Although a dilution factor could explain these findings, we fail to demonstrate a decrease of host lymphocyte alloreactivity on day zero which would be an efficient way to prove the effectiveness of this approach. However, we used similar treatment protocols, drug doses and treatment volumes as applied in the human system of ECP. Although DC function was not assessed in this study, the combination of high dose TBI and other immunosuppressive agents such as MTX may have impaired or even completely abrogate DC function or number, eliminating or decreasing ECP effects. However, patients with GVHD usually require several treatment courses of ECP over several weeks to months to achieve clinical response.12,36–38 Our abbreviated schedule of ECP on only two consecutive days prior HCT might be too short. It may well be possible that many cycles of ECP prior allogeneic HCT would be required to prevent GVHD. Perhaps even application of ECP for several treatment cycles prior and post HCT might be necessary. This could be especially true in DLA-nonidentical grafts where the high degree of histoincompatibility demands a higher degree of immunosuppression.
In conclusion our data demonstrate that the use of ECP alone or in combination with pentostatin was not potent enough to prevent GVHD in our protocol of DLA-mismatched HCT. Further studies are needed to prove the real contribution of these tools as GVHD prophylaxis in HCT settings using more intense or prolonged treatment protocols.
Acknowledgments
Grants: This study was supported by grants from the National Institutes of Health HL036444 and CA078902 and a grant from Therakos, Incorporated. WAB was supported by a fellowship from Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung für Krebsforschung. FRK was supported by a grant from FAPESP/Brazil and an award from the Oncology Research Faculty Development Program from the Department of Health and Human Services, NIH, Bethesda, MD.
The authors would like to thank George Sale, M.D. for performing the pathology studies, Michele Spector, D.V.M. for providing veterinary care along with the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center. We also would like to thank Stacy Zellmer for DLA-typing and the technicians of the hematology department for analyzing peripheral blood counts. We are also grateful for the assistance of Helen Crawford, Bonnie Larson, and Sue Carbonneau in manuscript preparation.
Footnotes
CONFLICTS OF INTEREST: None.
- Wolfgang A. Bethge designed the study, analyzed and interpreted data, drafted, revised, and approved the manuscript. No conflicts of interest.
- Fabio R. Kerbauy designed and conducted the study, analyzed and interpreted data, revised and approved the manuscript. No conflicts of interest.
- Erlinda B. Santos designed and conducted the study, analyzed and interpreted data, drafted, revised, and approved the manuscript. No conflicts of interest.
- Theodore Gooley did the statistical analysis of the data and revised and approved the manuscript. No conflicts of interest.
- Rainer Storb conducted the study, interpreted data, and revised and approved the manuscript. No conflicts of interest.
- Brenda M. Sandmaier conceived, designed, conducted the study, drafted, revised, and approved the manuscript, and supervised interpretation of data. No conflicts of interest.
References
- 1.Thomas’ Hematopoietic Cell Transplantation. Oxford, UK: Blackwell Publishing Ltd; 2004. [Google Scholar]
- 2.Martin PJ, Hansen JA, Torok-Storb B, Durnam D, Przepiorka D, O’Quigley J, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 3.Henslee-Downey PJ. Mismatched bone marrow transplantation. Curr Opin Oncol. 1995;7:115–121. doi: 10.1097/00001622-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Henslee-Downey PJ, Abhyankar SH, Parrish RS, Pati AR, Godder KT, Neglia WJ, et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood. 1997;89:3864–3872. [PubMed] [Google Scholar]
- 5.Teshima T, Matsuo K, Matsue K, Kawano F, Taniguchi S, Hara M, et al. Impact of human leucocyte antigen mismatch on graft-versus-host disease and graft failure after reduced intensity conditioning allogeneic haematopoietic stem cell transplantation from related donors. Br J Haematol. 2005;130:575–587. doi: 10.1111/j.1365-2141.2005.05632.x. [DOI] [PubMed] [Google Scholar]
- 6.Zic JA, Stricklin GP, Greer JP, Kinney MC, Shyr Y, Wilson DC, et al. Long-term follow-up of patients with cutaneous T-cell lymphoma treated with extracorporeal photochemotherapy. J Am Acad Dermatol. 1996;35:935–945. doi: 10.1016/s0190-9622(96)90118-8. [DOI] [PubMed] [Google Scholar]
- 7.Dall’Amico R, Rossetti F, Zulian F, Montini G, Murer L, Andreetta B, et al. Photopheresis in paediatric patients with drug-resistant chronic graft-versus-host disease. Br J Haematol. 1997;97:848–854. doi: 10.1046/j.1365-2141.1997.1092927.x. [DOI] [PubMed] [Google Scholar]
- 8.Foss FM, Gorgun G, Miller KB. Extracorporeal photopheresis in chronic graft-versus-host disease (Review) Bone Marrow Transplant. 2002;29:719–725. doi: 10.1038/sj.bmt.1703529. [DOI] [PubMed] [Google Scholar]
- 9.Giunti G, Schurfeld K, Maccherini M, Tanganelli P, Rubegni P, Alfani D, et al. Photopheresis for recurrent acute rejection in cardiac transplantation. Transplant Proc. 1999;31:128–129. doi: 10.1016/s0041-1345(98)01471-7. [DOI] [PubMed] [Google Scholar]
- 10.Horina JH, Mullegger RR, Horn S, Holzer H, Halwachs G, Kerl H, et al. Photopheresis for renal allograft rejection. Lancet. 1995;346:61. doi: 10.1016/s0140-6736(95)92696-8. [DOI] [PubMed] [Google Scholar]
- 11.Oliven A, Shechter Y. Extracorporeal photopheresis: a review (Review) Blood Rev. 2001;15:103–108. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 12.Greinix HT, Volc-Platzer B, Rabitsch W, Gmeinhart B, Guevara-Pineda C, Kalhs P, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 13.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 14.Chan GW, Foss FM, Klein AK, Sprague K, Miller KB. Reduced-intensity transplantation for patients with myelodysplastic syndrome achieves durable remission with less graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:753–759. doi: 10.1016/j.bbmt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Pavletic SZ, Bociek RG, Foran JM, Rubocki RJ, Kuszynski CA, Wisecarver JL, et al. Lymphodepleting effects and safety of pentostatin for nonmyeloablative allogeneic stem-cell transplantation. Transplantation. 2003;76:877–881. doi: 10.1097/01.TP.0000084869.08639.A0. [DOI] [PubMed] [Google Scholar]
- 16.Miller KB, Roberts TF, Chan G, Schenkein DP, Lawrence D, Sprague K, et al. A novel reduced intensity regimen for allogeneic hematopoietic stem cell transplantation associated with a reduced incidence of graft-versus-host disease. Bone Marrow Transplant. 2004;33:881–889. doi: 10.1038/sj.bmt.1704454. [DOI] [PubMed] [Google Scholar]
- 17.Foss FM. The role of purine analogues in low-intensity regimens with allogeneic hematopoietic stem cell transplantation (Review) Semin Hematol. 2006;43:S35–S43. doi: 10.1053/j.seminhematol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Bethge WA, Kerbauy FR, Santos E, Gooley TA, Storb R, Sandmaier BM. Extracorporeal photopheresis in addition to pentostatin in conditioning for canine hematopoietic cell transplantation: role in engraftment. Bone Marrow Transplant. 2011;46:1382–1388. doi: 10.1038/bmt.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 20.Wagner JL, Burnett RC, Works JD, Storb R. Molecular analysis of DLA-DRBB1 polymorphism. Tissue Antigens. 1996;48:554–561. doi: 10.1111/j.1399-0039.1996.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 22.Ladiges WC, Storb R, Graham T, Thomas ED. Experimental techniques used to study the immune system of dogs and other large animals. In: Gay WI, Heavener JE, editors. Methods of Animal Experimentation. Academic Press; New York, NY: 1989. pp. 103–133. [Google Scholar]
- 23.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- 24.Loughran TP, Jr, Deeg HJ, Storb R. Morphologic and phenotypic analysis of canine natural killer cells: Evidence for T-cell lineage. Cell Immunol. 1985;95:207–217. doi: 10.1016/0008-8749(85)90309-0. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 26.Francisco LV, Langston AA, Mellersh CS, Neal CL, Ostrander EA. A class of highly polymorphic tetranucleotide repeats for canine genetic mapping. Mamm Genome. 1996;7:359–362. doi: 10.1007/s003359900104. [DOI] [PubMed] [Google Scholar]
- 27.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 28.Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high density expression of CD4 by canine neutrophils. Tissue Antigens. 1992;40:75–85. doi: 10.1111/j.1399-0039.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 29.Sandmaier BM, Storb R, Appelbaum FR, Gallatin WM. An antibody that facilitates hematopoietic engraftment recognizes CD44. Blood. 1990;76:630–635. [PubMed] [Google Scholar]
- 30.Sandmaier BM, Schuening FG, Bianco JA, Rosenman SJ, Bernstein I, Goehle S, et al. Biochemical characterization of a unique canine myeloid antigen. Leukemia. 1991;5:125–130. [PubMed] [Google Scholar]
- 31.Denkers E, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol. 1985;135:2183–2186. [PubMed] [Google Scholar]
- 32.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Veterinary Immunology & Immunopathology. 1993;39:461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- 33.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 34.Storb R, Deeg HJ. Failure of allogeneic canine marrow grafts after total body irradiation: Allogeneic “resistance” vs transfusion induced sensitization. Transplantation. 1986;42:571–580. doi: 10.1097/00007890-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Raff RF, Sandmaier BM, Graham T, Loughran TP, Jr, Pettinger M, Storb R. “Resistance” to DLA-nonidentical canine unrelated marrow grafts is unrestricted by the major histocompatibility complex. Exp Hematol. 1994;22:893–897. [PubMed] [Google Scholar]
- 36.Owsianowski M, Gollnick H, Siegert W, Schwerdtfeger R, Orfanos CE. Successful treatment of chronic graft-versus-host disease with extracorporeal photopheresis. Bone Marrow Transplant. 1994;14:845–848. [PubMed] [Google Scholar]
- 37.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 38.Perfetti P, Carlier P, Strada P, Gualandi F, Occhini D, van Lint MT, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 39.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 40.Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 41.Biagi E, Di BI, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 42.Di Renzo M, Sbano P, De Aloe G, Pasqui AL, Rubegni P, Ghezzi A, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clin Exp Immunol. 2008;151:407–413. doi: 10.1111/j.1365-2249.2007.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction of ultraviolet light A and photochemotherapy in cutaneous T-cell Lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107:235–242. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- 44.Bladon J, Taylor PC. Extracorporeal photopheresis induces apoptosis in the lymphocytes of cutaneous T-cell lymphoma and graft-versus-host disease patients. Br J Haematol. 1999;107:707–711. doi: 10.1046/j.1365-2141.1999.01773.x. [DOI] [PubMed] [Google Scholar]