Abstract
Background
The ratio of 3′hydroxycotinine to cotinine, or nicotine metabolite ratio (NMR), is strongly associated with CYP2A6 genotype, CYP2A6-mediated nicotine and cotinine metabolism, and nicotine clearance. Higher NMR (faster nicotine clearance) is associated retrospectively with heavier smoking and lower cessation rates.
Methods
NMR as a predictive biomarker of cessation outcomes is being investigated (NCT01314001). In addition to strong CYP2A6-genetic influences on NMR, demographic and hormonal factors alter NMR. Here we analyzed, for the first time together, these sources of variation on NMR in smokers screened for this clinical trial (N=1672).
Results
Participants (mean age=45.9) were 65.1% Caucasian, 34.9% African American, and 54.8% male. Mean NMR (SD) was higher in Caucasians vs. African Americans (0.41(0.20) vs. 0.33(0.21); P<0.001), and in females vs. males (0.41(0.22) vs. 0.37(0.20); P<0.001). Among females, birth control pill use (N=17) and hormone replacement therapy (N=14) were associated with 19.5% (P=0.09) and 29.3% (P=0.06) higher mean NMR, respectively, albeit non-significantly. BMI was negatively associated with NMR (Rho=−0.14; P<0.001), while alcohol use (Rho=0.11; P<0.001) and cigarette consumption (Rho=0.12; P<0.001) were positively associated with NMR. NMR was 16% percent lower in mentholated cigarette users (P<0.001). When analyzed together in a linear regression model, these predictors (each ≤2%) accounted for <8% of total NMR variation.
Conclusions
While these factors significantly affected NMR, they contributed little (together <8%; each ≤2%) to total NMR variation.
Impact
Thus when using NMR, for example to prospectively guide smoking cessation therapy, these sources of variation are unlikely to cause NMR misclassification.
Keywords: Smoking, nicotine, metabolism, demographics, CYP2A6
Introduction
Tobacco use remains a leading cause of morbidity and mortality worldwide, and life expectancy is shortened by more than 10 years in smokers [1]. If current trends in smoking prevalence continue, tobacco use is projected to kill one billion people worldwide during the twenty-first century [2], underscoring the need for improved smoking prevention and cessation strategies. One approach to improving smoking cessation rates and reducing the global burden of disease from tobacco may involve the personalization of smoking cessation pharmacotherapies using validated biomarkers that predict treatment success [3]. A diagnostic and predictive biomarker of smoking cessation outcomes with potential clinical utility is the nicotine metabolite ratio (NMR) [3].
Nicotine is the major psychoactive compound in cigarette smoke responsible for the reinforcing properties associated with cigarette smoking and the development of tobacco addiction [4]. The majority (~80%) of nicotine is metabolically inactivated to cotinine, in a reaction predominantly catalyzed by CYP2A6 [5]. Cotinine undergoes further metabolism to 3′hydroxycotinine, in a reaction exclusively mediated by CYP2A6 [6, 7]. The ratio of 3′hydroxycotinine/cotinine, known as the NMR, is a biomarker of CYP2A6 genotype activity, as well as nicotine metabolism rate, and it correlates strongly with total nicotine clearance [7, 8]. The NMR has been shown retrospectively to be associated with smoking cessation success in multiple clinical trials involving heavy and light smokers, of Caucasian and African American descent, respectively [9–12]. Individuals with lower NMR, indicative of lower CYP2A6 activity and slower nicotine clearance, displayed higher quit rates on transdermal nicotine [9, 10] and nicotine gum [12], relative to individuals with higher NMR. In contrast, there were no differences in quit rates on bupropion (a non-CYP2A6 substrate) between NMR groups, however among those receiving counseling and placebo, those with lower NMR had higher quit rates [11].
In addition to cessation, NMR and CYP2A6 genotype are associated with smoking acquisition, the level of cigarette consumption, as well as nicotine dependence [13–19]. Those with slower nicotine metabolism rates, determined via NMR or CYP2A6 genotype, display lower self-reported cigarettes smoked per day [15–17], lower total nicotine intake [20–23], lower nicotine dependence [18, 19], and lower total puff volumes resulting in lower carcinogen exposure [24]. The relationship between lower NMR and reduced cigarette consumption/nicotine dependence scores may be more pronounced in men than in women [19] and in younger cohorts and smokers not seeking treatment [9, 15]. CYP2A6 genotype is also associated with lung cancer risk; those with reduced activity CYP2A6 genotypes (i.e., slower metabolizers) have a lower risk of developing lung cancer [16, 25–27]. The reduced lung cancer risk among slower metabolizers likely stems from both lower levels of smoking and lower metabolic activation of tobacco-specific nitrosamines [23].
One advantage to using NMR rather than CYP2A6 genotype as a biomarker of nicotine metabolism rate is that it includes both genetic and environmental sources of variation in nicotine metabolism and clearance. Here we investigate the influence of non-genetic sources (specifically non-CYP2A6 genetic variation) of variation on NMR. If these sources of variation have a relatively small impact on NMR, and NMR is shown to prospectively predict cessation outcomes, this would further support the utility of NMR as a prospective biomarker to guide treatment assignment. In addition to CYP2A6 genotype [28], a number of factors contribute to inter-individual variability in NMR, including ethnicity [20, 29, 30], sex [31, 32], birth control pill use [31], body mass index (BMI) [33], and potentially mentholated cigarette use [34, 35]. NMR is higher among Caucasians relative to African Americans and Asians [8, 20, 29, 30], reflecting the lower frequency of slower-activity CYP2A6 genetic variants in Caucasians relative to African and Asian populations [15, 36–38]. NMR is also higher among premenopausal women relative to men, and even higher among women taking estrogen-containing birth control pills [31, 32]. In contrast, there are no differences in NMR between men and menopausal or postmenopausal women [31].
While several smaller studies have investigated individual influences on NMR, a comprehensive analysis to characterize these relationships simultaneously in one large population has not been performed. Moreover, to date the relationship between NMR and alcohol use has not been investigated, despite the common co-use of smoking and alcohol and the impact of alcohol on smoking cessation success [39, 40]. In contrast to the well-characterized CYP2A6 genotype contribution to variation in NMR, this paper describes environmental influences that are less understood. We divided our analysis into three parts. We first examined previously known influences on NMR (i.e. ethnicity, gender, exogenous estrogen-based hormonal therapies, and BMI). We next characterized relationships between NMR, alcohol use, mentholated cigarette use, and the level of cigarette consumption. Our final objective was to quantify the overall influence of these predictors on NMR, to determine if they, alone or together, represent a substantial source of variation in this biomarker.
Materials and Methods
Study subjects and data collection
Treatment-seeking adults (aged 18–65) smoking ≥10 cigarettes per day for the past six months responded to advertisements for a smoking cessation clinical trial (NCT01314001). Exclusion criteria included the use of chewing tobacco, snuff or snus; recent treatment for substance abuse; current cocaine or opiate abuse; the consumption of >25 standard alcoholic drinks/week; current depression, mania, schizophrenia, or post-traumatic stress disorder; recent use of anti-psychotics, anti-depressants, prescription stimulants, metformin, cimetidine, cardiac medications, or other anti-coagulants; and the daily use of prescription opiates/inhalers. Those interesting in participating after meeting eligibility criteria provided a blood sample for NMR determination, collected when participants were smoking as usual. The detailed study protocol, including NMR determination, is described in a previous analysis of NMR and three self-report measures of nicotine dependence in a subset of the trial participants (N=833 of 1807 screened by NMR) [19]. Briefly, cotinine and 3′hydroxycotinine were assessed from whole blood by liquid chromatography-tandem mass spectrometry (LC/MS-MS) using a previously validated method [41, 42]. NMR data were available on a total of 1807 eligible participants screened at the four clinical sites: the University of Pennsylvania (N=487), the Centre for Addiction and Mental Health (CAMH) at the University of Toronto (N=430), the MD Anderson Cancer Center (N=443) and the University at Buffalo, SUNY (N=447). Survey data on demographic variables (including age, gender, ethnicity) and smoking history were collected, as well as height and weight measurements in order to compute BMI. Data were also collected from female participants on the use of oral contraceptives and hormone replacement therapies. Data on mentholated cigarette use was collected from the subset of participants in the intent-to-treat (ITT) group (N=1155), assessed when they received their study medication and completed the first counseling session. Informed consent was obtained from each participant. The study was approved by Institutional Review Boards at each site.
Statistical analysis
All statistical analyses were completed using SPSS Version 22 (IBM Corporation, Armonk, NY, USA). The Shapiro-Wilk test was used to determine whether continuous variables were normally distributed. Mann-Whitney U Tests (two-tailed) and chi-square tests were used to compare continuous and categorical outcome measures, respectively, between two groups. The strength of correlation between continuous variables was assessed using Spearman’s rank correlation coefficient.
A univariate analysis of variance model was used to determine whether ethnicity and gender interact to influence NMR (2 × 2 factorial design). Hierarchical linear regression models were used to determine whether cigarette consumption confounds the association 1) between BMI and NMR, or 2) between alcohol use and NMR. In these models, breath CO (a biomarker of cigarette consumption) was entered in block 1, and the predictor (BMI or alcohol use) was entered in block 2. Separate hierarchical linear regression models were also used to test whether BMI and alcohol use interact with ethnicity and/or gender to influence NMR. The single predictors (BMI and alcohol use) together with ethnicity or gender were entered in block 1, and the interaction term (e.g. BMI × gender) was entered in the second block. We used a univariate analysis of variance model to determine whether mentholated cigarette use and ethnicity interact to influence NMR (2 × 2 factorial design). A univariate analysis of variance model was also used to determine whether NMR stratum (faster vs. slower metabolism) interacts with either ethnicity and/or gender to influence CPD (2 × 2 × 2 factorial design).
A linear regression model was also used to assess the variation in NMR accounted for by each of the predictors, and their combined overall contribution to NMR variability. The predictors (ethnicity, gender, birth control pill use, hormone replacement therapy use, BMI, alcohol consumption (number of standard drinks/week), and cigarettes per day) were entered simultaneously into the model. The overall contribution of the predictors to NMR variation was assessed by examining the model R2 value. The unique and individual contribution of each predictor to overall NMR variation was assessed by squaring the part correlation coefficients and multiplying by 100%. A separate and similar model was also run in the ITT sub-group (N=1155), with and without the use of mentholated cigarettes as a predictor. A Pearson’s chi-square test was used to determine whether the prevalence of mentholated cigarette use in African Americans and Caucasians was different.
Results
Participant demographics
Of the 1807 eligible subjects with NMR, 55.7% were male with a mean age of 45.4 years and mean BMI of 29.4. The majority of the subjects were Caucasian (60.3%) and African American (32.3%), with a small number of subjects reporting Asian (3.4%), American Indian/Alaska Native (0.3%), Hawaiian/Polynesian (0.1%), or ‘more than one’ or ‘other’ race (3.7%). We restricted all further analyses herein to Caucasians and African Americans with NMR (N=1672), as the small numbers of subjects from the four additional racial groupings (N=135 total) precluded meaningful statistical analysis. Participant characteristics of the final analytic sample are shown in Table 1.
Table 1.
Demographic characteristics of Caucasian and African American participants with NMR (N=1672)
| Characteristic | Value |
|---|---|
| % Female, N=756 | 45.2 |
| Mean age (SD), N=1672 | 45.9 (11.0) |
| % Caucasian, N=1089 | 65.1 |
| % African American, N=583 | 34.9 |
| Mean BMI (SD), N=1672 | 29.5 (6.5) |
| Mean # drinks/week (SD), N=1672 | 3.3 (5.2) |
| Mean cigarettes per day (SD), N=1672 | 18.7 (7.5) |
| Mean breath CO, ppm (SD), N=1672 | 23.3 (10.2) |
| NMR in total group, N=1672 | |
| Mean (SD) | 0.38 (0.21) |
| Median | 0.35 |
| Range | 0.01–1.90 |
| Skewness | 1.51 |
| Kurtosis | 4.87 |
| NMR in ITT group, N=1155a | |
| Mean (SD) | 0.35 (0.20) |
| Median | 0.30 |
| Range | 0.01 – 1.31 |
| Skewness | 1.39 |
| Kurtosis | 2.63 |
Participants with lower NMR were oversampled in the clinical trial, reflecting the lower NMR values observed in the ITT group vs. in the total group
Abbreviations: BMI, body mass index; CO, carbon monoxide; ppm, parts per million; NMR, nicotine metabolite ratio
Factors (ethnicity, gender, exogenous estrogen, and BMI) known to influence NMR
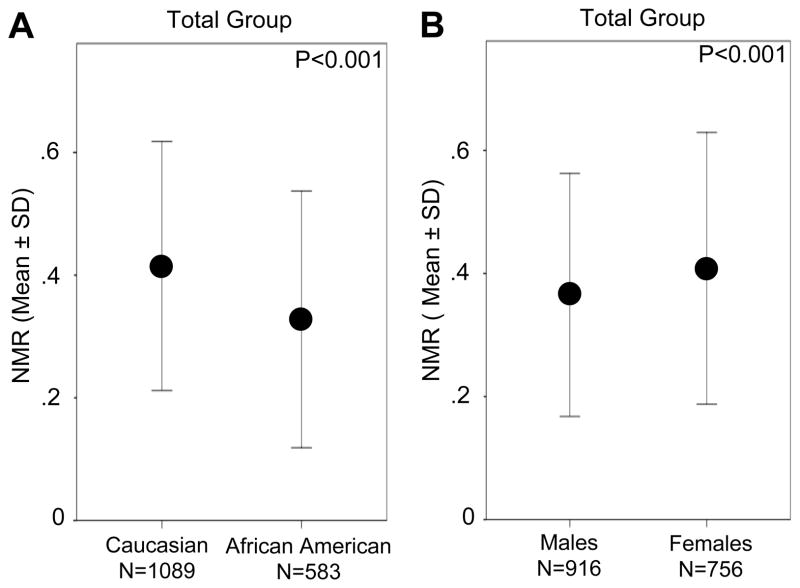
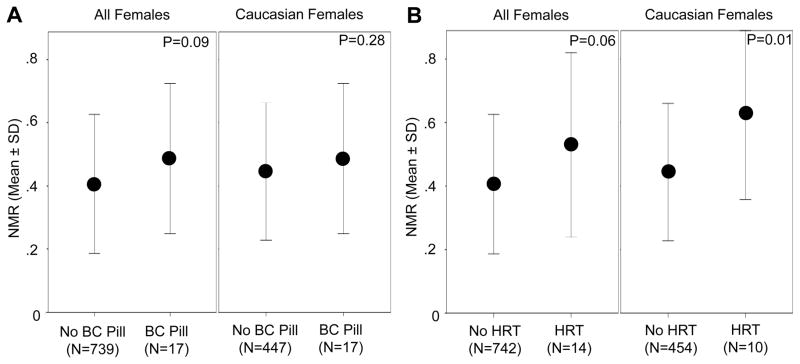
Mean NMR was higher in Caucasians than in African Americans (0.41 (0.20) vs. 0.33 (0.21), P<0.001) and in females compared to males (0.41 (0.22) vs. 0.37 (0.20), P<0.001) (Figure 1A and B). The interaction term (ethnicity × gender) was not significant (F(1,1668) = 1.06, P=.30). Next we examined the influence of estrogen-containing birth control pill use and estrogen-containing hormone replacement therapy use on NMR in women. Relative to non-users (N=739), females that reported current birth control pill use (N=17) had 19.5% higher mean (SD) NMR (0.49 (0.24) vs. 0.41 (0.22), respectively, P=0.09) (Figure 2A). Mean (SD) NMR was 29.3% higher among hormone replacement therapy users (N=14) relative to non-users (N=742) (0.53 (0.29) vs. 0.41 (0.22), respectively, P=0.06) (Figure 2B). We noted similar relationships in Caucasian females (Figure 2); in the African Americans, there were only four females total using either birth control pills or hormone replacement therapy, precluding our ability to assess this impact.
Figure 1.
Variation in the nicotine metabolite ratio (NMR) according to ethnicity and gender in Caucasian and African American adult smokers NMR is shown as a function of ethnicity (A) and gender (B) in the total group (Mann-Whitney U tests).
Figure 2.
Association between exogenous estrogen-containing therapy and NMR among females NMR is shown as a function of estrogen-containing birth control (BC) pill use in all females and Caucasian females (A) and as a function of estrogen-containing hormone replacement therapy (HRT) use in all females and Caucasian females (B) (Mann-Whitney U tests).
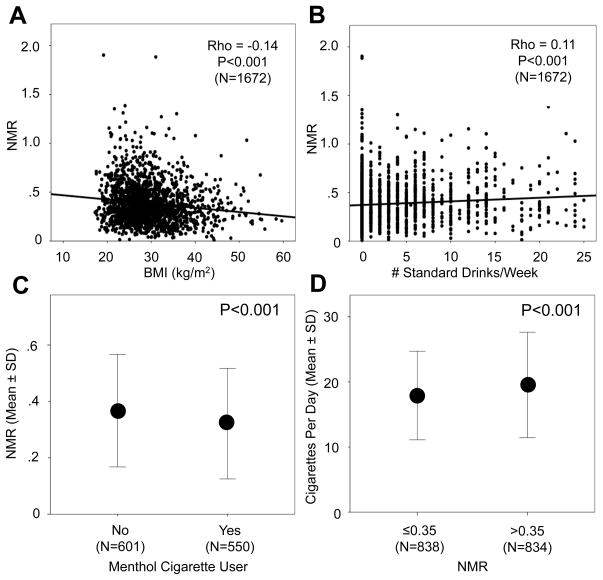
In the total group, BMI was negatively correlated with NMR (Rho=−0.14, P<0.001) (Figure 3A), which remained significant after controlling for breath CO, a biomarker of cigarette consumption (Linear regression model R2 change after controlling for CO = 0.018, P<0.001). In a separate linear regression model, after controlling for main effects (BMI and ethnicity), the interaction term (BMI × ethnicity) was not significant (P=0.91; Model R2 change = 0.0), suggesting the relationship between BMI and NMR is similar in Caucasians and African Americans. Likewise, after controlling for main effects, BMI and gender did not interact to influence NMR (P=0.68; Model R2 change = 0.0), suggesting a similar relationship between BMI and NMR in both males and females.
Figure 3.
Associations for NMR with BMI, alcohol consumption, cigarette use, and menthol Correlations between BMI and NMR (A) and alcohol use (# standard drinks/week) and NMR (B) are shown in the total group. The association between mentholated cigarette use and NMR in the intent-to-treat group (where it was available) is shown in (C), while the association between NMR, as a median split, and CPD is depicted in the total group in (D) (Mann-Whitney U tests).
Associations of alcohol use, mentholated cigarette use, and cigarette consumption with NMR
Alcohol use (range = 0–25 standard drinks/week) was positively associated with NMR in the total group (Rho=0.11, P<0.001) (Figure 3B), even after controlling for levels of smoking using breath CO (Linear regression model R2 change after controlling for CO = 0.008, P<0.001). In a linear regression model, after controlling for main effects (alcohol use and ethnicity), the interaction term (alcohol use × ethnicity) was not significant (P=0.73; Model R2 change = 0.0), suggesting a similar relationship in Caucasians and African Americans. Similarly, after controlling for alcohol use and gender, the interaction term (alcohol use × gender) was not significant (P=0.65; Model R2 change = 0.0), suggesting a similar relationship in males and females.
We next investigated the potential influence of mentholated cigarette use on NMR. Menthol inhibits CYP2A6 activity in vitro [34] and nicotine clearance in vivo [35], and therefore may result in lower NMR. In the ITT subgroup (N=1155), where menthol use data were available, the prevalence of mentholated cigarette use was 22.7% and 85.6% among Caucasian and African American smokers, respectively (P<0.001). Those smoking mentholated cigarettes (N=550) displayed significantly lower mean (SD) NMR compared to those smoking non-mentholated cigarettes (N=601) (0.32 (0.20) vs. 0.37 (0.20), respectively; P<0.001) (Figure 3C). We used a 2 × 2 factorial design to evaluate whether the association between mentholated cigarette use and NMR was similar across ethnicities. There was no significant main effect of mentholated cigarette use on NMR (F(1,1147) = 1.62, P=0.20), whereas a significant effect of ethnicity (F(1,1147) = 10.82, P=0.001) was observed. The interaction term (mentholated cigarette use × ethnicity) was not significant (F(1,1147) = 3.51, P=0.06).
We next evaluated the relationship between NMR and CPD. In contrast to the influence of selected variables on NMR described above, variation in CYP2A6 activity, measured by CYP2A6 genotype or NMR, has been previously shown to influence cigarette consumption (i.e., faster metabolizers smoke more heavily) [13, 14, 16, 19]. Thus we were interested in investigating a potential effect of NMR (slow vs. fast nicotine metabolism) on CPD. We included NMR (median split; ≤0.350 (N=838) vs. >0.350 (N=834)) as the predictor variable and CPD (continuous measure) as the outcome variable. Mean (SD) CPD was 17.9 (6.8) and 19.5 (8.1) in those with lower vs. higher NMR, respectively (P<0.001) (Figure 3D). We ran a 2 × 2 × 2 factorial model to evaluate potential interactions between ethnicity, gender, and NMR on CPD. There were significant main effects of NMR (F(1,1664) = 5.91, P=0.02), ethnicity (F(1,1664) = 89.03, P<0.001), and gender (F(1,1664) = 15.51, P<0.001) on CPD. The only significant interaction term in the model was ethnicity × gender (F(1,1664) = 6.16, P=0.01). The NMR cut-point of 0.31 was used in the clinical trial (NCT01314001) to differentiate slower from faster metabolizers, to randomize participants to treatment based on NMR, and to compare treatment outcomes from this clinical trial. Thus we also utilized an NMR cut-point of 0.31 to stratify participants into slower (≤0.31; N=679) and faster (>0.31; N=993) NMR groups and to evaluate potential interactions between ethnicity, gender, and NMR (2 × 2 × 2 factorial model) on CPD. There were significant main effects of NMR (F(1,1664) = 5.87, P=0.02), ethnicity (F(1,1664) = 84.93, P<0.001), and gender (F(1,1664) = 11.34, P=0.001) on CPD. The only significant interaction term in the model was ethnicity × gender (F(1,1664) = 6.16, P=0.01).
To further illustrate these various relationships with NMR, we compared alcohol consumption, BMI, as well as CPD and CO across NMR groups using the cut-point of 0.31. Consistent with the analyses above, slower metabolizers (lower NMR) displayed lower alcohol and cigarette consumption, but higher BMI, relative to faster metabolizers in the total group (Supplementary Table S1, also shown for the ITT subgroup).
Regression analysis identifying significant predictors of NMR
In the overall model, ethnicity, gender, HRT use, BMI, CPD, and number of alcohol drinks/week were significant predictors of NMR, while BC pill use trended towards significance (Table 2, men were coded as “0” for birth control pill use and HRT). The overall model R2 value was 0.076, indicating these variables accounted for 7.6% of the variation in NMR; each variable uniquely contributed ≤2% of the variation in NMR. We then ran the same model in females only (see footnote to Table 2). The overall R2 value for the model, which included all predictors except gender, was 0.066. The impact of birth control pill use and HRT on NMR was of a similar magnitude in the female-only group, as for the total group.
Table 2.
Linear regression analysis of the predictors of the nicotine metabolite ratio (NMR) in the total sample (N=1672)
| Nicotine Metabolite Ratio (NMR) R2 = 0.076a; P <0.001 |
|||||
|---|---|---|---|---|---|
| Predictor | B | Standard Error | β | P value | % Variationb |
| Ethnicityc | 0.071 | 0.011 | 0.162 | <0.001 | 2.3 |
| Genderd,e | 0.057 | 0.010 | 0.136 | <0.001 | 1.7 |
| Birth control pill use | 0.036 | 0.050 | 0.017 | 0.47 | 0.03 |
| Hormone replacement therapy use | 0.114 | 0.054 | 0.050 | 0.036 | 0.24 |
| Body mass index | −0.003 | 0.001 | −0.108 | <0.001 | 1.1 |
| Alcohol use (# drinks/week) | 0.003 | 0.001 | 0.070 | 0.004 | 0.46 |
| Cigarettes per day | 0.002 | 0.001 | 0.062 | 0.012 | 0.36 |
Together the predictors account for 7.6% of the variation in NMR
Calculated by squaring the part correlation coefficient (not shown), and multiplying by 100
African Americans and Caucasians were coded as ‘0’ and ‘1’, respectively, in the model
Males and females were coded as ‘0’ and ‘1’, respectively, in the model
When we restricted the model to females only (N=756), to further examine the effect of birth control pill and hormone replacement therapy use, the predictors (gender is excluded) together explained 6.6% of the variation in NMR. The standardized beta values for birth control pill and hormone replacement therapy use were 0.025 (P=0.48) and 0.069 (P=0.052), respectively, in the female-only group. They uniquely contributed 0.06% and 0.48% of the variation in NMR, respectively, in females.
We also ran a separate model examining the impact of these predictors on NMR among those in the ITT sub-group (N=1155; Table 3); a similar percent contribution (6.5%) to NMR variability was observed. Mentholated cigarette use (available in this subgroup) did not significantly contribute to NMR variation, and its inclusion in the model did not substantially alter the regression coefficients of the other variables (Table 3, footnote contains data on women only). Notably, HRT use was associated with a relatively large unstandardized (B) coefficient in both the total (B=0.11) and ITT (B=0.16) groups, suggesting a potential impact on NMR in those receiving HRT.
Table 3.
Linear regression analysis of the predictors of the nicotine metabolite ratio (NMR) in the intent-to-treat group (N=1155)
| Nicotine Metabolite Ratio (NMR) R2 = 0.065a; P<0.001 |
|||||
|---|---|---|---|---|---|
| Predictor | B | Standard Error | β | P value | % Variationb |
| Ethnicityc | 0.048 (0.053) | 0.015 (0.012) | 0.118 (0.130) | 0.002 (<0.001) | 0.81 (1.5) |
| Genderd,e | 0.058 (0.058) | 0.012 (0.012) | 0.144 (0.144) | <0.001 (<0.001) | 1.9 (1.9) |
| Birth control pill use | 0.031 (0.030) | 0.057 (0.057) | 0.016 (0.015) | 0.58 (0.60) | 0.03 (0.02) |
| Hormone replacement therapy use | 0.158 (0.158) | 0.062 (0.062) | 0.074 (0.073) | 0.010 (0.011) | 0.53 (0.53) |
| Body mass index | −0.003 (−0.003) | 0.001 (0.001) | −0.087 (−0.088) | 0.004 (0.003) | 0.69 (0.71) |
| Alcohol use (# drinks/week) | 0.002 (0.002) | 0.001 (0.001) | 0.056 (0.055) | 0.062 (0.062) | 0.29 (0.28) |
| Cigarettes | 0.002 (0.002) | 0.001 (0.001) | 0.077 (0.077) | 0.010 (0.011) | 0.55 (0.53) |
| Menthol | 0.007 | 0.015 | 0.018 | 0.627 | 0.02 |
Numbers in parentheses indicate values when the mentholated cigarette use variable is removed from the model to facilitate comparison to the total group in Table 2 where this variable is missing
Together the predictors account for 6.5% of the variation in NMR with and without mentholated cigarette use in the model
Calculated by squaring the part correlation coefficient (not shown), and multiplying by 100
African Americans and Caucasians were coded as ‘0’ and ‘1’, respectively, in the model
Males and females were coded as ‘0’ and ‘1’, respectively, in the model
When we restricted the model to females only (N=516), to further examine the effect of birth control pill and hormone replacement therapy use, the predictors (gender and menthol are excluded) together explained 5.3% of the variation in NMR. The standardized beta values for birth control pill and hormone replacement therapy use were 0.021 (P=0.63) and 0.10 (P=0.021), respectively, in the female-only group. They uniquely contributed 0.04% and 1% of the variation in NMR, respectively, in females.
Discussion
The NMR is currently being investigated prospectively as a predictive biomarker of response to smoking cessation treatments in an ongoing NMR-stratified clinical trial (NCT01314001). If NMR displays favorable efficacy and economic feasibility in predicting treatment response [3], the NMR could be used to tailor smoking cessation pharmacotherapy in treatment-seeking smokers. While CYP2A6 genotype, and potentially other genetic factors, cause substantial inter-individual variability in NMR [28], within-person variability is relatively minor as the NMR is stable and reproducible over time in cigarette smokers [33, 42].
We first examined known influences (i.e., ethnicity, gender, exogenous estrogen-based hormonal therapies, and BMI) on NMR. The higher NMR observed among Caucasians relative to African Americans is likely largely due to the lower frequency of reduced CYP2A6 activity variants among populations of Caucasian descent [36–38]. Among individuals without CYP2A6 variants (i.e., CYP2A6*1/*1 wild type individuals), there is no difference in NMR between Caucasians and African Americans [8], suggesting the variability in NMR observed between Caucasians and African Americans in the current study is likely attributable to variation in CYP2A6. Inter-ethnic variability in NMR may also arise when there are large differences between ethnicities in exposure to non-genetic factors that affect CYP2A6 expression and/or activity. For instance, the prevalence of mentholated cigarette use was much higher among African American relative to Caucasian smokers in our study, consistent with previous findings [43]. Menthol has been shown to inhibit CYP2A6 activity in vitro [34] and nicotine clearance in vivo [35], and may be associated with lower average NMR in African Americans compared to populations with lower prevalence of menthol cigarette use. This effect on NMR, while not a significant predictor of variation in overall NMR (Table 3), may represent a source of variability in NMR under certain circumstances.
We observed higher NMR among women relative to men, and even higher NMR in women taking estrogen-containing birth control pills or hormone replacement therapy. The higher NMR is likely attributable to enhancement of CYP2A6 transcriptional activity through estrogen binding of the estrogen response element located within the CYP2A6 gene [44]. We observed no interaction between gender and ethnicity on NMR, suggesting the influence of estrogen on NMR is similar between ethnicities.
BMI was negatively associated with NMR, consistent with previous reports [8, 12, 28, 33]. Negative associations were also observed for plasma cotinine (Rho=−0.10; P<0.001) and 3′hydroxycotinine (Rho=−0.18; P<0.001) with BMI, in line with prior findings [45, 46]. We postulate three potential explanations. First, when we controlled for the level of smoking (breath CO), as smoking is associated with lower body weight [47, 48], the negative association between BMI and NMR remained significant, suggesting the lower BMI observed among those with higher NMR may not result from heavier smoking in those with faster nicotine metabolism. In African Americans [49], we observe no association between CYP2A6 genotype group and BMI, despite observing significant correlations between NMR and BMI in both the total population and in reduced CYP2A6 metabolizers (Andy Z. X. Zhu; unpublished findings), further suggesting that this relationship is due to an effect of higher BMI (or obesity) on NMR rather than an effect of CYP2A6 activity or NMR on risk for obesity. Second, higher BMI may differentially affect the distribution pharmacokinetics of cotinine and 3′hydroxycotinine, resulting in an overall net reduction in NMR. However, it seems unlikely that this occurs through unique effects on the volume of distribution of cotinine versus 3′hydroxycotinine, as the pKa values of these compounds are similar (~4.4 vs. ~4.3, respectively [50]). Likewise, it seems unlikely that higher BMI would differentially affect the urinary excretion of these compounds, since negative associations for BMI with urinary cotinine and 3′hydroxycotinine are similar to those for plasma cotinine and 3′hydroxycotinine (Andy Z. X. Zhu; unpublished findings from another study [23]). Together these findings suggest the relationship between higher BMI and lower NMR is not mediated by differential effects of obesity on the volume of distribution or excretion of cotinine and 3′hydroxycotinine. Third, higher BMI and adiposity may uniquely affect the enzymes involved in the metabolism of cotinine and 3′hydroxycotinine, for example UDP-glucuronosyltransferases, however this remains to be explicitly tested in a pharmacokinetic study.
NMR was positively associated with cigarette consumption. This is likely an effect of NMR on the level of smoking, where faster metabolism is associated with heavier smoking and greater total nicotine intake [20, 23]. Consistent with this, the inhibition of CYP2A6 activity using oral methoxsalen treatment lead to reductions in both smoking and CYP2A6-mediated metabolic activation of the procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) [51]. We also observed a positive relationship between alcohol consumption and NMR, which could suggest CYP2A6 activity increases with alcohol use, or alternatively may represent an indirect effect of NMR on smoking, as nicotine is commonly used with alcohol [39, 52]. When we controlled for smoking level, the association between higher alcohol use and higher NMR remained significant (P<0.001) suggesting that the higher NMR in those consuming larger amounts of alcohol is not due exclusively to higher smoking among this group. In rodents, liver damage [53, 54] and three-week ethanol treatment [55] induced Cyp2a5, which is the murine orthologue of CYP2A6. In contrast, there was no impact on hepatic CYP2A6 levels or activity following five weeks of alcohol self-administration (~24 mM blood ethanol levels; equivalent to ~4 standard drinks/day) in African green monkeys [56]. The reason for the higher NMR in those with higher alcohol consumption remains to be determined. However, given that the number of drinks per week uniquely explained <1% of the total variation in NMR, typical variation in alcohol use is not likely to substantially alter the utility of NMR, in particular as a prospective biomarker to guide treatment.
In both Caucasians and African Americans, lower NMR is associated retrospectively with greater smoking cessation [9–12]. This study used NMRs collected during participant screening for the first prospectively stratified study of NMR as a predictive biomarker of cessation. This trial is currently underway (NCT01314001). We studied multiple sources of variation in NMR, which together accounted for <8% of NMR variation; the greatest unique contribution made by any one factor to NMR variation was <2%, suggesting these factors contribute little to overall variation in NMR on a population level. When NMR is used prospectively to guide therapy, relatively permanent or long-term sources of variation (e.g. ethnicity, gender, and BMI) are unlikely to cause treatment misclassifications, while potentially more transient influences on NMR (e.g. HRT and birth control pill use) may need to be considered if they are likely to change during the course of treatment. Together we extend our understanding of the type, and degree, of influence of demographic factors on NMR. Each factor examined, alone and together, contributed little variation to NMR, supporting the NMR as a stable, reliable and independent biomarker with potential clinical utility to guide smoking cessation pharmacotherapy.
Supplementary Material
Acknowledgments
Financial support: We acknowledge the support of the Endowed Chair in Addictions for the Department of Psychiatry (R.F. Tyndale), CIHR-CGSD and Ontario Graduate Scholarship (M.J. Chenoweth), NIH PGRN grant DA020830 (R.F. Tyndale and C. Lerman), CIHR grants MOP86471 (R.F. Tyndale) and TMH-109787 (R.F. Tyndale), CAMH, the CAMH Foundation, the Canada Foundation for Innovation (#20289 and #16014 to R.F. Tyndale and T.P. George) and the Ontario Ministry of Research and Innovation.
Footnotes
Conflicts of interest: Dr. George has consulted for Novartis. Dr. Tyndale has consulted for Apotex and McNeil. Dr. Schnoll has consulted for GlaxoSmithKline. Dr. Hawk has consulted on investigator-initiated smoking cessation studies funded by Pfizer and by the state of Florida. Drs. Lerman and Schnoll have received medication and placebo from Pfizer. Drs. Lerman, George, and Cinciripini have received research funding from Pfizer. The remaining authors declare no conflicts of interest.
References
- 1.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2.The World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package [Internet] Geneva: WHO Press; 2008. [cited 30 May 2014]. Available from: http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf. [Google Scholar]
- 3.Bough KJ, Lerman C, Rose JE, McClernon FJ, Kenny PJ, Tyndale RF, et al. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93:526–38. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24:1212–7. [PubMed] [Google Scholar]
- 6.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3′-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277:1010–5. [PubMed] [Google Scholar]
- 7.Dempsey D, Tutka P, Jacob P, 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Binnington MJ, Zhu AZ, Renner CC, Lanier AP, Hatsukami DK, Benowitz NL, et al. CYP2A6 and CYP2B6 genetic variation and its association with nicotine metabolism in South Western Alaska Native people. Pharmacogenet Genomics. 2012;22:429–40. doi: 10.1097/FPC.0b013e3283527c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 12.Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Loughlin J, Paradis G, Kim W, DiFranza J, Meshefedjian G, McMillan-Davey E, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–8. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–74. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 15.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–9. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 18.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–16. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnoll RA, George TP, Hawk L, Cinciripini P, Wileyto P, Tyndale RF. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-013-3421-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–4. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 21.Falcone M, Jepson C, Benowitz N, Bergen AW, Pinto A, Wileyto EP, et al. Association of the nicotine metabolite ratio and CHRNA5/CHRNA3 polymorphisms with smoking rate among treatment-seeking smokers. Nicotine Tob Res. 2011;13:498–503. doi: 10.1093/ntr/ntr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–30. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhu AZ, Binnington MJ, Renner CC, Lanier AP, Hatsukami DK, Stepanov I, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis. 2013;34:93–101. doi: 10.1093/carcin/bgs306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strasser AA, Benowitz NL, Pinto AG, Tang KZ, Hecht SS, Carmella SG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20:234–8. doi: 10.1158/1055-9965.EPI-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamaki Y, Arai T, Sugimura H, Sasaki T, Honda M, Muroi Y, et al. Association between cancer risk and drug-metabolizing enzyme gene (CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms in cases of lung cancer in Japan. Drug Metab Pharmacokinet. 2011;26:516–22. doi: 10.2133/dmpk.dmpk-11-rg-046. [DOI] [PubMed] [Google Scholar]
- 26.Fujieda M, Yamazaki H, Saito T, Kiyotani K, Gyamfi MA, Sakurai M, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25:2451–8. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 27.Ariyoshi N, Miyamoto M, Umetsu Y, Kunitoh H, Dosaka-Akita H, Sawamura Y, et al. Genetic polymorphism of CYP2A6 gene and tobacco-induced lung cancer risk in male smokers. Cancer Epidemiol Biomarkers Prev. 2002;11:890–4. [PubMed] [Google Scholar]
- 28.Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenet Genomics. 2009;19:388–98. doi: 10.1097/FPC.0b013e32832a404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derby KS, Cuthrell K, Caberto C, Carmella SG, Franke AA, Hecht SS, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:3526–35. doi: 10.1158/1055-9965.EPI-08-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol. 2007;165:901–10. doi: 10.1093/aje/kwm010. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–8. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Mwenifumbo JC, Sellers EM, Tyndale RF. Nicotine metabolism and CYP2A6 activity in a population of black African descent: impact of gender and light smoking. Drug Alcohol Depend. 2007;89:24–33. doi: 10.1016/j.drugalcdep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol Biomarkers Prev. 2008;17:1396–400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDougall JM, Fandrick K, Zhang X, Serafin SV, Cashman JR. Inhibition of human liver microsomal (S)-nicotine oxidation by (−)-menthol and analogues. Chem Res Toxicol. 2003;16:988–93. doi: 10.1021/tx0340551. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–15. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–97. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Haberl M, Anwald B, Klein K, Well R, Fuss C, Gepdiremen A, et al. Three haplotypes associated with CYP2A6 phenotypes in Caucasians. Pharmacogenet Genomics. 2005;15:609–24. doi: 10.1097/01.fpc.0000171517.22258.f1. [DOI] [PubMed] [Google Scholar]
- 38.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8:1385–402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 39.Bobo JK, Husten C. Sociocultural influences on smoking and drinking. Alcohol Res Health. 2000;24:225–32. [PMC free article] [PubMed] [Google Scholar]
- 40.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 41.Jacob P, 3rd, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–76. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P, 3rd, Aziziyeh A, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21:1105–14. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst. 2011;103:810–6. doi: 10.1093/jnci/djr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–41. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- 45.Ho MK, Faseru B, Choi WS, Nollen NL, Mayo MS, Thomas JL. Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:3426–34. doi: 10.1158/1055-9965.EPI-09-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Stable EJ, Benowitz NL, Marin G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24:171–9. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 47.Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–44. doi: 10.2105/ajph.77.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236–43. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 49.Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. 2012;104:290–8. doi: 10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li N, Li Y, Gorrod JW. Determination of partition coefficients and ionisation constants of (S)(−)-nicotine and certain metabolites. Med Sci Res. 1992;20:901–902. [Google Scholar]
- 51.Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF. The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res. 2003;5:891–9. doi: 10.1080/14622200310001615231. [DOI] [PubMed] [Google Scholar]
- 52.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–15. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 53.Gilmore WJ, Hartmann G, Piquette-Miller M, Marriot J, Kirby GM. Effects of lipopolysaccharide-stimulated inflammation and pyrazole-mediated hepatocellular injury on mouse hepatic Cyp2a5 expression. Toxicology. 2003;184:211–26. doi: 10.1016/s0300-483x(02)00581-4. [DOI] [PubMed] [Google Scholar]
- 54.Kirby GM, Nichols KD, Antenos M. CYP2A5 induction and hepatocellular stress: an adaptive response to perturbations of heme homeostasis. Curr Drug Metab. 2011;12:186–97. doi: 10.2174/138920011795016845. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y, Zhuge J, Wu D, Cederbaum AI. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab Dispos. 2011;39:330–6. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson CS, Miksys S, Palmour RM, Tyndale RF. Differential effects of nicotine treatment and ethanol self-administration on CYP2A6, CYP2B6 and nicotine pharmacokinetics in African green monkeys. J Pharmacol Exp Ther. 2012;343:628–37. doi: 10.1124/jpet.112.198564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.