Abstract
Background
Despite considerable use of make your own (MYO) cigarettes worldwide and increasing use in the United States, relatively little is known about how these cigarettes are smoked and the resultant toxicant exposure.
Methods
In a laboratory study, we compared two types of MYO cigarettes – roll your own (RYO) and personal machine made (PMM) – with factory made (FM) cigarettes in three groups of smokers who exclusively used RYO (n=34), PMM (n=23) or FM (n=20). Within each group, cigarettes were smoked in three conditions: 1) after confirmed overnight tobacco abstinence; 2) in an intense smoking paradigm; 3) and without restrictions. All cigarettes were smoked ad lib through a smoking topography unit.
Results
Plasma nicotine significantly increased after cigarettes in all conditions except PMM in the intense smoking paradigm. Puff volume, puff duration, total puff volume and puff velocity did not differ between cigarette types but the puffs per cigarette and time to smoke were significantly smaller for RYO compared to PMM and FM. Regardless of the cigarette, participants consumed the first three puffs more vigorously than the last three puffs.
Conclusions
Despite the belief of many of their consumers smoking MYO cigarettes are not a safe alternative to consumption of FM cigarettes. Like FM, MYO cigarettes expose their users to harmful constituents of tobacco smoke and despite differences in size and design their puffing profiles are remarkably similar.
Impact
These data are relevant to health and regulatory considerations on the MYO cigarettes.
Keywords: roll your own, MYO, cigarettes
Introduction
In response to increases in price and restrictions on the sale, marketing and advertising of conventional cigarettes the use of make your own (MYO) cigarettes has increased domestically and internationally (1-3). The United States (U.S.) prevalence of MYO smoking was reported at 6.7% in the 2006 ITC-4 survey (3) but reports in trade journals and the popular press suggest that current U.S. use may be substantially greater. Rosenberry et al. (4) found that in the U.S., MYO cigarettes could be divided into two general categories: roll your own (RYO) that are made by rolling tobacco in a paper leaf; and personal machine made (PMM) cigarettes made by injecting loose tobacco into a preformed, filtered cigarette tube, a cigarette quite unique to the U.S. market. Furthermore in the U.S. RYO cigarettes are typically made without a filter (4) whereas the use of a consumer-added filter to RYO cigarettes abroad is quite common (5).
There is limited literature on how MYO cigarettes are smoked and the exposure consequences that follow laboratory smoking. Shahab et al. studied RYO smokers in the UK (6, 7), Ayo-Yusuf and Olutola in South Africa (8) and Benjakul et al. in Thailand (9). There are no reported studies of smoking behavior or toxicant exposure from MYO cigarettes made and smoked by the U.S. smokers.
Smoking behavior is usually described by how cigarette smoke is drawn from the tobacco rod into the mouth of the smoker (puff). Puff topography can be used to characterize how established or new tobacco products are smoked. Smoking topography is typically assessed by the use of an orifice flow meter mouthpiece through which the smoke is drawn into the mouth by a study participant. Puff topography was used to quantify smoke exposure and is the basis of the parameters for International Organization for Standardization machine smoking method (10). Measures of smoking topography reflect and correlate with exposure to toxicants from tobacco smoke (11-13). Therefore, puff topography measures such as puff volume, number of puffs taken from a cigarette and the time taken to smoke, have been identified as measures that can reliably quantify smoke exposure from factory made (FM) cigarettes (14, 15), but there have been no topography studies of U.S. MYO smoking.
The present study assessed smoking topography associated with RYO, PMM, and conventional FM cigarette smoking in exclusive RYO, PMM and FM smokers. A central aim of this study was to determine the nicotine exposure and differences in smoking behavior among regular consumers of MYO and FM cigarettes while smoking cigarettes they had prepared themselves using their own tobacco, paper, tubes and other paraphernalia. Smoking behavior was assessed in conditions of tobacco abstinence, intense smoking and when there were no experimentally imposed restrictions. We also assessed the subjective experience from MYO and FM smoking.
The Family Smoking Prevention and Tobacco Control Act (2009) specifically authorized the Food and Drug Administration to regulate the MYO cigarette market. Fundamental to the regulatory process is an understanding of how MYO cigarettes are consumed and the consequent exposure to smoke-delivered toxicants. The present study was an initial attempt to capture that information in a laboratory-based clinical study.
Materials and Methods
Participants
Participants (N=77) were recruited from the Baltimore, MD, metropolitan area using local newspapers, direct mailers or Craigslist. Eligibility was determined through a telephone screener and screening visit in which smoking history was documented. Inclusion criteria were: 1) regular (daily) smoker for at least 2 years; 2) age from 18 to 65; 3) smoking at least 10 cigarettes per day (at least 80% of cigarettes smoked were either RYO, PMM or FM exclusively); 4) absence of smoking related illness or disease; and 5) not currently trying to quit smoking. Exclusion criteria were: 1) pregnancy or lactation; 2) high blood pressure or heart rate; 3) poor venous access; 4) general health problems (chronic bronchitis, asthma, etc.); 5) heart medications; and 6) history of blood draw complications. Participants were compensated $275 for completing three laboratory visits. Data collection occurred between June 2010 and May 2012 at Battelle's Human Exposure Assessment Laboratory (HEAL) in Baltimore, MD. Participants were assigned to one of three experimental groups based on the characteristics of their usual cigarette: RYO (n=34); PMM (n=23); and FM (n=20). All participants signed an IRB-approved informed consent form.
During all sessions participants smoked their usual cigarettes. MYO smokers (RYO and PMM) prepared their own cigarettes (under observation) using their own tobacco, paper and tubes as described previously (4). The study cigarettes (three) were selected from 25 as being close to the average weight of 25 cigarettes prepared. FM smoked their usual commercial brand of cigarette (i.e. Newport, Marlboro or Camel) which they also supplied. All of the PMM and FM cigarettes were filtered; 32 of the 34 of RYO participants smoked unfiltered cigarettes.
Study design and procedures
Every participant visited Battelle's HEAL for three separate experimental sessions:
Condition NR: participants smoked one of their usual cigarette (FM or MYO) without smoking restrictions prior to the experimental session;
Condition ABS: participants smoked one of their usual cigarettes after verified overnight tobacco abstinence (exhaled carbon monoxide≤12ppm);
Condition INT: participants came to the laboratory without any smoking restrictions before the visit and smoked two of their usual cigarettes within 1 hour and a third one 20 min later. All experimental measures were taken before and after the third cigarette.
Sessions for Conditions NR and ABS lasted about 1 hour and for INT about 2 hours. The interval between sessions was not shorter than 24 hours. The presentation of the conditions was randomized. Participants were familiarized with the study procedures and the equipment before the experimental sessions began. At the first visit, participants completed demographic and smoking history questionnaires. Before and after smoking, participants completed subjective questionnaires on cigarette craving and perceptions, blood samples were obtained from a forearm vein for nicotine assessments and baseline measures of exhaled CO (COex) were collected. Each participant smoked a cigarette through the mouthpiece of the puff analyzer.
Dependent measures
Self Report Subjective Measures
Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND) (16) and the total score from Nicotine Dependence Syndrome Scale (NDSS) (17). Tobacco craving was measured using the short version of the Questionnaire on Smoking Urges (QSU) (18-20). The appeal and subjective effects of the cigarettes were assessed using the Duke Sensory Questionnaire (DSQ) (21) and the Cigarette Evaluation Scale (CES) (22). Both questionnaires employ a 7-points Likert scale. The DSQ queries: 1) puff liking you just took? 2) puff satisfaction? 3) puff nicotine levels? 4) similar to your own brand? 5) strength of puffs on the tongue 6) nose 7) mouth & throat 8) windpipe and 9) chest? Responses to Questions 5-9 were summed to create a composite score of strength (23). The CES is an 11-item questionnaire that assesses whether cigarettes are: 1) satisfying; 2) taste good; 3) make you dizzy; 4) calm you down; 5) help you concentrate; 6) feel more awake; 7) reduce hunger for food; 8) make you nauseous; 9) feel less irritable; 10) enjoy the sensations of the smoke in your throat and chest; 11) immediately reduce your craving for cigarettes. For data analyses, Question 1 (Satisfaction) and Question 11 (Craving Relief) were analyzed alone. Composite variables were made from the following items: Question 2 and 10; Questions 4, 5, 6 and 9; and Question 3 and 8 to quantify constructs of interest: Peripheral Sensation, Psychological Reward and Negative Effect (23, 24).
Smoking topography measurements
Puff volume, puff duration, inter-puff interval and puff velocity, time to smoke and number of puffs were measured with Clinical Research Support System (CReSS®, Borgwaldt KC, Richmond, VA). A series of mouthpiece adaptors (BorgWalt, Richmond VA) were used to accommodate the smaller size cigarette RYO cigarettes. Before the study, we confirmed the accuracy of the CReSS device on puff count, puff duration and puff volume using a calibrated syringe to pull “false” puff while the mouthpiece was fitted with various sizes of RYO cigarettes. For statistical analyses we considered puff volumes≥15mL and puff durations≥0.2sec using “puff clean up” methods described elsewhere (14).
Plasma nicotine assessments
The change in plasma nicotine level is an excellent proximate marker of smoke exposure that has been used in laboratory studies of nicotine delivery from alternative and FM cigarettes (25-28). Venous blood samples (7 mL each) were collected before and 2 min after smoking. The samples were centrifuged and the plasma was stored frozen until analyses for nicotine. Tubes were frozen at -20°C for short-term storage and shipped on dry ice overnight to LabStat International ULC (Kitchener, Ontario, Canada), where gas chromatography/thermal specific ionic detection was used to determine plasma nicotine levels (LOD = 1.2ng/mL; LOQ = 4.1ng/mL). Nicotine boost was determined by calculating score between post- and pre-smoking nicotine measures.
Exhaled carbon monoxide (COex)
Exhaled carbon monoxide is a widely used biomarker of recent tobacco smoke exposure which is correlated with nicotine dependence scales (29-33). COex was measured before and within 10 min after smoking using a BreathCO monitor (Vitalograph Inc, Lenexa, KS). CO boost was determined by calculating score between post- and pre-smoking CO measures.
Statistical analyses
All statistical analyses were conducted with StatSoft, Inc. (2013). STATISTICA (data analysis software system), version 12. www.statsoft.com. Analysis Of Variance (ANOVA) was performed to find differences among participants as a function of age, cigarettes smoked per day, FTND score, NDSS score, QSU, DSQ, CES, biomarkers levels and smoking topography parameters (grouping variables: smoking condition or type of cigarettes smoked). Additionally, we used ANOVA methods to identify differences in smoking topography parameters (puff volume, duration and velocity) between the first three (1, 2 and 3) and the last three (X, Y and Z) puffs taken during each smoking session (grouping variables: type of cigarette and puff number). Post-hoc Tukey's Honest Significant Difference (THSD) test was used for identifying differences in variables within each cigarette group but across the various smoking conditions. For post-hoc comparisons between various types of cigarettes, we used THSD with the Spjøtvoll-Stoline modification for unequal N.
Sample size
Method described by Bausell and Li was used for sample size calculation for this study (34). A sample size large enough to detect an interaction between smoker group (FM, RYO, PMM) and smoking conditions (no restrictions (NR), abstinent (ABS), and intense (INT)) with sufficient power given that the patterns in the group vectors are truly nonparallel is required. For this study, the null hypothesis can be expressed as: HO: μp, j- μq, j = 0 j = NR, ABS, and INT; p,q = FM, RYO,PMM. Sample size for this design is estimated at 17-30 participants per group to detect effect moderate to large effect sizes. Moderate to large standardized differences were reported in puffing behavior (volume, average puff flow rates, peak flow rates) when cigarettes of differing tar levels (35) and differences in tobacco products (RYO versus FM) (7).
Results
Participants
The study was completed by 77 participants who met eligibility criteria and attended all three sessions. Participants' characteristics are presented in Table 1.
Table 1. Participants' characteristics.
| RYO (n=34) | PMM (n=23) | FM (n=20) | TOTAL (N=77) | ||
|---|---|---|---|---|---|
| Sex | |||||
| Male | 88.2% (30) | 73.9% (17) | 80.0% (16) | 81.8% (63) | |
| Female | 11.8% (4) | 26.1% (6) | 20.0% (4) | 18.2% (14) | |
|
| |||||
| Race | |||||
| African American | 29.4% (10) | 4.3% (1) | 55.0% (11) | 28.6% (22) | |
| Caucasian | 61.8% (21) | 87.0% (20) | 40.0% (8) | 63.6% (49) | |
| Other | 8.8% (3) | 8.7% (2) | 5.0% (1) | 7.8% (6) | |
|
| |||||
| Age | |||||
| Mean (SD) | 38 (12) | 41 (12) | 36 (11) | 39 (12) | |
|
| |||||
| Cigarettes per day | |||||
| Mean (SD) | 18 (6) | 22 (11) | 15 (4) | 18 (8) | |
|
| |||||
| Baseline plasma cotinine (ng/mL) | |||||
| Mean (SD) | NR | 197 (97) | 190 (88) | - | - |
| ABS | 135 (82) | 128 (85) | - | - | |
| INT | 217 (110) | 201 (115) | - | - | |
|
| |||||
| FTND Score | |||||
| Mean (SD) | 6 (2) | 6 (2) | 5 (2) | 6 (2) | |
|
| |||||
| NDSS Overall Score | |||||
| Mean (SD) | 0.12 (1.09) | -0.08 (0.97) | -0.36 (0.80) | 0.07 (0.99) | |
RYO – roll your own cigarettes; PMM – personal machine made cigarettes; FM – factory made cigarettes; FTND - Fagerström Test for Nicotine Dependence; NDSS - Nicotine Dependence Syndrome Scale; Mean (SD) – arithmetic mean with standard deviation; Baseline plasma cotinine level for subgroups (n=26 for RYO and n=10 for PMM)
More whites (n=49) than African Americans (n=22) participated in the study and 6 persons reported more than one race or “other”. The self-reported amount of cigarettes smoked per day was significantly higher for the PMM than the FM group (p<0.01). The groups did not differ on age or the level of nicotine dependence. However, there were significant differences between the gender and racial composition of the groups. Specifically, there were more men than women in all groups and more white than African Americans participants in the PMM group. These differences are generally reflective of the participant characteristics of the MYO smokers in the Baltimore area (4) and in the U.S. (3).
Study cigarettes
The average weight of the RYO cigarettes used in this study was 0.4±0.2g and was significantly less (p<0.01) than the PMM, 1.0±0.2g, and the FM, 0.9±0.1g cigarettes. The PMM and FM cigarettes were all filtered, whereas 94% of RYO cigarettes were unfiltered.
Self Report Subjective Measures
Nicotine dependence
All participants were moderately dependent on nicotine. Nicotine dependence measured with FTND ranged from 5 to 6 across the experimental groups. Furthermore, there were no significant differences in the NDSS overall score among smokers across all types of cigarettes. NDSS overall scores correlated well with FTND scores (r=0.58, p<0.01) which was consistent with findings in other studies (36-38).
Cigarette craving
Results of cigarette craving measured with the QSU questionnaire and the summary of statistical analyses are presented in Table 2. Overnight tobacco abstinence (Condition ABS) led to significant increases in the positive desire to smoke for reward (PRE smoking QSU Factor 1), increases in the need to smoke for relief (PRE smoking QSU Factor 2) as well as PRE smoking Total QSU Score. RYO smoking significantly reduced cigarette craving (expressed as a difference between POST- and PRE Total QSU Score) across all three smoking conditions whereas PMM and FM significantly reduced craving in NR and ABS conditions. After smoking sessions participants reported similar craving for cigarettes (assessed with POST Total QSU Score) across all cigarette types and all smoking conditions. Additionally, within the same condition (i.e. NR, ABS and INT), participants reported similar cravings across all cigarettes types (p>0.05) before the session began.
Table 2. Questionnaire of Smoking Urges results.
| Type of cigarettes | Condition | Factor 1 | Factor 2 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| PRE | POST | p | PRE | POST | p | PRE | POST | p | ||
| RYO (n=34) | NR | 3.8 (1.7) | 1.5 (1.7) | † | 2.2 (1.7) | 0.8 (1.4) | * | 6.0 (3.2) | 2.3 (2.9) | † |
| ABS | 5.1 (1.4) | 2.4 (1.8) | † | 3.1 (1.7) | 1.4 (1.6) | † | 8.2 (2.8) | 3.7 (3.2) | † | |
| INT | 3.0 (1.8) | 1.0 (1.4) | † | 1.7 (1.5) | 0.7 (1.3) | NS | 4.7 (3.1) | 1.7 (2.6) | † | |
|
| ||||||||||
| ABS>NR†; INT† | ABS>INT† | ABS>INT† | - | ABS>NR†; INT† | ||||||
|
| ||||||||||
| PMM (n=23) | NR | 3.4 (1.9) | 1.3 (1.5) | † | 1.8 (1.5) | 0.9 (1.4) | NS | 5.2 (3.0) | 2.2 (2.7) | † |
| ABS | 5.2 (1.2) | 2.5 (1.7) | † | 3.3 (1.8) | 1.5 (1.5) | † | 8.5 (2.7) | 4.0 (2.9) | † | |
| INT | 2.6 (1.7) | 1.3 (1.7) | NS | 1.3 (1.5) | 0.8 (1.4) | NS | 4.0 (3.0) | 2.1 (2.9) | NS | |
|
| ||||||||||
| ABS>NR†; INT† | ABS>NR*; INT* | ABS>NR†; INT† | - | ABS>NR†; INT† | ||||||
|
| ||||||||||
| FM (n=20) | NR | 4.9 (1.1) | 1.3 (1.8) | † | 2.4 (1.4) | 0.6 (1.2) | † | 7.2 (2.3) | 2.0 (2.9) | † |
| ABS | 5.6 0.6) | 2.4 (1.9) | † | 3.4 (1.5) | 1.3 (1.4) | † | 9.0 (1.9) | 3.7 (3.2) | † | |
| INT | 2.6 (1.6) | 1.0 (1.2) | * | 1.3 (1.3) | 0.7 (1.1) | NS | 3.8 (2.7) | 1.7 (2.3) | NS | |
|
| ||||||||||
| ABS>INT† | ABS>INT* | ABS>INT† | - | ABS>INT† | ||||||
|
| ||||||||||
| NR=ABS=INT | ||||||||||
RYO – roll your own; PMM – personal machine made; FM – factory made; NR – No restrictions; ABS – Overnight tobacco abstinence (12h, exhaled CO≤12ppm); INT – intense smoking; PRE / POST – before / after smoking; Means with standard deviations are given p – post-hoc ANOVA test for PRE/POST difference;
p<0.05;
p<0.01;
NS – statistically non significant
The appeal and effects of the cigarettes
Analysis of the Duke Sensory Questionnaire results showed no differences in MYO and FM perceptions across all smoking conditions. The participants: equally liked puffs just taken; were equally satisfied with puffs just taken; assessed that during all sessions the puffs delivered similar dose of nicotine; reported that puffs were similar to their own brand; and experienced similar strength of puffs taken (p>0.05). Cigarette Evaluation Scale results analysis showed that neither type of cigarettes nor condition influenced participants' Satisfaction, Craving for Relief and Peripheral Sensation. However, RYO smokers in Condition ABS scored higher than PMM (p<0.01) in Psychological Reward (CES parameter that assessed calmness, concentration, awake and irritation after smoking). According to the CES results, MYO and FM smokers also reported unequal Negative Effects (dizziness and nauseous) across analyzed smoking conditions. RYO smokers in ABS scored higher than RYO in NR (p<0.05) as well as RYO in INT (p<0.01). PMM in ABS scored higher than PMM in INT (p<0.05).
Smoking topography
The results of smoking topography measurements are summarized in Table 3.
Table 3. Smoking topography parameters by cigarette type and condition.
| Cigarette type | Condition | Number of puffs | Puff volume (mL) | Total puff volume (mL) | Puff duration (sec.) | Puff velocity (mL/sec.) | Time to smoke (sec.) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean (SD) | |||||||
| RYO (n=34) | NR | 11.0 (5.1) | 53.1 (20.1) | 584 (332) | 1.68 (0.48) | 38.4 (14.3) | 234 (87) |
| ABS | 11.5 (6.0) | 54.4 (24.5) | 633 (382) | 1.60 (0.53) | 42.0 (15.5) | 221 (107) | |
| INT | 12.4 (5.9) | 63.3 (33.4) | 745 (440) | 1.84 (0.69) | 40.7 (16.9) | 241 (92) | |
| PMM (n=23) | NR | 15.3 (6.6) | 61.5 (16.1) | 935 (453) | 1.90 (0.40) | 41.1 (10.4) | 340 (107) |
| ABS | 15.8 (6.4) | 61.9 (17.8) | 947 (357) | 1.90 (0.49) | 40.9 (9.4) | 341 (113) | |
| INT | 14.7 (5.1) | 58.3 (13.6) | 840 (291) | 1.81 (0.28) | 40.2 (11.5) | 330 (114) | |
| FM (n=20) | NR | 13.3 (3.6) | 58.7 (18.2) | 740 (179) | 1.94 (0.48) | 37.8 (12.1) | 312 (69) |
| ABS | 13.1 (3.7) | 53.3 (19.6) | 656 (194) | 1.77 (0.49) | 37.7 (13.8) | 316 (74) | |
| INT | 13.3 (3.9) | 55.7 (20.1) | 694 (198) | 1.87 (0.50) | 37.0 (12.9) | 322 (64) | |
RYO – roll your own; PMM – personal machine made; FM – factory made; NR – No restrictions; ABS – Overnight tobacco abstinence (12h, exhaled CO≤12ppm); INT – intense smoking; Mean (SD) – arithmetic mean with standard deviation
Puff volume, puff velocity, puff duration
Average puff volume, puff velocity and puff duration were not influenced by the type of cigarettes smoked. No statistically significant differences were found for these smoking topography parameters compared across different smoking conditions or the different cigarettes.
Total puff volume
Average total puff volumes were similar for the same type of cigarettes across different smoking conditions. Average total puff volume was significantly (p<0.05) larger during PMM smoking than during RYO smoking in Conditions NR and ABS. Additionally, average total puff volume was higher during PMM smoking than FM smoking in Condition ABS (p<0.05).
Number of puffs
Participants took similar numbers of puffs to smoke their cigarette (RYO, PMM or FM) regardless of the experimental condition. When compared across condition, RYO smokers took significantly (p<0.05) fewer puffs than PMM smokers in Conditions NR and Condition ABS (both p<0.05).
Time to smoke
Average time to smoke (TTS) was similar for the same type of cigarettes within various smoking conditions. However, for all three smoking conditions, average TTS was significantly longer for PMM and FM than for RYO (p<0.05).
Puff by puff analysis
Puff by puff profiles of the first three (1, 2, 3) and last three (X, Y, Z) puffs during smoking across experimental cigarettes are presented in Figure 1. Regardless of the cigarette, participants consumed the first three puffs more vigorously than the last three puffs. Specifically, puff volume and puff duration were generally larger and inter-puff interval was shorter at the beginning of a cigarette than at its end. Results showed that puff volumes, durations, intervals and velocities were not constant throughout smoking. Results of ANOVA and post-hoc analyses contrasting significant differences between the first and last three puffs are summarized in Table 4.
Figure 1.
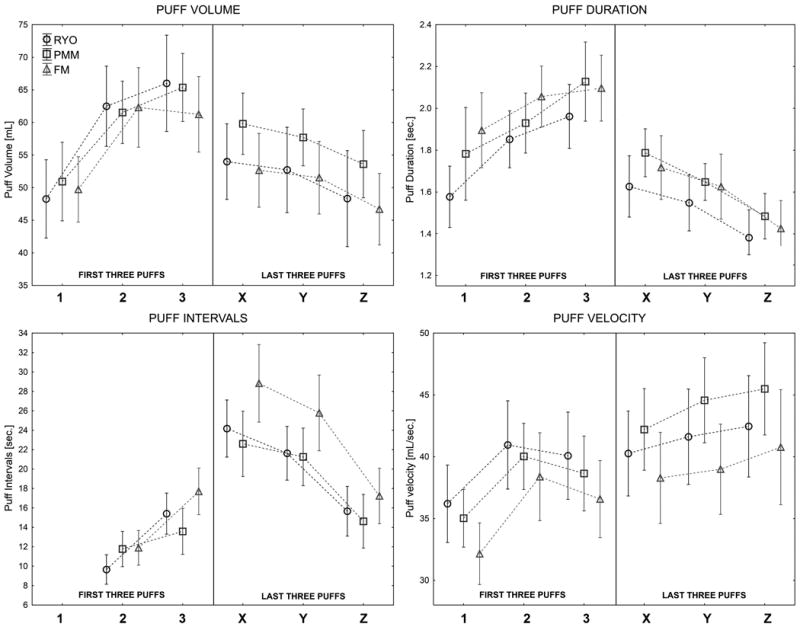
Puff by puff smoking topography profiles: puff volumes, durations, intervals, and velocities illustrating topography in first three puffs (1, 2 and 3) and the last three puffs (X, Y and Z) (Means with Confidence Intervals are presented; Grouping variable: cigarette type; experimental conditions (NR, ABS, INT) are combined).
Table 4. Puff by puff smoking profiles by cigarette type and puffing parameter.
| First three puffs | Last three puffs | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cigarette type | Smoking topography parameter | ANOVA (F) | 1 | 2 | 3 | X | Y | Z |
| RYO (n=34) | Volume | † (5.01) | 2*, 3† | 1*, Z* | 1†, Y*, Z† | - | 3* | 2*, 3† |
| Duration | † (8.75) | 3† | Y*, Z† | 1†, X*, Y†, Z† | 3* | 2*, 3† | 2†, 3† | |
| Intervals | † (21.86) | - | 3†, X†, Y†, Z† | 2†, X†, Y† | 2†, 3†, Z† | 2†, 3†, Z† | 2†, X†, Y† | |
| Velocity | NS | - | - | - | - | - | - | |
|
| ||||||||
| PMM (n=23) | Volume | † (4.31) | 2*, 3† | 1* | 1†, Z* | - | - | 3* |
| Duration | † (8.56) | 3* | Z† | 1*, X*, Y†, Z† | 3* | 3† | 2†, 3† | |
| Intervals | † (12.88) | - | X†, Y† | X†, Y† | 2†, 3†, Z† | 2†, 3†, Z† | X†, Y† | |
| Velocity | † (6.26) | X*, Y†, Z† | - | Z* | 1* | 1† | 1†, 3* | |
|
| ||||||||
| FM (n=20) | Volume | † (5.11) | 2*, 3* | 1*, Z† | 1*, Z† | - | - | 2†, 3† |
| Duration | † (11.41) | Z† | X*, Y†, Z† | X†, Y†, Z† | 2*, 3† | 2†, 3† | 1†, 2†, 3† | |
| Intervals | † (19.68) | - | X†, Y† | X†, Y† | 2†, 3†, Z† | 2†, 3†, Z† | X†, Y† | |
| Velocity | * (2.73) | Z† | - | - | - | - | 1† | |
Grouping variable: cigarette type; all conditions are combined; RYO – roll your own; PMM – personal machine made; FM – factory made; 1, 2, 3 - the first three; and X, Y, Z – the last three puffs ANOVA / post-hoc ANOVA test:
Toxicant exposure
The results of biomarkers measurements and statistical analyses results are summarized in Table 5.
Table 5. Nicotine and exhaled carbon monoxide measurements by cigarette type and condition.
| Plasma nicotine (ng/mL) | Exhaled CO (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Cigarette type | Condition | PRE | POST | p | BOOST | PRE | POST | p | BOOST |
| RYO (n=34) | NR | 12.3 (8.1) | 26.6 (13.3) | † | 14.3 (10.2) | 29 (17) | 34 (16) | NS | 4 (3) |
| ABS | 2.4 (2.0) | 21.8 (16.6) | † | 19.4 (16.9) | 9 (3) | 14 (4) | NS | 6 (3) | |
| INT | 16.2 (8.1) | 25.7 (12.2) | † | 9.5 (8.2) | 32 (12) | 36 (12) | NS | 4 (3) | |
|
| |||||||||
| ABS<NR†; INT† | - | ABS>INT† | ABS<NR†; INT† | ABS<NR†; INT† | ABS>INT* | ||||
|
| |||||||||
| PMM (n=23) | NR | 13.3 (7.1) | 33.1 (19.8) | † | 19.8 (18.3) | 27 (13) | 32 (11) | NS | 5 (3) |
| ABS | 2.8 (1.9) | 20.4 (17.4) | † | 17.6 (17.8) | 9 (2) | 15 (3) | NS | 6 (3) | |
| INT | 22.1 (11.9) | 31.0 (17.7) | NS | 8.9 (17.9) | 36 (13) | 40 (13) | NS | 4 (3) | |
|
| |||||||||
| ABS<NR†; INT† INT>NR† | - | - | ABS<NR†; INT† INT>NR† | ABS<NR†; INT† INT>NR† | ABS>INT* | ||||
|
| |||||||||
| FM (n=20) | NR | 10.3 (7.5) | 29.1 (13.1) | † | 18.8 (12.2) | 22 (14) | 39 (26) | † | 9 (5) |
| ABS | 1.6 (1.0) | 23.5 (11.9) | † | 21.9 (11.6) | 8 (3) | 16 (4) | NS | 9 (3) | |
| INT | 18.2 (9.2) | 31.0 (12.2) | † | 12.2 (9.9) | 33 (13) | 44 (20) | NS | 7 (5) | |
|
| |||||||||
| ABS<NR†; INT† INT>NR† | - | ABS>INT* | ABS<NR†; INT† INT>NR† | ABS<NR†; INT† | - | ||||
Arithmetic means with standard deviations are presented RYO – roll your own; PMM – personal machine made; FM – factory made; PRE – biomarker level before smoking; POST – biomarker level after smoking; BOOST – difference between biomarker levels after and before smoking; NR – No restrictions; ABS – Overnight tobacco abstinence (12h, exhaled CO≤12ppm); INT – intense smoking; p – post-hoc ANOVA test
Plasma cotinine/nicotine levels
Comparisons of the baseline plasma cotinine levels in a subset of RYO and PMM smokers indicated similar levels suggesting their nicotine exposure was equivalent at baseline (Table 1). Across all cigarettes, overnight tobacco abstinence (Condition ABS) significantly lowered plasma nicotine levels. Nicotine levels after smoking were similar across all cigarette types and conditions. There were no statistically significant differences between RYO, PMM and FM cigarettes in nicotine levels before smoking for the same smoking condition. All cigarettes, across all smoking conditions increased plasma nicotine levels, except PMM in Condition INT. Condition ABS for RYO and FM cigarettes resulted in significantly higher nicotine boost than Condition INT. Of all 462 plasma nicotine measures about 20% were below LOQ. As expected most (2/3) of the samples below LOQ were those from participants after overnight tobacco abstinence (pre ABS).
Exhaled carbon monoxide levels
Across all cigarette types, overnight tobacco abstinence resulted in significantly decreased COex level. In all three conditions, smoking FM cigarettes resulted in significantly higher COex boost than RYO and PMM cigarettes. After smoking in Condition ABS, participants had significantly lower COex than after NR and INT, for all types of cigarettes.
Discussion
This study is the first to examine differences in smoking topography between MYO and FM cigarettes among US smokers. By manipulating pre-experimental smoking conditions we were able to evaluate ad lib smoking topography and toxicant exposure in RYO, PMM, and FM smokers across a wide range of baseline cigarette cravings. In spite of significant differences in cigarette craving, many variables of smoking topography remained constant. For example, regardless of the type of cigarette or the experimental condition puff volume, puff duration, time to smoke, and interpuff interval and puff velocity were similar. Some of the variables (time to smoke and number of puffs) differed between the smaller RYO cigarette and the larger PMM and FM cigarettes. In addition to the similarity in puffing, there were similar increases in plasma nicotine and COex.
Dependence and Cigarettes Smoked per Day (CPD)
Analyses of the NDSS and FTND results confirmed that all participants (across all types of cigarettes) were similarly, dependent on nicotine and had similar levels of plasma cotinine (Table 1). However, the PMM group smoked significantly more (22 CPD) compared to FM smokers (15 CPD) while having similar plasma nicotine levels before smoking within the same smoking condition. This finding cannot be explained by smoking topography variables or the average weight of the cigarette (these factors were also similar in both groups). One of the possible explanations for that difference in CPD between those two groups might be the lower capability of PMM cigarettes to deliver nicotine, which might reflect different cigarette construction, tobacco characteristics (nicotine content in raw product before smoking) or different combusting processes during smoking.
Exposure to nicotine
Before smoking sessions, PMM smokers had slightly (not significantly) higher plasma nicotine levels than RYO and FM smokers which might reflect that this group smoked significantly more cigarettes than FM smokers (22 vs. 15 CPD) and slightly more than RYO smokers (18 CPD). Before smoking in Condition ABS (overnight tobacco abstinence) nicotine plasma levels decreased significantly comparing to Condition NR (no restrictions) and INT (intense smoking), which confirms participants' compliance with the study protocol. Within the same smoking conditions but across different types of cigarettes, participants were able to extract almost similar amount of nicotine, which confirms that all examined types of cigarettes were equally efficient in nicotine delivery. Similar and substantial quantities of nicotine delivery across all types of cigarettes, despite differences in their size (RYO significantly smaller than FM or PMM) might be explained in cigarette design. RYO are usually not filtered (4) and as a result more nicotine (as well as other toxicants) is delivered with smoke. This finding is consistent with research by Darrall and Figgins (5) who studied RYO in United Kingdom. The authors reported that adding a filter to RYO had little effect on CO yield, but reduced nicotine and tar levels by 48% and 46%, respectively.
Exposure to carbon monoxide
Exposure to CO (expressed as CO boost) was significantly higher during FM than RYO and PMM cigarettes smoking. The average weight of the FM cigarette was significantly higher than RYO cigarettes, which might explain the FM/RYO differences in CO exposure as a function of the amount of tobacco consumed during smoking (the higher mass of the cigarette, the higher exposure to CO). However, PMM and FM cigarettes had similar weights but different CO delivery. The finding cannot be explained by differences in smoking behavior, because smoking topography parameters were similar across these both types of cigarettes. Taken together these findings suggest that CO exposure depends not only on the mass of combusted tobacco but also additional factors must play a role. Toxicant generation during smoking is a very complex process that depends on cigarette construction, presence and characteristics of the filler and temperature-oxygen conditions during smoking. Tobacco in FM cigarettes is packed denser, and FM cigarettes have filters (RYO are usually unfiltered). As a result, the combustion processes might be different (lower oxygen delivery during FM comparing to PMM smoking might result in higher CO generation and exposure).
Smoking topography
Smoking topography parameters may be divided into two groups: 1) those that are independent of the size the smoking article (puff volume, puff duration and velocity) and 2) those that are dependent on the size of the article (time to smoke, puffs per cigarette). Three average topography parameters (puff volume, puff duration and puff velocity) were similar across all three types of cigarettes and three smoking conditions. Although the puffing was similar between MYO and FM cigarettes studied here, puffing patterns may not be consistent with other smoking articles such as cigars, little cigars and cigarillos (39).
Smoking topography parameters reported in this study are similar to those measured by others using FM(11, 14, 40-44) and MYO cigarettes (6). There have been reports in the literature (40,45-49) that smoking topography may change over the course of smoking a single cigarette. In adults (40, 45-47) and adolescents (48, 49) initial puffs are larger, longer and more closely spaced than the last puffs of a cigarette. The usual explanation for differences is that the smokers are trying (with their initial puffs) to satisfy a nicotine “need” that becomes satisfied over the course of a cigarette. Our results support that trend in both RYO and PMM smokers across all experimental conditions. However, if the pattern of smoking truly changes in response to nicotine need, we would expect to see greater differences when comparing across experimental conditions. For example, in the intense condition, INT, (full tobacco satiation) there should be fewer differences between the first and last puffs of a cigarette whereas in the overnight abstinent condition, ABS, there might be more difference between the first and last puffs. This expectation was not evident - all cigarettes in all conditions were smoked with the same pattern suggesting that the pattern of cigarette puffing in established smokers is constant regardless of immediate nicotine need. The smoking history of the participant may influence this pattern. In the present study almost all (96%) of the RYO and PMM participants had begun cigarette smoking as FM smokers (and had later changed to exclusive MYO smoking). It must be considered that the patterns of smoking were established very early in the smoking history (with FM cigarettes) and persisted years later smoking while smoking MYO cigarettes. Because the speed of nicotine delivery appears to be directly related to the addiction potential of the product (50, 51) the constancy of the puffing profile may be an example of behavioral autonomism suggesting that once established a pattern of smoking persists regardless of the nicotine need. It would be interesting to compare puffing patterns and their relation to nicotine need in new smokers or in smokers using novel products such as little cigars or electronic cigarettes.
Subjective effects of cigarettes
Overall there were very few significant differences in the subjective experience associated with smoking the experimental cigarettes. That is participants reported moderate to high levels of satisfaction, craving relief and sensations across products. Furthermore, the conditions of the experiment did not change the subjective evaluation of the cigarettes (e.g. the cigarettes were no better liked in the abstinence than in the intense smoking conditions). In a previous study comparing novel cigarettes of differing nicotine content, the delivery of nicotine (but not menthol flavoring) was related to the subjective experiences (23). Others have shown differences in subjective response to novel cigarettes (52,53). That there were few differences in subjective evaluations in the present study may be because the assessments were made using a familiar cigarette that delivered equivalent and expected quantities of nicotine.
MYO smoking and ISO standard
Measured average puff volumes for all tested cigarettes, in all smoking conditions were higher than standard puffing regime required by ISO method for cigarette testing (35mL). The differences ranged from +51% (RYO) to +80% (RYO) in NR (no restrictions) and INT (intense smoking) conditions, respectively. Average puff duration in all smoking conditions and for all cigarettes were lower than required by ISO method (2sec.). The differences were not high, but still ranged from -20% (RYO) in NR condition to -6% (FM, INT condition). Measured average puff velocities were also higher than in ISO method (17.5mL/sec.) in all analyzed cases. They ranged from +111% (RYO, ABS condition) to +150% (FM, INT condition). Our data confirm again that current ISO testing regime is an inappropriate standard for evaluating cigarette toxicity and setting regulatory restrictions not only for FM cigarettes, but also cannot be used for accurate assessment of toxicants intake from RYO and PMM cigarettes. Other standard methods for cigarette testing: Massachusetts method (puff volume: 45mL; puff duration 2sec.; puff velocity: 22.5mL/sec.) and Canadian regime (puff volume: 55mL; puff duration 2sec.; puff velocity: 27.5mL/sec.) also do not correspond well to the smoking topography parameters we measured in this study. Although the Canadian regime has been called a ‘maximum’ smoking regime, the puffing parameters of some participants exceeded those of the Canadian intense method.
Summary
The study demonstrated that despite differences in their size, tobacco weight and design, RYO, PMM and FM cigarettes delivered similar amounts of nicotine, CO and presumably other toxicants. Toxicant delivery and smoking patterns were similar across a wide range of experimentally manipulated conditions of tobacco craving. Participants of the present study were exclusive RYO, PMM or FM smokers; puffing profiles, toxicant exposure and subjective responses may differ among people who do not exclusively use these products. Furthermore, the participants were largely white men. While that is the typical demographic of smokers of self-made cigarettes in the Baltimore area, the results must be viewed as a characteristic of this group. Our study group was not racially homogenous, but we observed no differences in puffing behavior. The study described above was conducted in a laboratory conditions, thus, we cannot generalize to smoking in a natural environment. Smoking thru topography devices are commonly used. Computer based smoking topography proven to be a reliable measure of smoking behavior and toxicant exposure (14, 15) but some experimental evidence suggests that a portable topography device (not used in this study) may increase smoking difficulty, reduce smoking enjoyment and alter cigarette taste (54). Our results indicate that MYO cigarettes deliver toxicants similar to FM cigarettes and contradict the consumer perceptions that MYO cigarettes are safer or less toxic than FM cigarettes (55-57). The results of this clinical study support the conclusions from machine smoking of MYO cigarettes (58, 59) where MYO cigarette delivered substantial levels of nicotine, CO and “tar”. Machine smoking and clinical studies of MYO cigarettes and cigarette smoking affirm their delivery of toxicants and strengthen the need for their continued regulation.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number 1R01CA138973-01, W.B. Pickworth)
Footnotes
The authors report no competing interests
Reference List
- 1.Centers for Disease Control and Prevention. Drop in cigarette consumption offset by increases in other forms of smoked tobacco. 2012 Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6130a1.htm.
- 2.Hanewinkel R, Isensee B. Opinion on tobacco tax increase: factors associated with individuals' support in Germany. Health Policy. 2008;86:234–8. doi: 10.1016/j.healthpol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Young D, Yong HH, Borland R, Shahab L, Hammond D, Cummings KM, et al. Trends in roll-your-own smoking: findings from the ITC Four-Country Survey (2002-2008) J Environ Public Health. 2012;2012:1–7. doi: 10.1155/2012/406283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberry ZR, Strasser AA, Canlas LL, Potts JL, Pickworth WB. Make Your Own Cigarettes: Characteristics of the Product and the Consumer. Nicotine Tob Res. 2013;15:1453–7. doi: 10.1093/ntr/nts271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darrall KG, Figgins JA. Roll-your-own smoke yields: theoretical and practical aspects. Tob Control. 1998;7:168–75. doi: 10.1136/tc.7.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahab L, West R, McNeill A. The feasibility of measuring puffing behaviour in roll-your-own cigarette smokers. Tob Control. 2008;17(Suppl 1):i17–23. doi: 10.1136/tc.2007.021824. [DOI] [PubMed] [Google Scholar]
- 7.Shahab L, West R, McNeill A. A comparison of exposure to carcinogens among roll-your-own and factory-made cigarette smokers. Addict Biol. 2009;14:315–20. doi: 10.1111/j.1369-1600.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 8.Ayo-Yusuf OA, Olutola BG. ‘Roll-your-own’ cigarette smoking in South Africa between 2007 and 2010. BMC Public Health. 2013;13:597. doi: 10.1186/1471-2458-13-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjakul S, Termsirikulchai L, Hsia J, Kengganpanich M, Puckcharern H, Touchchai C, et al. Current manufactured cigarette smoking and roll-your-own cigarette smoking in Thailand: findings from the 2009 Global Adult Tobacco Survey. BMC Public Health. 2013;13:277. doi: 10.1186/1471-2458-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. Routine analytical cigarette-smoking machine -- Definitions and standard conditions: ISO 3308:2012. 2012 [Google Scholar]
- 11.Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14:1370–5. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- 12.Bridges RB, Combs JG, Humble JW, Turbek JA, Rehm SR, Haley NJ. Puffing topography as a determinant of smoke exposure. Pharmacol Biochem Behav. 1990;37:29–39. doi: 10.1016/0091-3057(90)90037-i. [DOI] [PubMed] [Google Scholar]
- 13.Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 82:320–9. doi: 10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–9. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 15.Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–4. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004 Apr;6:327–48. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- 18.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001 Feb;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 19.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991 Nov;86:1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 20.West R, Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology (Berl) 2010;208:427–32. doi: 10.1007/s00213-009-1742-x. [DOI] [PubMed] [Google Scholar]
- 21.Behm FM, Rose JE. Reducing craving for cigarettes while decreasing smoke intake using capsaicin-enhanced low-tar cigarettes. Experimental and Clinical Psychopharmacology. 1994;(2):143–153. [Google Scholar]
- 22.Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clinical Research. 1992;40:871A. [Google Scholar]
- 23.Pickworth WB, Moolchan ET, Berlin I, Murty R. Sensory and physiologic effects of menthol and non-menthol cigarettes with differing nicotine delivery. Pharmacol Biochem Behav. 2002;71:55–61. doi: 10.1016/s0091-3057(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 24.Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 25.Malson JL, Lee EM, Moolchan ET, Pickworth WB. Nicotine delivery from smoking bidis and an additive-free cigarette. Nicotine Tob Res. 2002;4:485–90. doi: 10.1080/1462220021000018498. [DOI] [PubMed] [Google Scholar]
- 26.Malson JL, Lee EM, Murty R, Moolchan ET, Pickworth WB. Clove cigarette smoking: biochemical, physiological, and subjective effects. Pharmacol Biochem Behav. 2003;74:739–45. doi: 10.1016/s0091-3057(02)01076-6. [DOI] [PubMed] [Google Scholar]
- 27.Malson JL, Pickworth WB. Bidis--hand-rolled, Indian cigarettes: effects on physiological, biochemical and subjective measures. Pharmacol Biochem Behav. 2002;72:443–7. doi: 10.1016/s0091-3057(02)00709-8. [DOI] [PubMed] [Google Scholar]
- 28.Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–64. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- 29.Muhammad-Kah RS, Hayden AD, Liang Q, Frost-Pineda K, Sarkar M. The relationship between nicotine dependence scores and biomarkers of exposure in adult cigarette smokers. Regul Toxicol Pharmacol. 2011;60:79–83. doi: 10.1016/j.yrtph.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Lindner D, Smith S, Leroy CM, Tricker AR. Comparison of exposure to selected cigarette smoke constituents in adult smokers and nonsmokers in a European, multicenter, observational study. Cancer Epidemiol Biomarkers Prev. 2011;20:1524–36. doi: 10.1158/1055-9965.EPI-10-1186. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Li L, Smith M, Guo Y, Whitlock G, Bian Z, et al. Exhaled carbon monoxide and its associations with smoking, indoor household air pollution and chronic respiratory diseases among 512000 Chinese adults. Int J Epidemiol. 2013;42:1464–75. doi: 10.1093/ije/dyt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp Toxicol Pathol. 2006;58:101–24. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Zielinska-Danch W, Goniewicz ML, Szoltysek-Boldys I, Czogala J, Koszowski B, Slodczyk E, et al. Estimation of optimal levels of tobacco biomarkers to distinguish active and passive smokers using ROC analysis. Przegl Lek. 2009;66:636–40. [PubMed] [Google Scholar]
- 34.Bausell RB, Li Y. Power Analysis for Experimental Research: A Practical Guide for Biological, Medical, and Social Sciences. New York: Cambridge University Press; 2002. [Google Scholar]
- 35.Pickworth WB, Houlgate P, Schorp M, Dixon M, Borgerding M, Zaatari G. A review of human smoking behaviour data and recommendations for a new ISO standard for the machine smoking of ciagrettes. A Report of the Ad Hoc WG9 Smoking Behaviour Review Team to ISO/TC 126 WG9. 2005 [Google Scholar]
- 36.Broms U, Madden PA, Heath AC, Pergadia ML, Shiffman S, Kaprio J. The Nicotine Dependence Syndrome Scale in Finnish smokers. Drug Alcohol Depend. 2007;89:42–51. doi: 10.1016/j.drugalcdep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuyemi KS, Pulvers KM, Cox LS, Thomas JL, Kaur H, Mayo MS, et al. Nicotine dependence among African American light smokers: a comparison of three scales. Addict Behav. 2007;32:1989–2002. doi: 10.1016/j.addbeh.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark DB, Wood DS, Martin CS, Cornelius JR, Lynch KG, Shiffman S. Multidimensional assessment of nicotine dependence in adolescents. Drug Alcohol Depend. 2005;77:235–42. doi: 10.1016/j.drugalcdep.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Fabian LA, Canlas LL, Potts J, Pickworth WB. Ad lib smoking of Black & Mild cigarillos and cigarettes. Nicotine Tob Res. 2012;14:368–71. doi: 10.1093/ntr/ntr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czogala J, Goniewicz ML, Czubek A, Koszowski B, Sobczak A. “How does smoker really smoke?”--preliminary report on smoking topography among Polish smokers. Przegl Lek. 2008;65:657–62. [PMC free article] [PubMed] [Google Scholar]
- 41.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–11. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- 42.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004;13:1800–4. [PubMed] [Google Scholar]
- 43.Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2006;15:154–7. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- 44.Pickworth WB, Lee EM, Abreu ME, Umbricht A, Preston KL. A laboratory study of hydromorphone and cyclazocine on smoking behavior in residential polydrug users. Pharmacol Biochem Behav. 2004;77:711–5. doi: 10.1016/j.pbb.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Gust SW, Pickens RW, Pechacek TF. Relation of puff volume to other topographical measures of smoking. Addict Behav. 1983;8:115–9. doi: 10.1016/0306-4603(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt AR, Kirkham AJ, Baldry AG, Dixon M, Cumming G. How does puffing behavior alter during the smoking of a single cigarette? Pharmacol Biochem Behav. 1989;33:189–95. doi: 10.1016/0091-3057(89)90449-8. [DOI] [PubMed] [Google Scholar]
- 47.Kolonen S, Tuomisto J, Puustinen P, Airaksinen MM. Puffing behavior during the smoking of a single cigarette in a naturalistic environment. Pharmacol Biochem Behav. 1992;41:701–6. doi: 10.1016/0091-3057(92)90215-2. [DOI] [PubMed] [Google Scholar]
- 48.Veilleux JC, Kassel JD, Heinz AJ, Braun A, Wardle MC, Greenstein J, et al. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. J Adolesc Health. 2011;48:176–81. doi: 10.1016/j.jadohealth.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins CC, Epstein DH, Parzynski CS, Zimmerman D, Moolchan ET, Heishman SJ. Puffing behavior during the smoking of a single cigarette in tobacco-dependent adolescents. Nicotine Tob Res. 2010;12:164–7. doi: 10.1093/ntr/ntp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fant RV, Pickworth WB, Henningfield JE. The addictive effects of nicotine are related to the speed of delivery. In: Opitz K, editor. Nicotine as a Therapeutic Agent, Immunity and the Environment. Stuttgart:Gustav-Fisher; 1997. pp. 53–61. [Google Scholar]
- 51.Pickworth WB, Henningfield JE. Smokable drugs: Pharmacologic basis for consumer appeal. Addiction. 1997;92:691–692. [Google Scholar]
- 52.Rose JE, Behm FM. There is more to smoking than the CNS effects of nicotine. In: Adlkofer F, Thurau K, editors. Effects of nicotine on biological systems II Advances in pharmacologic sciences. Birkhauser: Springer-Verlag; 1991. pp. 239–246. [Google Scholar]
- 53.Robinson ML, Houtsmuller EJ, Moolchan ET, Pickworth WB. Placebo cigarettes in smoking research. Exp Clin Psychopharmacol. 2000;8:326–32. doi: 10.1037//1064-1297.8.3.326. [DOI] [PubMed] [Google Scholar]
- 54.Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nosa V, Glover M, Min S, Scragg R, Bullen C, McCool J, Kira A. The use of the ‘rollie’ in New Zealand: preference for loose tobacco among an ethnically diverse low socioeconomic urban population. N Z Med J. 2011;124:25–33. [PubMed] [Google Scholar]
- 56.Young D, Wilson N, Borland R, Edwards R, Weerasekera D. Prevalence, correlates of, and reasons for using roll-your-own tobacco in a high RYO use country: findings from the ITC New Zealand survey. Nicotine Tob Res. 2010;12:1089–98. doi: 10.1093/ntr/ntq155. [DOI] [PubMed] [Google Scholar]
- 57.Young D, Borland R, Hammond D, Cummings KM, Devlin E, Yong HH, O'Connor RJ. Prevalence and attributes of roll-your-own smokers in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii76–82. doi: 10.1136/tc.2005.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaiserman MJ, Rickert WS. Handmade cigarettes: it's the tube that counts. Am J Public Health. 1992;82:107–9. doi: 10.2105/ajph.82.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaiserman MJ, Rickert WS. Carcinogens in tobacco smoke: benzo[a]pyrene from Canadian cigarettes and cigarette tobacco. Am J Public Health. 1992;82:1023–6. doi: 10.2105/ajph.82.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]


