Abstract
We examined the visual processing of a social learning stimulus and the ways in which visual attention was distributed to objects as well as to the examiner’s face during word learning under conditions that varied only in the presence or absence of a label. The goal of the current study, then, was to evaluate the effects of differentially providing pointing and labeling during exposure to a novel target object in males with fragile X syndrome (FXS) (n = 14, ages 4.33–10.02), autism spectrum disorder (ASD) (n = 17, ages 4.04–10.4), or typical development (TD) (n = 18, ages 2.05–5.33). In particular, the present study examined attention to the examiner’s face as well as target and distracter objects that were presented as video stimuli. An eye-tracker captured gaze to the video stimuli as they were shown in order to examine the way in which children with FXS, ASD, or TD distributed their gaze toward the examiner and the objects. Results indicated that no group showed increased gaze toward the target object compared to the distracter object. However, results revealed that participants with FXS showed significantly increased face gaze compared to the novel objects, whereas children with ASD and TD both showed similar amounts of relative gaze toward the face and objects. Furthermore, the act of pointing at the target object was found to increase gaze toward the target objects compared to when there was no pointing in all groups. Together, these findings suggest that social cues like those employed in a word-learning task, when presented with video, may relate to gaze in FXS in context- or task-dependent ways that are distinct from those expected during live interaction.
Keywords: Fragile X Syndrome, Autism, Eye Tracking, Word Learning
1. Introduction
Over the course of development, children attend to and gather information from a large variety of social cues when learning new words (Hollich et al., 2000). A conversational partner’s direction of gaze, indicating gestures, speech prosody, facial expression, and labels inform the child’s understanding of the association between a novel label and an intended referent (Baldwin & Moses, 2001). Previous research has shown that the attentional abilities supporting word learning are established relatively early in typical development as children learn to notice and respond to adult social cues that provide referential information (Carpenter, Nagell, & Tomasello, 1998; Scaife & Bruner, 1975; Striano & Stahl, 2005). By 20 months of age, for example, typically developing children will avoid making a mapping based upon temporal contiguity between label and object unless the label is accompanied by some social indication that the conversational partner intends to name that particular object (Baldwin, et al., 1996). The present study examined the way in which the social cues of labeling and pointing affected attention toward novel objects in children with neurodevelopmental disorders.
Given their unique phenotypic characteristics, attention to the types of social cues that facilitate word learning is likely to be a challenge for children with neurodevelopmental disorders, such as fragile X syndrome (FXS) (Brady, Warren, Keller, Fleming, & Sterling, 2014; Warren, Brady, Sterling, Fleming, & Marquis, 2010). In the present study, we focused on fast mapping by children with FXS. The term “fast mapping” refers to the initial associative learning process by which a novel label is paired with an object referent during word learning (cf. Carey & Bartlett, 1978; Dollaghan, 1987; Heibeck & Markman, 1987). Adult social cues provided within a word-learning context serve the function of directing the child’s attention to the novel object while the novel label is being presented, thereby providing an opportunity for accurate associative learning to take place. The current study was designed to examine, within the context of an experimental fast mapping paradigm, the responsiveness of children with FXS to two specific types of adult social cues, pointing gestures and verbal labeling.
1.1. Pointing
Numerous findings demonstrate the importance of pointing gestures in directing children’s attentional resources during word learning (Baldwin & Markman, 1989; Briganti & Cohen, 2011; Deak, Flom, & Pick, 2000). For example, evidence has indicated that adult pointing toward an object results in more attention toward the object than when the adult merely looks at the object without pointing (Deak et al., 2000; Doherty & Anderson, 1999). There is a convergence of evidence to suggest that pointing gestures are important in directing young children’s attention toward objects within referential contexts (Grassmann & Tomaselo, 2010; Leibal, Behne, Carpenter, & Tomasello, 2009). The phenotypic characteristics of young males with FXS, including gaze aversion (Garrett, Menon, MacKenzie, & Reiss, 2004; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006), social anxiety (Cordiero, Ballinger, Hagerman, & Hessl, 2011), inattention (Cornish & Wilding, 2010) and symptoms of ASD (Roberts, Weisenfeld, Hatton, Heath, & Kauffman, 2007), can be expected to negatively affect their ability to attend to, and understand, the relevance of pointing gestures in supporting the word learning process.
1.2. Verbal Labeling of Novel Objects
Another critical social cue that facilitates children’s attention to an adult’s intended referent is the presence of a verbal label within an interactive context that includes a novel (i.e., nameless) object (see Baldwin & Moses, 2001 for review). The presence of labels has been shown to support attention following in TD children (Moore, Angelopoulos & Bennett, 1999; Pruden, Hirsh-Pasek, Golinkoff, & Hennon, 2006) and children with ASD (McDuffie, Yoder, & Stone, 2006). That is, young children direct a greater proportion of their visual attention to an object that is accompanied by a verbal label from a speaker than to an object that is accompanied by other types of cues designed to increase the salience of a novel object (e.g., object movement, handling of the object, talking without labeling). Presumably, the presence of a verbal label carries privileged information about a speaker’s intention to name the novel object. Little is known about whether the presence of verbal labels increases the salience of a novel object for children with FXS; that is, whether these children will attend more to a novel object that is labeled than to one that is merely talked about without labeling. The phenotypic characteristics of young males with FXS, however, can be expected to negatively impact their ability to attend to and understand the relevance of verbal labels in supporting the acquisition of new words.
1.3. Fragile X Syndrome
FXS, the most common inherited cause of intellectual disability (Crawford, Acuna, & Sherman, 2001), is caused by a CGG trinucleotide expansion on the FMR1 gene on the X-chromosome. This expansion results in the lack or reduction of FMRP, a protein critical for synaptic development and experience-dependent learning (Bassell & Warren, 2008). Although FXS affects both males and females, it is more common in males, and males are more severely affected on average. Over 95% of males with FXS have cognitive abilities within the intellectual disability range (IQ of < 70; Hessl et al., 2009). In addition, males with FXS often display a behavioral phenotype that includes gaze avoidance, repetitive behaviors, inattention, hyperarousal, and social anxiety (Hessl, Glaser, Dyer-Friedman, & Reiss, 2006; Wolff et al., 2012).
Language is generally delayed relative to age-expectations in males with FXS (Abbeduto, Brady, & Kover, 2007; Brady, Skinner, Roberts, & Hennon, 2006). There is recent evidence, however, that boys with FXS are less impaired, on average, in the process of learning new words than age-matched boys with ASD despite the former having lower levels of nonverbal cognitive functioning (McDuffie et al., 2013; Blinded for Review, under review). These findings raise the possibility that the social skills necessary for word learning are less impaired in FXS than in boys with ASD. The current study was designed to examine specific aspects of the social and behavioral phenotypes of males with FXS that might shed light on their word learning.
1.4. Autism Spectrum Disorder
There is a large body of research demonstrating early and persistent deficits in social and joint attention abilities in children with ASD (Adamson et al., 2009; Adamson, Deckner, & Bakeman, 2010; Leekam, Lopez, & Moore, 2000; Mundy, 2003; Mundy, Sigman, & Kasari, 1990; Osterling, Dawson, & Munson, 2002; Paparella, Goods, Freeman, & Kasari, 2011). Additionally, children with ASD often fail to respond appropriately to adults’ head turns and other attention directing cues (Leekam, Baron-Cohen, Perrett, Milders, & Brown, 1997; Leekam, Hunnisett, & Moore, 1998; Moore, Angelopoulos & Bennett, 1999; Mundy, Mastergeorge, & McIntyre, 2012) that are known to facilitate word learning (Baldwin, 1993b; Baron-Cohen, Baldwin, & Crowson, 1997; Gliga, Elsabbagh, Hudry, Charman, & Johnson, 2012).
Children with ASD also demonstrate delays in language development and deficits in social communication skills relative to TD children (Charman et al., 2003; Charman, Drew, Baird, & Baird, 2003; Dawson et al., 2004). In contrast to FXS, there is a well-established literature examining the relationship between social cognitive abilities and word learning as measured by interactive, social learning paradigms in children with ASD. In one study, McDuffie and colleagues (2006) used a fast-mapping paradigm to examine whether the provision of labels would result in increased attention toward a target object relative to talking about an object with connected speech without explicit labeling. These authors (2006) found that toddlers with ASD and TD both showed increased gaze toward labeled objects relative to objects that were talked about but not labeled. These findings suggest that, within a highly structured word learning context, providing a label increased attention to a novel object even for children with ASD, who face challenges in responding to referential cues during word learning (Adamson et al., 2009, 2010; Toth, Munson, Meltzoff, & Dawson, 2006). In this study, we examined the capacity of children with FXS to use social cues in word learning relative to children with ASD.
1.5. Attention to Faces in FXS
A commonly observed feature in FXS is that affected individuals are characterized by aberrant gaze patterns with respect to social stimuli, such as the faces of others when compared to typically developing children (Dalton, Holsen, Abbeduto, & Davidson, 2008; Dalton et al., 2005; Farzin, Rivera, & Hessl, 2009; Spezio, Adolphs, Hurley, & Piven, 2007a, 2007b). In live social interactions (Dawson et al., 2004; Hessl et al., 2006), when viewing images, and watching videos of social scenes (Farzin, Rivera, & Hessl, 2009; Klin, Jones, Schulz, Volkmar, & Cohen, 2002), children with FXS often show less attention to the face, which can be an important source of information during learning opportunities. Thus, such avoidant behaviors become important in the context of a learning setting in which the face of an examiner can serve as a cue for learning or directing attention. We were interested in the ability of children with FXS to attend to faces in word learning relative to children with ASD, who also have well-documented problems in attending to faces (Chawarska, Macari, & Shic, 2012; Leekam, Lopez, & Moore, 2000).
1.6. Word Learning Abilities in Children with FXS
The majority of males with FXS display some symptoms of ASD, and as many as 60% meet diagnostic criteria for an ASD according to gold standard diagnostic measures used in research (Hall, Lightbody, Hirt, Rezvani, & Reiss, 2010; Harris et al., 2008; Rogers, Wehner, & Hagerman, 2001). However, the nature of features of ASD observed in individuals with FXS continues to generate controversy (Bailey, Hatton, & Skinner, 1998; Hall et al., 2010; Harris et al., 2008; McDuffie, Thurman, Hagerman, & Abbeduto 2014; Blinded for Review, under review; Wolff et al., 2012). Indeed, some have suggested that ASD symptoms reflect different underlying psychological mechanisms in the two disorders (e.g. anxiety in FXS, lack of interest and insight in the social world in ASD). Such findings provide a rationale for continuing to examine the social and learning processes of individuals with FXS; that is, findings that may accurately characterize the behavioral profiles of individuals with nonsyndromic ASD may not offer broad applicability toward understanding FXS.
To date there has been only one study examining the process of fast mapping in males with in FXS (McDuffie et al., 2013). McDuffie and colleagues (2013) provided a comparison of word learning by young males with FXS relative to age-matched males with ASD and younger males with typical development. In this study, a target and distracter object were presented to each participant during four different trials. The trials were divided into a teaching phase, during which the target and distracter objects were presented individually, and a testing phase during which the child was requested to identify the labeled object using a forced-choice paradigm. Object presentation during the teaching phase was accompanied by head turns, gaze shifts, object movement, and gestures designed to highlight the salience of the novel objects. The only difference between the cues used during presentation of the target and distracter objects during the teaching phase of each trial was that the target object’s presentation was accompanied by a label presented five times during child-directed speech. The distracter object, on the other hand, was accompanied by an equivalent amount of talking using connected speech but no labeling. Results indicated that, despite having lower levels of nonverbal cognitive ability, participants with FXS outperformed those with ASD in target object selection, though both groups performed at above chance levels. The conclusions from the McDuffie et al. (2013) study are important for understanding absolute levels of task performance. What is less well understood is how individuals with FXS learned in this experimental paradigm; that is, how their patterns of attention may have differed between conditions (label, vs. no label) and how the duration of attention to the labeled object was related to target object selection. Understanding the ways in which individuals with FXS process social stimuli in real time relative to children with TD and ASD has important implications for identifying targets for treatment and intervention in FXS.
Recently, novel approaches for understanding the ways in which children with ASD process social stimuli have emerged, including the use of eye-tracking methodology. There have even been efforts to extend these methods the study the word learning of children with ASD (e.g. Akechi et al., 2011; Akechi, Kikuchi, Tojo, Osanai, & Hasegawa, 2013; Gillespie-Lynch, Elias, Escudero, Hutman, & Johnson, 2013; Gliga et al., 2012; Norbury, Griffith, & Nation, 2010). However, few eye-tracking studies have focused on individuals with FXS (but see Dalton, Holsen, Abbeduto, & Davidson, 2008; Farzin, Rivera, & Hessl, 2009; Shaw & Porter, 2013; Williams, Porter, & Langdon, 2013).
1.7. Assessing Word Learning through Eye Tracking
A number of eye-tracking studies have sought to better understand how TD children and those with ASD visually process and learn from social stimuli (e.g. Akechi et al., 2011, 2013; Gillespie-Lynch et al., 2013; Gliga et al., 2012; Norbury et al., 2010). These eye-tracking paradigms have largely used adaptations of interactive paradigms investigating the ways in which social cues guide learning in a setting of referential ambiguity, (i.e. when multiple novel objects are present, but only a single novel label is expressed; Baldwin, 1993a, 1993b, Baron-Cohen et al., 1997; Luyster & Lord, 2009). These studies have provided evidence of atypical attention following in young children at risk for, or diagnosed with, ASD (e.g. Gillespie-Lynch et al., 2013; Norbury et al., 2010) and have demonstrated that attention following alone is not sufficient to enable word-learning success (Gliga et al., 2012). Akechi et al. (2011) used an eye-tracking paradigm to demonstrate that participants with ASD were not able make object-label pairings when the target object was presented and labeled outside of their focus of attention (i.e. during a discrepant labeling trial). If the object was highlighted by motion, however, learning was improved (Akechi et al., 2011). Introduction of a pointing hand similarly increased attention to objects presented during a discrepant labeling trial in participants with ASD (Akechi et al., 2013).
Together, these eye-tracking studies have contributed to our understanding of the ways in which children with ASD attend to and process social learning stimuli. These studies, however, have used paradigms that involve the presentation of multiple cues aside from object labels, including social cues such as gaze, pointing, and head turns that aided directing attention to novel objects, making it difficult to isolate the effects of single cues, such as labeling, on visual attention to a novel object. Isolating the effect that the use of labels has on visual attention toward a novel object represents an important opportunity to further understand the ways in children with neurodevelopmental disorders attend to and learn from social cues.
The current study was designed to examine attention following in males with FXS within a referential context and to evaluate the effects of differentially providing pointing and labeling during exposure to a novel target object. We employed the McDuffie et al. (2006) paradigm to examine the visual processing of a social learning stimulus and examined the ways in which visual attention was distributed to objects as well as to the examiner’s face during fast mapping under conditions which varied only in the presence or absence of a label. A comparison group of boys with nonsyndromic ASD (i.e. boys who had received a behavioral diagnosis of an ASD and for whom a known genetic etiology had been ruled out) and younger TD boys were included to allow the examination of between-group differences in patterns of attention-following and subsequent word learning performance. The specific questions addressed in this study were: (1) Within each group, is there evidence of increased attention toward the labeled target object relative to the unlabeled distracter object? (2) Are there between-group differences in the amount of attention shown toward the target objects, distracter objects, and examiner’s face? (3) Within each group, does pointing result in increased attention toward the target object? (4) Are there between-group differences in target-object gaze when pointing occurs? and (5) Within each group, does the proportion of gaze to the target object relate to measures of cognitive ability, vocabulary, and ASD symptomatology?
2. Method
2.1. Participants
Participants were recruited as part of a larger study on the role of social and affective cues in word learning in males with FXS, ASD, and TD. Participants described in the present study overlap with those in several published and in-press samples also drawn from the larger project (e.g. Blinded for Review, 2013; Blinded for Review, in press; Blinded for Review, 2014; Blinded for Review, in press; Blinded for Review, under review). Participation in this larger study required that participants: (1) were between 4 and 10 years of age and had FXS or ASD or between 2 and 5 years of age with TD; (2) had nonverbal mental ages on the Leiter-R (described below) between 2 and 5;11 years; (3) were native English speakers with fluent English speaking parents; (4) lived with the biological mother; (5) used speech as the primary means of communication according to parent report; (6) had no uncorrected sensory or physical impairment that would impede performance in the study; (7) passed a pure tone air-conduction threshold of 30 dB HL or better in each ear (averaged across 500, 1000, and 2000Hz). In addition, children with TD did not meet criteria for ASD based on the Social Communication Questionnaire (described below), and had no current participation in special education services. Children with ASD had a community diagnosis of ASD had tested negative for a diagnosis of FXS previously. Participants with FXS had undergone genetic testing and had been shown to have the FMR1 full mutation (i.e. greater than 200 CGG repeats).
2.1.1. Participant selection for the present study
Participants selected for the present study were a subset of those participating in the larger study. Because FXS is an X-linked disorder and males are both more commonly and more severely affected than females, only males were included. Those in the present study were able to complete at least one valid trial of the eye-tracking paradigm (described below). These participants included 14 males with FXS ranging from 4.33 to 10.02 years (M = 7.68, SD = 1.85), 17 males with ASD ranging from 4.04 to 10.40 years (M = 6.92, SD = 1.89), and 18 males with TD ranging from 2.05 to 5.33 years (M = 3.70, SD = .88). Participants were selected from the larger study so that they were matched groupwise for nonverbal cognitive ability on the Leiter-R (described below).
2.2. Standardized Measures
2.2.1. Leiter International Performance Scale – Revised (Leiter; Roid & Miller, 1997)
The Leiter-R is a nonverbally administered test of cognitive ability that requires only nonverbal responses. The Brief IQ subtests of this measure were administered (i.e. Figure Ground, Form Completion, Sequential Order, and Repeated Patterns). Scores computed using the Leiter-R were standard scores (IQ), growth scores, and age-equivalent scores. Standard score indicates the child’s cognitive ability relative to peers, whereas the growth score provides an index of absolute ability levels. Due to the matching strategy, participants did not differ significantly on Leiter-R growth scores, F(2, 46) = .48, p = .62, ηp2 = 02, or nonverbal mental age, F(2, 46) = 0.63, p = .54, ηp2 = .03.. Participants with ASD, however, were observed to have somewhat higher IQs than those with FXS, a difference that just failed to reach significance t(29) = 2.02, p = .052, d = .74, which is not unexpected given roughly half of individuals with ASD do not have an intellectual disability (CDC, 2014). See Table 1 for means and standard deviations of Leiter IQ scores, growth scores, and age equivalent scores for each participant group.
Table 1.
Means and (Standard Deviation) on Standardized Measures
| FXS | ASD | TD | ||||
|---|---|---|---|---|---|---|
| Leiter NVIQ | 58.57 | (13.48) | 69.29 | (15.60) | 111.44 | (14.67) |
| Leiter Age Equivalent | 4.17 | (1.08) | 4.55 | (1.10) | 4.25 | (0.86) |
| Leiter Growth Scores | 453.93 | (13.04) | 457.82 | (11.00) | 455.50 | (9.57) |
| PPVT Raw | 70.71 | (34.52) | 55.00 | (27.72) | 73.33 | (26.62) |
| ADOS Severity/SCQ* | 6.36 | (2.59) | 8.06 | (1.56) | 3.74 | (2.27) |
Note: ADOS Severity for FXS or ASD, SCQ for TD
2.2.2. Peabody Picture Vocabulary Test – 4th edition (PPVT-4; Dunn & Dunn, 2007)
The PPVT-4 was used as a measure of receptive vocabulary. It is a norm-referenced tool standardized for use in individuals as young as 2 years old and with a diverse sample of individuals representative of the demographic makeup of the United States population. The test items are colorized pages, each with four full-color pictures on a page. The examiner produces a target word request (e.g. “Point to the bus”), and the participant responds by pointing to or otherwise selecting the picture that represents the word’s meaning. Raw scores from the PPVT-4 were used in the present study. A group comparison did not reveal significant differences in PPVT-4 raw scores, F(2, 46) = 1.96, p = .16, ηp2 = 08. See Table 1 for means and standard deviations of PPVT-4 raw scores for each participant group.
2.2.3. Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003)
The SCQ is a parent-report questionnaire based on the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) consisting of questions about a child’s developmental history. It was used as a screening tool for ASD symptoms and was administered to the parents of participants thought to be typically developing. A cutoff of 12 or above points was chosen to screen out participants for a possible ASD (Corsello et al., 2007). See Table 1 for means and standard deviations of SCQ Raw scores for TD participants.
2.2.4. Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 2002)
Participants with ASD or FXS were administered the ADOS, which is a play-based and semi-structured assessment designed to give children the opportunity to respond to a variety of social and interaction skills. The ADOS is a gold-standard measure of ASD symptoms, and in the present study was used to verify ASD classifications in participants with ASD as well as to characterize ASD symptom severity in participants with FXS or ASD. The ADOS was administered by research-reliable examiners. As expected, participants with ASD were observed to have higher ASD symptom severity than those with FXS, t(29) = 2.26, p = .03, d = .84. See Table 1 for means and standard deviations of ADOS Severity scores for participants with FXS or ASD.
2.3. Eye-tracking Stimuli
The video-recorded word-learning stimuli used in the current study were modeled after a live interactive word-learning task (McDuffie et al., 2006; McDuffie et al., 2013), that emphasized the use of a variety of social and affective cues (i.e., eye gaze, pointing, facial expressions, and intonation) while sequentially presenting two novel objects during each of four trials. During each trial, one object (the target) was labeled with one of four CVCV nonsense labels (boomee, geenay, mowfoo, and teedah) and a second object (the distracter) was not labeled, but was talked about for an equivalent amount of time using connected speech. Within a trial, only a single object was visible to the child at a time. The video-recorded stimuli were presented on a table-mounted eye tracker (see below for technical specifications), and the participant sat in a chair in front of the eye tracker or on a parent’s lap to watch the videos. All participants were able to complete the calibration to the manufacturer’s specifications and recommendations.
The novel objects were all made of common materials such as plastic, wood, and metal, and had a distinctive appearance. The objects were chosen based on their novelty, and object pairs were selected such that they contrasted in color. Target objects were consistently referred to by the same label and pairs of objects remained consistent across participants.
In each video-recorded trial, an actor presented one object of the pair individually while facing the camera. The video clips were constructed so that, to a child watching the screen, they would appear similar to what a child would see and experience during an interactive version of the task. The actor was dressed in plain clothes in front of a plain background. The presentation of the object pairs was randomized across participants. Within trials, the order of presentation of objects (target vs. distracter) and the side of presentation of the objects (right vs. left) was systematically varied across the four trials. In two of the trials, the target object was introduced first, whereas the distracter object was introduced first in the other two trials. Whether the target object appeared on the left or right of the examiner also varied, such that each of the four trials represented a unique combination of the order and side of presentation of the target object. Thus, the inclusion of four trials guarded against any bias toward the side or order of presentation of the target objects
2.3.1. Trial Pointing Segment
While introducing each of the novel objects, the actor picked up and gazed at the object, held it to his side at shoulder height, and pointed to the object saying, “Look, it’s a [novel label]” for the target, or, “Look, wow! See what I have?” for the distracter. During this portion of the trial, data were collected for gaze toward the following areas of interest (AOIs): object (i.e., target or distracter), the examiner’s face, and the examiner’s hand, which was pointing toward the object. This segment of the video stimulus is referred to as the Pointing Segment and lasted approximately three seconds.
2.3.2. Trial Elaboration Segment
Following the Pointing Segment, the object was kept to the side, but lowered slightly to chest height and the pointing hand was lowered out of sight. The examiner said either, “A [novel label]!” (for the target), or “See what I have?!” (for the distracter). The examiner then spoke three additional phrases. For the target, these short phrases included the novel label in sentence-final position (e.g., “I like showing you this modi. What a great modi. This is a special modi!”). In contrast, the three phrases offered for the distracter object did not include a label (e.g., “Look at this one. I like showing this to you. What a nice one!”). As this portion of the script was delivered, the examiner moved the objects slowly in place and alternated gaze several times between the object and camera. During this portion of the trial, data were collected for gaze toward the following AOIs: examiner’s face and the object visible on the screen. This segment of the video stimulus is referred to as the Elaboration Segment, and lasted approximately thirteen seconds. After the script was completed, the object was moved out of the video frame. See Table 2 for a visual depiction of the trial stimuli.
Table 2.
Visual Depiction of Eye Tracking Stimulus Trial (Order: Distracter/Target)
| 1) Pointing Segment: Examiner points to and draws attention to novel distracter object. “Look! Wow!” Examiner then lowers pointing hand out of sight, looks toward camera, and says, “See what I have?” |  |
| 2) Elaboration Segment: Examiner describes distracter object while shifting gaze between the object and camera. Examples of unlabeled descriptions: “What a nice one!” “I like showing you this.” A total of three descriptors are used. |  |
| 3) No objects are visible between presentation of 1st object and 2nd object of a pair. |  |
| 4) Pointing Segment: Examiner points to and labels a target object. “Look! It’s a Boomee!” Examiner then lowers pointing hand out of sight, looks toward camera, and says, “A boomee!” |  |
| 5) Elaboration Segment: Examiner describes a target object while gazing between the object and camera. Examples of labeled descriptions” “What a great Boomee!” “I love showing this Boomee.” A total of three labeling phrases are used. |  |
2.3.3. Trial Exclusion
If a participant showed no gaze to any AOI during either segment of a trial, the participant’s data for that trial was excluded from all further analyses. Table 3 indicates the number of participants who had trials excluded for this reason. One participant with ASD and one participant with FXS showed no gaze toward the AOIs in any of the four trials and thus, were completely excluded from further analyses. All remaining participants contributed data for at least one trial.
Table 3.
Trials by Group Eliminated due to no Gaze
| Novel Labels |
FXS | ASD | TD |
|---|---|---|---|
| Boomee | 2 | 1 | 0 |
| Geenay | 2 | 3 | 1 |
| Mowfoo | 3 | 2 | 1 |
| Teedah | 1 | 1 | 0 |
2.4. Procedure
Data were collected at two university sites in the United States. Trained examiners at both sites administered the eye-tracking and standardized measures. Participants completed several word-learning tasks as a part of their participation in a larger study, including an interactive version of this task, though different words were used. In all cases, the eye-tracking task was implemented following the completion of all other word learning tasks and standardized testing. At one site, the eye-tracking stimuli were presented on a Tobii 1750 eye tracker monitor. At the other site, the video stimuli were presented on a Tobii 2150 eye tracker monitor using identical software. At the site using the Tobii, the monitor resolution was adjusted prior to collecting each participant’s data to make the stimulus presentations identical and the data output comparable to the Tobii 1750. The eye-tracking device consists of a monitor that tracks eye gaze that is connected to a computer that allows the video stimuli to be played. The eye-tracker emits infrared light that is reflected off the pupils of participants, and cameras on the eye-tracker are able to detect these reflections and use them to indicate where on the video stimuli a participant was looking at a given point.
Prior to starting the eye-tracking trials, participants were led into the eye-tracking room and shown a brief three-page booklet describing the procedure in child-friendly language, with pictures and text reminding them to be still and quiet during the procedure, as well as a reminder of a prize afterward. Each participant then went through a calibration routine on the eye-tracking screen to contribute to the unique calibration profile maintained for each individual. Following the calibration, each participant viewed the four pre-recorded word-learning video stimulus clips. Each full teaching trial was approximately 32 seconds in length, and participants were shown the four trials with minimal time between trials to minimize distraction. The order of presentation of the four stimuli clips was varied across participants using randomly generated presentation orders.
For each of the four video clips, and in each of the segments (i.e., target presentation, distracter presentation; see Table 1), AOIs were selected and drawn in the eye-tracking software around the examiner’s eyes, mouth, face (exclusive of the eyes and mouth areas), the target object, and the distracter object. For the present study, the eye, mouth, and other face gaze was combined for one “face” variable that captured all gaze to all face regions. Depictions of these areas of interest are shown below from a still image of one of the four stimuli for demonstration purposes in Figure 1. Clearview software version 2.7 (Tobii Technology, Sweden) was used to create the AOIs for the video stimuli as well as calculate gaze characteristics for each participant for each of the four video clips. This software automatically tracks gaze in real time throughout the stimulus presentations, and when a participant’s gaze enters an AOI, the software calculates how much time is spent fixating on that AOI in milliseconds. These AOIs were used to create variables to examine different aspects of the child’s initial recognition of an object, sustained attention to target objects, as well as compliance with on-screen instructions.
Figure 1.
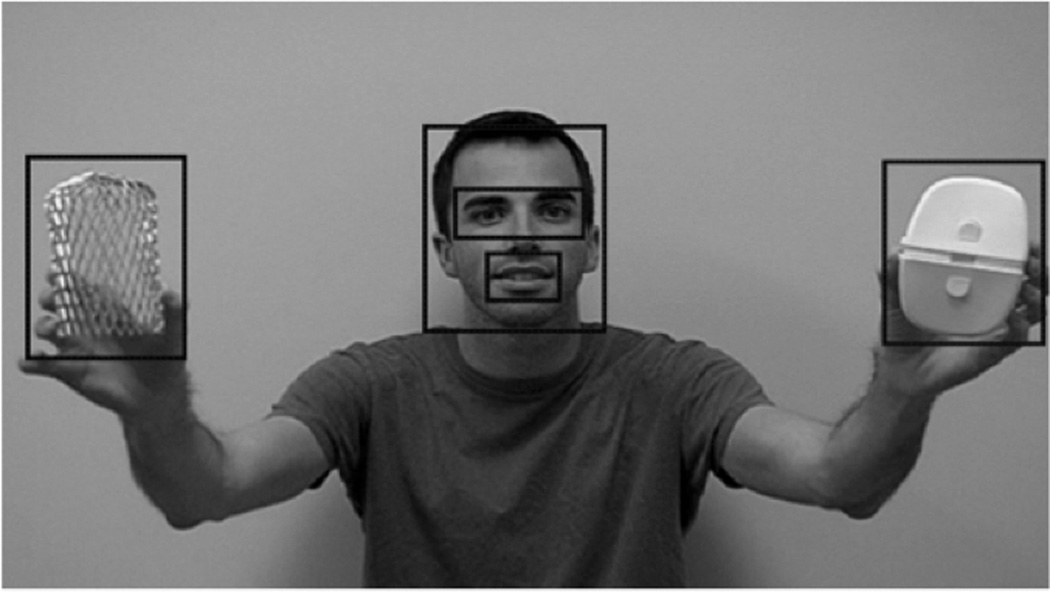
AOIs Used to Collect Gaze
Total looking time to all AOIs in each group was examined to determine whether the groups showed similar amounts of overall attention to the stimuli. Although a significant main effect of group was not observed, F(2, 46) = 2.77, p = .07, the TD group tended to show more gaze toward the stimuli than the other two participant groups on average. As a result, proportions were created for each participant and for each stimulus video separately, and then collapsed across stimuli to create final gaze variables. These variables were created using cumulative gaze toward a particular AOI as the numerator, and that participant’s total gaze toward all AOIs in that scene as the denominator. Thus, the final variables represent each participant’s gaze toward an AOI in question expressed as a proportion of his total gaze toward all of the AOIs in that scene.
3. Results
3.1. Participant Gaze to the Target Object Relative to Distracter Object
In order to evaluate the effect of labeling on proportion of gaze to the target object, the first research question focused on whether participants within each group directed a greater proportion of their gaze toward the target object than they did toward the distracter object across both segments of the video-recorded stimuli. Paired-samples t-tests revealed that neither the FXS participants, t(13) = −1.73, p = .11, nor the TD participants, t(18) = −1.11, p = .28, directed more gaze toward the target than the distracter object. In children with ASD, the tendency to show more distracter object gaze than target object gaze approached, but did not reach, significance, t(17) = −1.92, p = .07. An additional analysis comparing total gaze during the target presentation and total gaze during the distracter presentation (i.e. total gaze to all AOIs, not proportions) revealed that, overall gaze toward the video-recorded stimuli was similar during presentations of the target objects and distracter objects across all three groups (FXS: t(13) = 1.15, p = .27, TD: t(18) = 1.65, p = .12, and ASD: t(17) = .04, p = .97). In other words, differences in proportions of gaze toward one object cannot be explained by differences in the amount of time participants in each group spent viewing the stimuli.
3.2. Between-Group Differences in Gaze to Areas of Interest
To address the question of whether between-group differences existed in proportion of gaze to the AOIs, three separate one-way ANOVAs compared the proportion of attention to (a) the target objects, (b) the distracter objects, and (c) the face of the examiner during the target and distracter object presentations. A significant main effect of Group was observed with respect to both target object gaze, F(2, 46) = 8.47, p = .001, ηp2 = .27, and distracter object gaze, F(2, 45) = 3.67, p = .03, ηp2 = .14. Fisher’s LSD pairwise analyses revealed that participants with TD and ASD, who did not differ from each other, showed greater proportions of gaze toward both the target and distracter objects than did participants with FXS. Next, a significant main effect of Group was observed with respect to gaze toward the face during the target object presentation, F(2, 46) = 5.52, p = .007, ηp2 = .19, as well as distracter object presentation, F(2, 45) = 3.55, p = .04, ηp2 = .14. Fisher’s LSD pairwise tests revealed that participants with FXS showed a significantly greater proportion of gaze to the face than did participants with ASD or TD, who did not differ from each other.
To further characterize the ways in which the groups differed in their gaze distributions toward the stimuli, two separate repeated-measures ANOVAs were conducted. In the first analysis, object gaze and face gaze during the target object presentation were examined as the repeated measures and group was the between-subjects measure. A main effect of Group was not found, F(2, 46) = 3.17, p = .051, ηp2 = .12; however, there was a significant effect for AOI, F(1, 46) = 24.45, p < .001, ηp2 = .35, indicating that participants were proportionally more likely to direct their gaze to the face rather than to the target object. The main effect of AOI was qualified by a statistically significant interaction between AOI and Group, F(2, 46) = 7.14, p = .002, ηp2 = .24, revealing that males with FXS directed a significantly greater proportion of their gaze toward the face than the target object relative to the other groups (see Figure 2).
Figure 2.
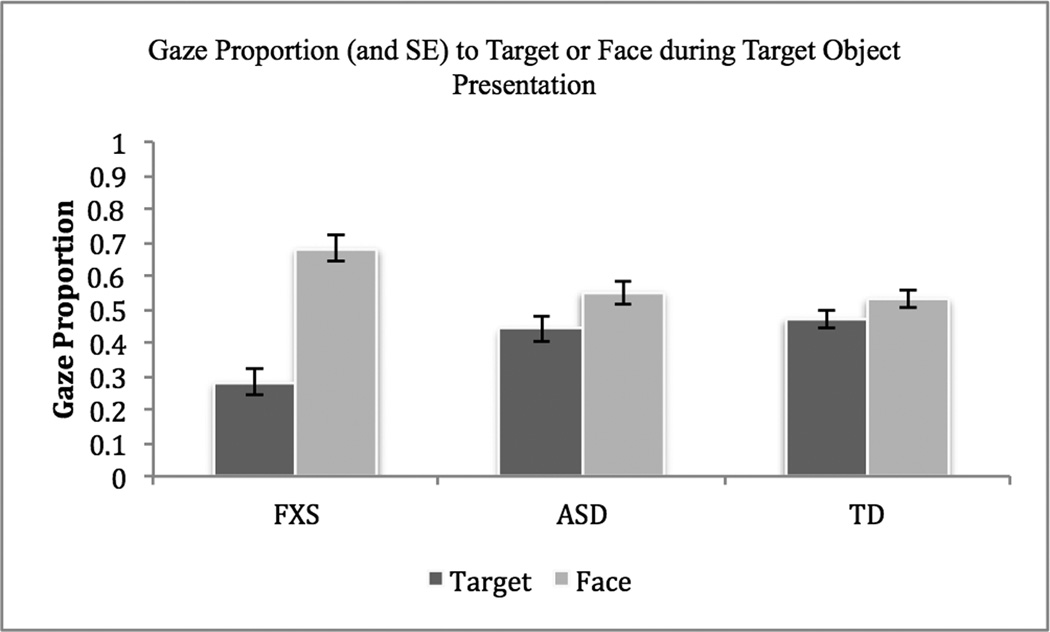
In the second analysis, object gaze and face gaze during the distracter object presentation was examined as the repeated measures and group was the between-subjects measure. Neither the main effect for Group, F(2, 45) = .42, p = .66, ηp2 = .02, nor AOI, F(1, 45) = 3.01, p = .09, ηp2 = .06, was significant. A statistically significant interaction between AOI and Group, however, was observed, F(2, 45) = 3.62, p = .04, ηp2 = .14, indicating once again that participants with FXS directed a significantly greater proportion of their gaze toward the face of the examiner than the distracter object (See Figure 3). Follow-up paired t-tests comparing object gaze and face gaze revealed that only for participants with FXS was gaze to the face significantly greater than object gaze for both the target, t(14) = −5.44, p < .001, and distracter objects, t(13) = −2.26, p = .04.
Figure 3.
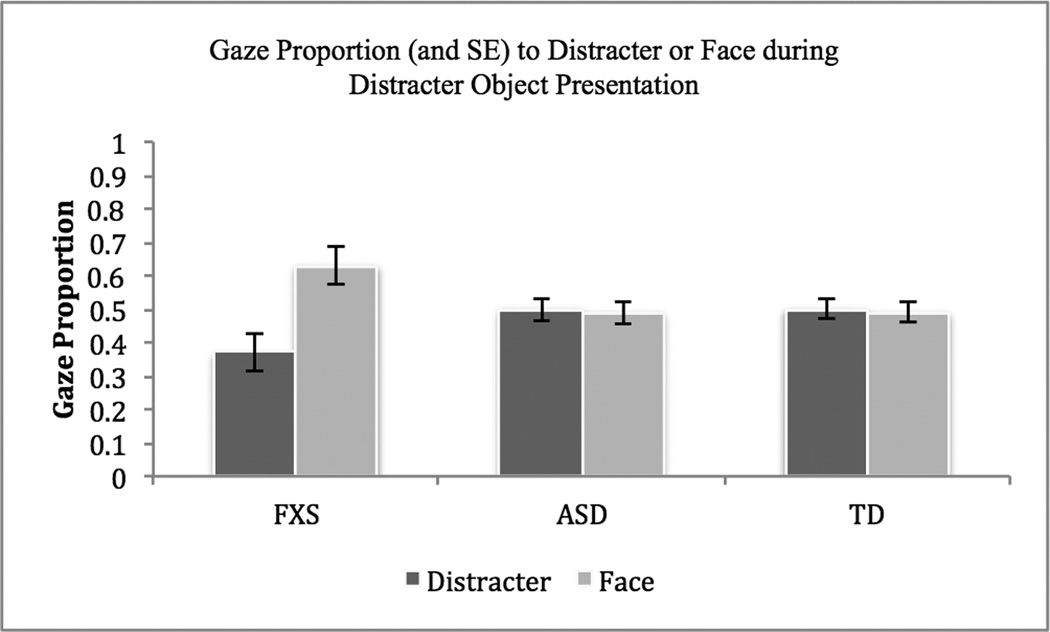
3.3. Effect of Pointing on Target-Object Gaze
The third research question focused on whether pointing led to a greater proportion of gaze toward the target objects during the Pointing Segment of each trial (i.e., the portion of the stimulus video during which the examiner used pointing to direct attention to the novel object) relative to the Elaboration Segment of each trial (i.e., the portion of the stimulus video during which no additional attention-directing gestural cues were provided). These comparisons were conducted using within-group paired-samples t-tests. Results indicated a significant effect of pointing for all participant groups; that is, participants with FXS, t(13) = 3.09, p = .009, ASD, t(17) = 4.17, p = .001, and TD, t(18) = 2.79, p = .01, all directed a greater proportion of their gaze toward the target object during the Pointing Segment of the stimulus video than they did during the Elaboration Segment of the stimulus video. Table 4 shows mean values of target object gaze and standard deviations for these analyses.
Table 4.
Proportion of Gaze to Target Objects during Pointing and Elaboration Segments of Stimulus Video: Means (and SD)
| Group | Pointing Segment | Elaboration Segment | p value |
|---|---|---|---|
| FXS | .41 (.20) | .20 (.14) | p = .01 |
| ASD | .56 (.16) | .38 (.13) | p = .001 |
| TD | .56 (.17) | .44 (.14) | p = .009 |
3.4. Between-Group Differences in Target Object Gaze During Pointing Segment
The next research question focused on whether the groups differed in their gaze to the target objects during the Pointing Segment of the experimental task. Between-group analyses using a one-way ANOVA revealed a significant main effect for group, F(2, 46) = 3.72, p = .03, ηp2 = .14, with post-hoc tests using Fisher’s LSD indicating that participants with TD and ASD, while not differing from each other, both showed a greater proportion of gaze toward the target objects than those with FXS during the initial Pointing Segment of the task.
3.5. Concurrent Correlates of Fast-Mapping Task Performance
The final research question focused on whether, within each group, proportion of gaze to the target object was concurrently correlated with measures of nonverbal cognitive ability, vocabulary understanding, or ASD symptomatology. Raw scores from the PPVT-4, growth scores from the Leiter-R, ADOS severity scores for the groups with ASD and FXS, and total SCQ scores for TD participants, were correlated with proportion of gaze shown toward the target objects separately for each segment of the teaching phase. One-tailed tests were used.
For participants with ASD, a significant positive correlation was observed between proportion of target object gaze during the Pointing Segment and PPVT-4 raw scores (r = .46, p = .03) and a significant negative correlation was observed between proportion of target object gaze during the Pointing Segment and ADOS Severity scores (r = −.46, p = .03). No other associations between participant characteristics and proportion of target-object gaze reached significance (see Table 5). In addition, no significant relationships were observed for any participant group between nonverbal cognitive ability, receptive vocabulary, or ASD symptomatology and proportion of gaze shown toward the distracter object.
Table 5.
Means, Standard Deviations, and Correlations between Task Performance and Child Characteristics
| FXS | M | SD | 1. | 2. | 3. | |
| Label-Point Target Object Gaze | .41 | .21 | .30 | .38 | .27 | |
| Label Describe Target Object Gaze | .20 | .14 | .30 | .42 | −.42 | |
| Child Variables | ||||||
| 1. PPVT-4 | 70.71 | 34.52 | -- | .82** | −.04 | |
| 2. Leiter-R GS | 453.93 | 13.04 | -- | −.16 | ||
| 3. ADOS Severity | 6.36 | 2.59 | -- | |||
| ASD | M | SD | 1. | 2. | 3. | |
| Label-Point Target Object Gaze | .56 | .16 | .46* | .23 | −.46* | |
| Label Describe Target Object Gaze | .38 | .13 | .09 | .00 | −.40 | |
| Child Variables | ||||||
| 1. PPVT-4 | 55.00 | 27.72 | -- | .65** | −.02 | |
| 2. Leiter-R GS | 457.82 | 11.00 | -- | .04 | ||
| 3. ADOS Severity | 8.06 | 1.56 | -- | |||
| TD | M | SD | 1. | 2. | 3. | |
| Label-Point Target Object Gaze | .56 | .17 | −.25 | −.42 | −.08 | |
| Label Describe Target Object Gaze | .44 | .14 | .23 | .21 | .29 | |
| Child Variables | ||||||
| 1. PPVT-4 | 73.33 | 26.62 | -- | .74** | −.09 | |
| 2. Leiter-R GS | 455.50 | 9.57 | -- | .06 | ||
| 3. SCQ Total | 3.75 | 2.27 | -- | |||
p < .05
p < .01
4. Discussion
The present study examined how videotaped word learning stimuli that included a label, head turns, and pointing gestures delivered by an examiner affected attention toward novel objects in young boys with FXS. A group boys with ASD and a group of younger TD boys were used as developmental-level matched comparison groups. Specifically, the present study was designed to examine whether labeling cues would increase attention toward target objects. Results indicated that labeling cues did not result in increased looking toward a target object over an unlabeled distracter object for any group. Given the non-contingent nature of the eye-tracking stimuli, it could be that the video stimuli were not as engaging as a live examiner, and thus did not elicit the same responsiveness to the labeling cues seen in interactive studies.
4.1. Gaze to Face
Several unexpected and informative findings, however, did emerge that add to our understanding of profiles of social attention in FXS, such as increased face gaze in FXS relative to the other two participant groups. In our dynamic stimulus videos, there were many competing visual cues and aspects that could draw attention. Although no group showed increased target object versus distracter object gaze, the different ways in which the participant groups distributed their gaze across the stimuli provide useful insights into how their different attention profiles may facilitate learning. First, participants with TD and ASD showed more gaze to both target and distracter objects than did participants with FXS. During the presentation of the objects, participants with TD and ASD distributed their gaze evenly between the target or distracter object and the examiner’s face. This is in contrast to participants with FXS, who in both instances showed not only more gaze to the face than did participants with TD or ASD did, but also more face gaze than object gaze.
This is a particularly interesting finding in light of the fact that many consider ASD and FXS to have qualitatively similar deficits in social approach and response behaviors. In fact, this finding corresponds with recent findings regarding subtle but important social differences between individual with FXS and those with ASD. For example,McDuffie et al. (2014) utilized the Autism Diagnostic Interview-Revised and demonstrated that boys with FXS were less impaired in social smiling than boys with ASD who were matched on chronological age and severity of ASD affectedness. Similarly, several groups of investigators have suggested that boys with FXS are less impaired in Reciprocal Social Interaction, as measured by the ADOS, than are boys with ASD (Clifford et al., 2007; Hall et al., 2010; Wolff et al., 2012). McDuffie, Thurman, Hagerman, and Abbeduto (2014) also demonstrated that, in direct observations, even boys with FXS who met criteria for a comorbid diagnosis of ASD showed less impairment in facial expressions, quality of social overtures, and shared enjoyment than age matched boys with ASD. These authors have argued that such differences may reflect the fact that the underlying psychological (and neuropsychological) mechanisms producing ASD-like symptoms are different for the two disorders.
Previous studies have often focused on gaze aversive behaviors, or anxiety in response to faces, for individuals with FXS, and have shown either decreased duration and fixations to faces (e.g. Farzin et al., 2009), or have suggested that face aversion may be a strategy to reduce anxiety in FXS (e.g. Hessl et al., 2006). The present findings, however, indicate a more face-centric focus of attention in FXS. Thus, in the FXS participants, it may be that the context of the task led to a distribution of attention toward the face of the examiner instead of the novel objects. This finding is interesting given that even studies using static facial imagery have demonstrated an aversion to gazing at faces (Farzin et al., 2009), although there is recent evidence that while there may be initial disengagement from faces, individuals with FXS may spend similar amounts of time viewing them (Williams, Porter, & Langdon, 2013). Thus, the context of the task, which was focused on directing attention to an object and characterized by gaze shifting, as well as the amount of time the face was available to view, may have contributed to a situation in which participants with FXS less anxious about looking at the face of the examiner.
Attention regulation difficulties, especially toward dynamic stimuli, have long been recognized as an important phenotypic characteristic of individuals with FXS. Disruption of attention is a frequently cited aspect of the behavioral phenotype of children with FXS (see Cornish & Wilding, 2010). In particular, there is evidence that individuals with FXS may have increased difficulty switching their attention from one stimulus to another (Woodcock, Oliver, & Humphreys, 2009) and sustaining attention (Sullivan et al., 2007), which could impact learning in a dynamic setting. In the present study, the examiner’s face was the visible prior to both the target and distracter objects emerging, and it may be that without in-vivo cues to redirect attention toward an object and away from a face, attention shifting for children with FXS was less successful than for children with TD or ASD. The ability to coordinate attention between a social partner who may be giving important pragmatic cues such as labels or points, and the novel object these cues are intended to highlight, is an important ability in naturalistic word learning contexts (McDuffie et al., 2006; McDuffie et al., 2013). Thus, the present findings suggest that there may be some attention regulation involvement underlying the challenges that individuals with FXS face in learning words in such settings. Although there is evidence from a similar paradigm that children with FXS may be better equipped to make new object-label pairings in such a task than age-matched children with ASD (McDuffie et al., 2013), there is also evidence that a difficulty in attention coordination may be behind some of the learning and cognitive deficits observed in FXS (Cornish et al., 2001; Cornih, Cole, Longhi, Karmiloff-Smith, & Scerif, 2012; Munir, Cornish, & Wilding, 2000; Wilding, Cornish, & Munir, 2002).
Thus, it may be that the logistics of an on-screen paradigm combined with the attentional characteristics of the FXS behavioral phenotype together contributed to a pattern of gaze and attention that remained relatively more fixated on the examiner’s face than on the objects that were subsequently introduced. In the absence of real-time supportive cues, it may be that the underlying deficits in the processing of gaze (e.g. Dalton et al., 2008; Garrett et al., 2004; Watson, Hoeft, Garrett, Hall, & Reiss, 2008) or in the integration of bimodal audio-visual cues (e.g. Scerif, Longhi, Cole, Karmiloff-Smith, & Cornish, 2012) contributed to a pattern of gaze in FXS that was not conducive to object attentiveness.
A comparison of these findings with a paradigm examining gaze characteristics of a FXS group during a “live” interactive task would be important for a number of reasons. First, given the relative strength of participants with FXS compared to ASD in both receptive vocabulary skills (Abbeduto et al., 2007) as well as fast-mapping abilities (McDuffie et al., 2013), similar findings across paradigms might indicate a different strategy employed in FXS during such a word-learning setting, where bi-modal speech cues may be important for consolidating the pairing between the object and novel spoken word (e.g. Lewkowicz & Hansen-Tift, 2012). Second, such a comparison could indicate that video presentations of otherwise social material may offer a way to present this material to individuals with FXS while avoiding the face aversion typically observed in during in-person interactions with individuals with FXS, leading to a better view of the upper bounds of their competence.
4.2. Gaze to Objects in the Context of Pointing
One result that united all participant groups was the finding that the action of pointing toward the target object was related to significantly increased attention toward that object as compared to labeling the object without pointing at it. These findings corroborate other eye-tracking findings in TD and ASD, in which the introduction of either additional motion (Akechi et al, 2011) or pointing (Akechi et al., 2013) increased attention in ASD during a referential word-learning task. Thus, the finding in the present study that children with FXS along with children with ASD and TD showed increased attention toward labeled target objects in the presence of a pointing cue highlights a potential strategy for improving learning in both in-vivo and on-screen modalities. This finding has important implications for interventions aimed at increasing the effectiveness of interactive-based teaching opportunities, whether delivered by parents or teachers, where using additional pointing cues could be a strategy to increase attention following toward desired teaching targets whether in-vivo or on-screen.
4.3. Correlates of Performance
One of the goals of the present study was to examine the association between target-object gaze and standardized language measures in order to examine the construct validity of the eye-tracking measure. A previous study examining such a connection (Akechi et al., 2011) was unable to demonstrate a link between verbal mental age and target object gaze in a non-ostensive word-learning paradigm. In our paradigm, the proportion of gaze shown toward the target object correlated significantly with PPVT-4 raw scores in the group with ASD, thereby supporting the potential utility of eye tracking measures of word learning as construct-valid approaches to assessing vocabulary. Furthermore, the additional finding that target object gaze was negatively correlated to ASD symptom severity in the ASD group but not in the FXS group adds to the literature suggesting that there are different underlying neuropsychological and neurobiological mechanisms or pathways behind the social and joint attention profiles of these two groups of children (e.g. McDuffie et al., 2010, McDuffie et al. 2014; Blinded for Review, under review; Wolff et al., 2012).
4.4. Limitations
In this study, increased target object gaze relative to distracter object gaze was conceptualized as a way to measure whether a participant succeeded in recognizing the importance of the pragmatic cue of labeling. Other eye-tracking studies in ASD have also used a proportional distribution measure when examining attention to a target versus distracter object (Gliga et al., 2012). In the present study, however, the target object and distracter object were not visible together and thus, not competing for the child’s attention, so the use of this metric might not have been the most effective way to measure relative attention toward the target objects. This seems to be especially true in the FXS participant group, where a high proportion of gaze was shown toward the examiner’s face instead of to either of the novel objects. Despite the logistical constraints of the current paradigm, then, it is a promising finding that significant correlations were found between target-object gaze and demonstrated language abilities for participants with ASD. Given this constraint, the main measure of attention in the current study was the proportion of gaze participants showed toward the target objects versus the distracter objects. The task parameters, however, also did not allow participants an opportunity to follow gaze to an incorrect location, as only one object was introduced at a time, and the screen offered nothing else to view aside from the examiner and one object. Successful task performance was defined as an object-oriented pattern of gaze, but there are a number of reasons the novel objects, and the target objects in particular, may not have received larger proportions of gaze. First, it may be that the face was more interesting to participants with FXS despite the novelty of the objects. Throughout the object presentation, the examiner was talking and shifting gaze between the camera and object, in an attempt to make eye contact with the participant. This combination of movement as well as audio-visual cues provided by the examiner may account for either the lack of object-focused gaze in the ASD and TD groups, or the face-centric viewing by the FXS group. Second, it may be that, in the absence of encouraging words or the opportunity to engage with the objects, as was the case in the McDuffie et al., (2013) study, longer durations of gaze toward the objects were not as appealing for the participants as other aspects of the stimuli. In addition to these logistical realities, the two novel objects were never presented together. Although this was an intentional manipulation aimed at simplifying the word-learning task in the original McDuffie et al. (2006) paradigm, the combined effects of these task parameters may have led to a pattern of viewing that was contrary to what was expected.
The passive viewing of video stimuli without any further interaction from an examiner that constituted the present task is unlike what most children are likely to encounter with a communicative partner in a naturalistic word-learning setting. In such a context, it is likely that children will also encounter additional cues that will guide their gaze toward target objects, which was not the case in the present study. Nevertheless, these findings represent a first account of the gaze patterns of individuals with FXS during a fast-mapping paradigm, and suggest that the type of gaze aversive behaviors long reported in FXS may be context dependent instead of universal.
As a result of the small sample of participants, especially in the FXS group, an analysis of gaze patterns of participants with FXS with and without a classification of ASD was not possible. Although ASD severity scores were not as high, on average, as in the non-syndromic ASD group, most FXS participants in the current study had an ASD classification based on the administration of the ADOS and ADI-R. The nature of ASD symptoms in FXS is an ongoing controversy in the literature (e.g. Hall et al., 2010; McDuffie et al., 2014; Wolff et al., 2012). However, it should be noted that the gaze patterns of individuals with FXS in the current study were significantly different from those displayed by participants with non-syndromic ASD. It will be important for future studies with larger participant samples to examine patterns of gaze in FXS and ASD for groups of participants who are matched on ASD classification or symptom severity.
4.5. Future Directions
The present study represents the first use of eye-tracking methodology to examine gaze during a fast-mapping paradigm in FXS, as well as to examine the effect of labeling on gaze directed to a target object. An important next step for this research will be to examine the effects of object gaze on subsequent comprehension of novel labels. McDuffie et al. (2006) demonstrated that attention following during the teaching phase of an interactive fast-mapping paradigm was significantly related to performance measured during a forced choice comprehension probe. Additionally, this same study demonstrated that fast-mapping ability as measured during comprehension probes was significantly related to spoken vocabulary as measured by parent report. Patterns of gaze obtained during the present study suggest an interactive component may be important to encourage on-task participation. This could be especially important for participants with FXS due to the problems they can experience with the coordination of and sustaining attention (Cornish, Sudhalter, & Turk, 2004). Furthermore, the present study only examined gaze in males with FXS and with ASD; future studies including females are warranted to determine whether the same pattern of findings observed would apply to females as well. In FXS as in ASD, understanding the ways in which phenotypic characteristics can interfere with language acquisition can be informative for designing teaching strategies that can optimally direct the cognitive resources needed for learning. Understanding the ways in which individuals with neurodevelopmental disorders visually process teaching scenes containing learning information can be valuable in representing a baseline for designing teaching opportunities that can more effectively direct attention toward valuable early learning opportunities.
Highlights.
Verbal labeling did not increase attention to target objects in any group.
Males with FXS showed increased face gaze relative to those with TD or ASD.
Pointing increased visual attention to the target objects in all groups.
Target object gaze was related to vocabulary and autism severity in males with ASD.
Acknowledgements
This work was supported by NIH grants R01 HD054764, P30 HD03352, and U54 HD079125 from the National Institute of Child Health and Human Development. We wish to thank the children and their families for their participation in this study. Leonard Abbeduto has received financial support to develop and implement outcome measures for fragile X syndrome clinical trials from F. Hoffman-LaRoche, Ltd., Roche TCRC, Inc., and Neuren Pharmaceuticals Limited. Randi J. Hagerman has received funding from Novartis, Roche Pharmaceuticals, Curemark, Forest, and Seaside Therapeutics to carry out treatment studies in FXS and autism. She also has consulted with Roche/Genentech and Novartis regarding treatment studies in fragile X syndrome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other authors have financial disclosures to make.
Contributor Information
David P. Benjamin, Email: dpbenjamin@ucdavis.edu.
Ann M. Mastergeorge, Email: amastergeorge@u.arizona.edu.
Andrea S. McDuffie, Email: andrea.mcduffie@ucdmc.ucdavis.edu.
Sara T. Kover, Email: skover@uw.edu.
Randi J. Hagerman, Email: randi.hagerman@ucdmc.ucdavis.edu.
Leonard Abbeduto, Email: leonard.abbeduto@ucdmc.ucdavis.edu.
References
- Abbeduto L, Brady N, Kover ST. Language development and fragile X syndrome: profiles, syndrome-specificity, and within-syndrome differences. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13(1):36–46. doi: 10.1002/mrdd.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson LB, Bakeman R, Deckner DF, Romski M. Joint engagement and the emergence of language in children with autism and Down syndrome. Journal of Autism and Developmental Disorders. 2009;39(1):84–96. doi: 10.1007/s10803-008-0601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson LB, Deckner DF, Bakeman R. Early interests and joint engagement in typical development, autism, and Down syndrome. Journal of Autism and Developmental Disorders. 2010;40(6):665–676. doi: 10.1007/s10803-009-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akechi H, Kikuchi Y, Tojo Y, Osanai H, Hasegawa T. Brief Report: Pointing Cues Facilitate Word Learning in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-012-1555-3. [DOI] [PubMed] [Google Scholar]
- Akechi H, Senju A, Kikuchi Y, Tojo Y, Osanai H, Hasegawa T. Do children with ASD use referential gaze to learn the name of an object? An eye-tracking study. Research in Autism Spectrum Disorders. 2011;5:1239–1242. doi: 10.1016/j.rasd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal of Mental Retardation. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Moses LJ. Links between social understanding and early word learning: Challenges to current accounts. Social Development. 2001;10(3):309–329. [Google Scholar]
- Baldwin DA. Early Referential Understanding: Infants' Ability to Recognize Referential Acts for What They Are. Developmental Psychology. 1993a;29(5):822–843. [Google Scholar]
- Baldwin DA. Infants' ability to consult the speaker for clues to word reference. Journal of Child Language. 1993b;20(2):395–418. doi: 10.1017/s0305000900008345. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Markman EM. Establishing word-object relations: a first step. Child Development. 1989;60(2):381–398. doi: 10.1111/j.1467-8624.1989.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Baldwin DA, Markman EM, Bill B, Desjardins RN, Irwin JM, Tidball G. Infants' reliance on a social criterion for establishing word-object relations. Child Development. 1996;67(6):3135–3153. [PubMed] [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M. Do children with autism use the speaker's direction of gaze strategy to crack the code of language? Child Development. 1997;68(1):48–57. [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N, Skinner D, Roberts J, Hennon E. Communication in young children with fragile X syndrome: a qualitative study of mothers' perspectives. American Journal of Speech-Language Pathology. 2006;15(4):353–364. doi: 10.1044/1058-0360(2006/033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N, Warren SF, Fleming K, Keller J, Sterling A. Effect of Sustained Maternal Responsivity on Later Vocabulary Development in Children With Fragile X Syndrome. Journal of Speech Language and Hearing Research. 2014;57:212–226. doi: 10.1044/1092-4388(2013/12-0341). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briganti AM, Cohen LB. Examining the role of social cues in early word learning. Infant Behavior and Development. 2011;34(1):211–214. doi: 10.1016/j.infbeh.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Carey S, Bartlett E. Acquiring a Single New Word. Papers and Reports on Child Language Development. 1978;15:17–29. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63(4):i–vi. 1–143. [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, United States, 2010. Morbidity & Mortality Weekly Report (MMWR) 2014;63(SS02):1–21. [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Drew A, Cox A. Predicting language outcome in infants with autism and pervasive developmental disorder. International Journal of Language and Communication Disorders. 2003;38(3):265–285. doi: 10.1080/136820310000104830. [DOI] [PubMed] [Google Scholar]
- Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) Journal of Child Language. 2003;30(1):213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Shic F. Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012 doi: 10.1111/j.1469-7610.2012.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37(4):738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3(1):57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Cole V, Longhi E, Karmiloff-Smith A, Scerif G. Does attention constrain developmental trajectories in fragile×syndrome? A 3-year prospective longitudinal study. American Journal on Intellect and Developmental Disabilities. 2012;117(2):103–120. doi: 10.1352/1944-7558-117.2.103. [DOI] [PubMed] [Google Scholar]
- Cornish K, Munir F, Wilding J. A neuropsychological and behavioural profile of attention deficits in fragile X syndrome. Revista de Neurologia. 2001;33(Suppl 1):S24–S29. [PubMed] [Google Scholar]
- Cornish K, Sudhalter V, Turk J. Attention and language in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):11–16. doi: 10.1002/mrdd.20003. [DOI] [PubMed] [Google Scholar]
- Cornish K, Wilding J. Attention, genes, and developmental disorders. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Corsello C, Hus V, Pickles A, Risi S, Cook EH, Jr, Leventhal BL, Lord C. Between a ROC and a hard place: decision making and making decisions about using the SCQ. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(9):932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze fixation during facial-emotion processing in fragile X and autism. Autism Research. 2008;1(4):231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Deak GO, Flom RA, Pick AD. Effects of gesture and target on 12- and 18-month-olds' joint visual attention to objects in front of or behind them. Developmental Psychology. 2000;36(4):511–523. [PubMed] [Google Scholar]
- Doherty MJ, Anderson JR. A new look at gaze: Preschool children's understanding of eye-direction. Cognitive Development. 1999;14(4):549–571. [Google Scholar]
- Dollaghan CA. Fast mapping in normal and language-impaired children. Journal of Speech and Hearing Disorders. 1987;52(3):218–222. doi: 10.1044/jshd.5203.218. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test. 4th ed. ed. Circle Pines, MN: American Guidance Service; 2007. [Google Scholar]
- Farzin F, Rivera SM, Hessl D. Brief Report: Visual Processing of Faces in Individuals with Fragile X Syndrome: An Eye Tracking Study. Journal of Autism and Developmental Disorders. 2009 doi: 10.1007/s10803-009-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here's looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Archives of General Psychiatry. 2004;61(3):281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Gillespie-Lynch K, Elias R, Escudero P, Hutman T, Johnson SP. Atypical Gaze Following in Autism: A Comparison of Three Potential Mechanisms. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Elsabbagh M, Hudry K, Charman T, Johnson MH. Gaze Following, Gaze Reading, and Word Learning in Children at Risk for Autism. Child Development. 2012;83(3):926–938. doi: 10.1111/j.1467-8624.2012.01750.x. [DOI] [PubMed] [Google Scholar]
- Grassmann S, Tomasello M. Young children follow pointing over words in interpreting acts of reference. Developmental Science. 2010;13(1):252–263. doi: 10.1111/j.1467-7687.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Hirt M, Rezvani A, Reiss AL. Autism in fragile X syndrome: a category mistake? Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):921–933. doi: 10.1016/j.jaac.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Hagerman RJ. Autism profiles of males with fragile X syndrome. American Journal of Mental Retardation. 2008;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heibeck TH, Markman EM. Word learning in children: an examination of fast mapping. Child Development. 1987;58(4):1021–1034. [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2006;47(6):602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, Hall S. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. Journal of Neurodevelopmental Disorders. 2009;1(1):33–45. doi: 10.1007/s11689-008-9001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollich GJ, Hirsh-Pasek K, Golinkoff RM, Brand RJ, Brown E, Chung HL, Rocroi C. Breaking the language barrier: an emergentist coalition model for the origins of word learning. Monographs of the Society for Research in Child Development. 2000;65(3):i–vi. 1–123. [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Leekam S, Baron-Cohen S, Perrett DI, Milders M, Brown S. Eye-direction detection: A dissociation between geometric and joint attention skills in autism. British Journal of Developmental Psychology. 1997;15(1):77–95. [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Hansen-Tift AM. Infants deploy selective attention to the mouth of a talking face when learning speech. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(5):1431–1436. doi: 10.1073/pnas.1114783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebal K, Behne T, Carpenter M, Tomasello M. Infants use shared experience to interpret pointing gestures. Developmental Science. 2009;12(2):264–271. doi: 10.1111/j.1467-7687.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luyster R, Lord C. Word learning in children with autism spectrum disorders. Developmental Psychology. 2009;45(6):1774–1786. doi: 10.1037/a0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Thurman AJ, Hagerman RJ, Abbeduto L. Symptoms of Autism in Males with Fragile X Syndrome: A Comparison to Nonsyndromic ASD Using Current ADI-R Scores. Journal of Autism and Developmental Disorders. 2014 doi: 10.1007/s10803-013-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie AS, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, Brown WT. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. American Journal on Intellectectual and Developmental Disabilities. 2010;115(4):307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie AS, Kover ST, Hagerman R, Abbeduto L. Investigating Word Learning in Fragile X Syndrome: A Fast-Mapping Study. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-012-1717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie AS, Yoder PJ, Stone WL. Labels increase attention to novel objects in children with autism and comprehension-matched children with typical development. Autism. 2006;10(3):288–301. doi: 10.1177/1362361306063287. [DOI] [PubMed] [Google Scholar]
- Moore C, Angelopoulos M, Bennett P. Word learning in the context of referential and salience cues. Developmental Psychology. 1999;35(1):60–68. doi: 10.1037//0012-1649.35.1.60. [DOI] [PubMed] [Google Scholar]
- Mundy P. Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(6):793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Mastergeorge AM, McIntyre NS. Effects of Autism on Social Learning and Social Attention. In: Mundy P, Mastergeorge AM, editors. Educational Interventions for Students with Autism. San Francisco, CA: Jossey-Bass; 2012. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38(9):1261–1270. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Griffiths H, Nation K. Sound before meaning: word learning in autistic disorders. [Research Support, Non-U.S. Gov't] Neuropsychologia. 2010;48(14):4012–4019. doi: 10.1016/j.neuropsychologia.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14(2):239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Paparella T, Goods KS, Freeman S, Kasari C. The emergence of nonverbal joint attention and requesting skills in young children with autism. Journal of Communication Disorders. 2011;44(6):569–583. doi: 10.1016/j.jcomdis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Pruden SM, Hirsh-Pasek K, Golinkoff RM, Hennon EA. The birth of words: ten-month-olds learn words through perceptual salience. Child Development. 2006;77(2):266–280. doi: 10.1111/j.1467-8624.2006.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LA, Hatton DD, Heath M, Kaufmann WE. Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2007;37(9):1748–1760. doi: 10.1007/s10803-006-0305-9. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Scaife M, Bruner JS. The capacity for joint visual attention in the infant. Nature. 1975;253(5489):265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Scerif G, Longhi E, Cole V, Karmiloff-Smith A, Cornish K. Attention across modalities as a longitudinal predictor of early outcomes: the case of fragile X syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53(6):641–650. doi: 10.1111/j.1469-7610.2011.02515.x. [DOI] [PubMed] [Google Scholar]
- Shaw TA, Porter MA. Emotion recognition and visual-scan paths in Fragile X syndrome. Journal of Autism and Developmental Disorders. 2013;43(5):1119–1139. doi: 10.1007/s10803-012-1654-1. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Analysis of face gaze in autism using "Bubbles". Neuropsychologia. 2007;45(1):144–151. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. Journal of Autism and Developmental Disorders. 2007;37(5):929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Striano T, Stahl D. Sensitivity to triadic attention in early infancy. Developmental Science. 2005;8(4):333–343. doi: 10.1111/j.1467-7687.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton DD, Hammer J, Sideris J, Hooper S, Ornstein PA, Bailey DB., Jr Sustained attention and response inhibition in boys with fragile X syndrome: measures of continuous performance. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B(4):517–532. doi: 10.1002/ajmg.b.30504. [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman R, Abbeduto L. Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities. 2014;35(5):1072–1086. doi: 10.1016/j.ridd.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The Social-Pragmatic Theory of Word Learning. Pragmatics. 2000;10(4):401–413. [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36(8):993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SF, Brady N, Sterling A, Fleming K, Marquis J. Maternal responsivity predicts language development in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115(1):54–75. doi: 10.1352/1944-7558-115.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Archives of General Psychiatry. 2008;65(11):1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J, Cornish K, Munir F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia. 2002;40(8):1343–1349. doi: 10.1016/s0028-3932(01)00212-3. [DOI] [PubMed] [Google Scholar]
- Williams TA, Porter MA, Langdon R. Viewing social scenes: a visual scan-path study comparing fragile X syndrome and Williams syndrome. Journal of Autism and Developmental Disorders. 2013;43(8):1880–1894. doi: 10.1007/s10803-012-1737-z. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(12):1324–1332. doi: 10.1016/j.jaac.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock KA, Oliver C, Humphreys GW. Task-switching deficits and repetitive behaviour in genetic neurodevelopmental disorders: data from children with Prader-Willi syndrome chromosome 15 q11-q13 deletion and boys with Fragile X syndrome. Cognitive Neuropsychology. 2009;26(2):172–194. doi: 10.1080/02643290802685921. [DOI] [PubMed] [Google Scholar]


