Abstract
PURPOSE:
To conduct a Phase I trial of a Modified Vaccinia Ankara vaccine delivering wild type human p53 (p53MVA) in patients with refractory gastrointestinal cancers.
EXPERIMENTAL DESIGN:
Three patients were vaccinated with 1.0 × 108 pfu p53MVA followed by nine patients at 5.6 × 108 pfu. Toxicity was classified using the NCI Common Toxicity Criteria and clinical responses were assessed by CT scan. Peripheral blood samples were collected pre- and post-immunization for immunophenotyping, monitoring of p53MVA induced immune response and examination of PD-1 checkpoint inhibition in vitro.
RESULTS:
p53MVA immunization was well tolerated at both doses, with no adverse events above grade 2. CD4+ and CD8+ T cells showing enhanced recognition of a p53 overlapping peptide library were detectable after the first immunization, particularly in the CD8+ T cell compartment (p=0.03). However in most patients this did not expand further with the second and third immunization. The frequency of PD-1+ T cells detectable in patients PBMC was significantly higher than in healthy controls. Furthermore, the frequency of PD-1+ CD8+ T cells showed an inverse correlation with the peak CD8+ p53 response (p=0.02) and antibody blockade of PD-1 in vitro increased the p53 immune responses detected after the second or third immunizations. Induction of strong T cell and antibody responses to the MVA backbone were also apparent.
CONCLUSION:
p53MVA was well tolerated and induced robust CD8+ T cell responses. Combination of p53MVA with immune checkpoint inhibition could help sustain immune responses and lead to enhanced clinical benefit.
Keywords: immunotherapy, MVA, p53, clinical trial, GI cancers
Introduction
Immune based therapies have been most extensively studied in cancers such as melanoma, but there is evidence that anti-tumour immune responses in gastrointestinal (GI) cancer patients correlate with improved prognosis (1, 2). The obstacles to stimulating effective anti tumor immunity are considerable, but there is encouraging evidence that immunotherapy can improve outcomes in cancer patients (3-5). Furthermore, it is now recognized that radiotherapy and some chemotherapy agents confer therapeutic benefit, at least partly, by stimulating immune responses that directly target cancer cells or induce immunostimulatory side effects (6).
Wild type p53 protein maintains normal cell division and mutations in this gene are present in the majority of solid tumors (7). p53 gene mutations result in the accumulation of high levels of oncogenic p53 protein within tumor cells. In contrast, the concentration of normal p53 in healthy cells is low, making p53 an attractive target for immunotherapy of a wide range of malignancies. Immune recognition of p53 in tumor cells has been demonstrated both in vitro (8, 9) and in mouse models (10, 11). Furthermore, clinical trials targeting p53 by administration of synthetic peptides and dendritic cell based vaccines have yielded promising results (12, 13). Most notable are trials utilizing dendritic cells infected with a p53 adenoviral vector (Advexin), which showed evidence of clinical benefit when administered to lung cancer patients (14). However the p53 vaccines tested to date are restricted to patients with certain tissue types, or require individual manufacture for each recipient and hence are laborious and costly to produce.
We have developed a strategy using the genetically engineered version of the MVA virus (Modified Vaccinia Ankara) to immunize patients with the wild type p53 antigen (p53MVA). Using a viral vector to deliver full-length p53 has the potential to generate sustained antigen expression and the presentation of numerous antigenic determinants on different HLA molecules. In pre-clinical studies, Hupki mice (Human p53 Knock-In) were engineered to substitute the mouse p53 gene with the human form, enabling tolerance, thereby developing an immunological milieu similar to what the human vaccine will encounter clinically. Hupki mice immunized with p53MVA showed regression of established 4TI syngeneic breast tumors with murine p53 knockout and engineered human p53 expression, and generation of systemic anti-tumor immunity (15). Finally, studies with PBMC collected from cancer patients with solid tumors showed that specific recall immune responses to p53 could be stimulated in vitro with p53MVA (16).
MVA has a demonstrated safety record, being used in numerous clinical trials with only mild side-effects. The initial vaccine dose of 1.0 × 108 pfu was chosen because a previous trial using MVA expressing IL-2 and MUC1 reported low toxicity, as well as disease stabilization and cellular immune responses (17). In the MVA-5T4 trials for colorectal cancer which used doses of 5.0 × 108 pfu, immunological and clinical responses were achieved in the absence of toxicity (18). Murine studies conducted by us (10) and others (19) have demonstrated that p53 based immunotherapy is most effective when used in combination with anti-CTLA4. Furthermore, comparable human data was reported in prostate cancer patients treated with a combination of a PSA-fowlpox vaccine and ipilimumab™ (Bristol-Myers Squibb, New York City, NY) (20). This adds weight to the rationale of combining viral based vaccines with other immunostimulatory agents. However, since this was a first-in-human trial of p53MVA, a single agent study was optimal to assess properties of the vaccine construct. Here we report the findings of this study in regard to safety, clinical response and immunological endpoints.
Methods
p53MVA Vaccine Formulation
The therapeutic agent tested in this study was a Modified Vaccinia Ankara vector expressing full length wild type human p53. The p53MVA vaccine product was manufactured at the Center for Biomedicine and Genetics at City of Hope using GMP-grade materials and the final formulation was diluted in phosphate-buffered saline (PBS) and 7.5% lactose. The p53MVA vaccine was previously evaluated in an IND-directed toxicology study in mice. There was no significant toxicity in terms of weight loss, physical exam, activity level, or chemical or hematologic studies (data not shown). p53MVA was vialed at two different concentrations, 1.3 × 108 pfu/ml and 7.0 × 108 pfu/ml and stored at −80°C. Vaccine doses were thawed at room temperature and administered within 1 hour of thawing. Previous studies showed that the vaccine was stable at room temperature for 4 hours (data not shown). Patients received injections in a volume of 0.8ml. There were no other therapeutic products involved.
Patients and Eligibility Criteria
Participants were recruited from GI cancer patients attending Medical Oncology clinics at the City of Hope Medical Center between December 2012 and June 2013. The IRB-approved study was conducted under IND14716 and registered as NCT01191684 at ClinicalTrials.gov. Prior to treatment, all patients received and signed the informed consent. Patients with unresectable and chemotherapy resistant primary or recurrent carcinoma of colorectal, gastric or pancreatic origin were eligible. Patients with colorectal cancer who had failed to respond to 5-FU based therapy with oxaliplatin and irinotecan as well as EGFR directed therapies for KRAS wild type patients; gastric cancer patients that failed standard first line treatment including herceptin for patients Her2+ or pancreatic cancer patients who had failed to respond to at least one chemotherapy regimen were eligible. Only patients with no clinically evident brain metastasis, with an anticipated survival of at least 3 months and a performance status of 80-100 (Karnofsky Performance Status) were admitted to the trial. Evidence of tumoral p53 over expression by immunohistochemistry or p53 mutational analysis was required. Patients with immunodeficiency (including HIV and organ graft related), prior radiation to more than 50% of all nodal groups, or those receiving concurrent corticosteroids were excluded. In addition, patients with a history of autoimmune disease, severe environmental allergies, myopericarditis, allergy to egg proteins or a known family history of Li-Fraumeni syndrome were ineligible. Patients could not receive chemotherapy or radiotherapy within the four weeks preceding enrollment. The characteristics of all enrolled patients are detailed in Table 1.
Table 1.
Patients receiving p53MVA
| Patient | Age | Sex | Cancer | Rounds of CT /RT |
Performance status |
p53MVA dose |
Highest grade AE |
Types of p53MVA related AE |
3 month CT scan result |
Immunizations + blood draws |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | M | pancreatic | 6/2 | 90 | LD | 1 | ISR, fatigue | PD | 3 imm >4 bd |
| 2 | 64 | F | colon | 6/2 | 90 | LD | 1 | ISR | PD | 3 imm >4 bd |
| 3 | 45 | F | colon | 5/1 | 90 | LD | 2 | lymphopenia, fatigue, myalgia, arthralgia, ISR, chills, diarrhea |
PD | 3 imm >4 bd |
| 4 | 64 | F | colon | 9/2 | 90 | HD | 2 | headache, fatigue, fever, ISR, nausea, anemia |
PD | 3 imm 4 bd |
| 5 | 55 | M | pancreatic | 4/2 | 90 | HD | 1 | ISR, fatigue | PD | 3 imm 4 bd |
| 6 | 48 | M | pancreatic | 3/1 | 80 | HD | 1 | ISR, fever | PD | 3 imm 4 bd |
| 7 | 53 | M | colon | 3/0 | 90 | HD | 2 | ISR, nausea, fatigue, fever |
PD | 2 imm 3 bd |
| 8 | 51 | F | colon | 6/0 | 90 | HD | 1 | hypertension, ISR, fatigue, cough |
PD | 3 imm 3 bd |
| 9 | 57 | M | pancreatic | 3/1 | 90 | HD | 1 | ISR | PD | 3 imm 4 bd |
| 10 | 59 | M | colon | 3/0 | 100 | HD | 1 | Chills, bone pain, fever, ISR, fatigue, pain |
PD | 3 imm 4 bd |
| 11 | 58 | M | pancreatic | 2/0 | 80 | HD | 1 | ISR | PD | 1 imm 2 bd |
| 12 | 58 | F | colon | 9/0 | 80 | HD | 2 | Fatigue, fever, ISR, pain, headache, malaise |
PD | 3 imm 4 bd |
M/F= male/female, CT/RT = chemotherapy/radiotherapy, LD/HD = Low Dose/ High Dose , AE= adverse event, ISR = injection site reaction, PD= progressive disease, imm= immunizations, bd= blood draws
Dose Escalation
The study included 12 patients treated at escalating doses of 1.0 ×108 followed by 5.6 ×108 pfu p53MVA, following a standard 3+3 design. No intra-patient dose escalation was carried out. The first 3 patients received subcutaneous injections of 108 pfu p53MVA every three weeks for a total of three injections. Dose limiting toxicity (DLT) was classified using the NCI Common Toxicity Criteria version 4.0. Patients treated in the low dose group did not experience dose limiting toxicity, hence treatment in the high dose group was initiated. A second group of three patients received three cycles of subcutaneous injections of 5.6 × 108 pfu p53MVA. As none of these patients experienced DLT, an additional six patients were then treated at this dose level.
Clinical Procedure
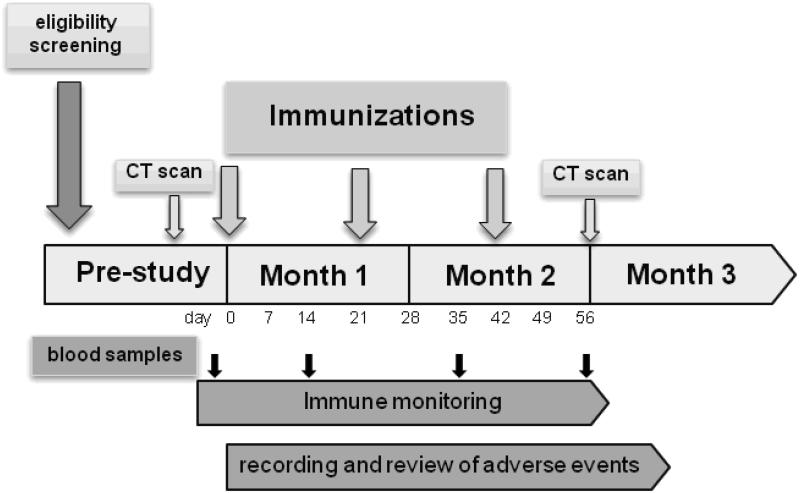
Phlebotomy was performed for biochemical, hematological and immunological assays prior to vaccination and two weeks following each immunization according to the schedule shown (Fig 1). A CT scan and physical exam were carried out pre-study to establish the extent of disease and was repeated two months following the initial injection. Vaccine injections were administered by a study nurse in the subcutaneous tissue of the upper arm over the deltoid muscle on days 0, 21 and 42. All subjects were monitored for one hour in the clinic after each immunization for temperature changes and local reactions at the injection site. All subjects were contacted 24 and 48 hours after each immunization in order to record any vaccine related complications. Two patients were unable to complete the vaccination schedule due to disease progression, but all 12 enrolled patients provided a pre and post-vaccine blood draw for immunological analysis.
Figure 1.
Clinical Trial Schema: vaccination and phlebotomy schedule for a p53MVA phase I clinical trial.
Cell Culture
Peripheral blood samples were collected from patients by venipuncture in ACD tubes and processed within an hour. PBMCs were purified by density gradient separation using Ficoll-Paque Plus (GE Healthcare). CD3+ cells were isolated from PBMCs using human CD3 negative selection microbeads (Miltenyi Biotec) as per manufacturer instructions. All primary cells were cultured in serum free conditions in X-VIVO 20 medium (Lonza). The CD3− fraction was retained to generate dendritic cells as follows: After 2 hours plastic adherence, non adherent cells were removed and the adherent population cultured in X-VIVO 20 media supplemented with IL-4 (50 ng /ml) and GM-CSF (100 ng/ml) for 5 days. Non-adherent cells were washed and infected with Adp53 (a kind gift from Scott Antonia, Moffit Cancer Centre) or control adenoviral vector (AdGFP) at an MOI of 15. After 2 hours, additional media containing polyinosinic-polycytidylic acid (50μg/ml) was added. After 48 hours, transduced, matured DC were washed and used to set up co-cultures with autologous CD3+ cells at an effector to stimulator ratio of 10:1. For PD-1 blocking experiments, azide free αPD-1 (eBioscience) or IgG1k isotype control (BD Pharminigen) antibodies were added at 5μg/ml. In vitro stimulation was carried out for 7 days, with 10u/ml IL-2 added on day 2. Stimulated cells were then washed and reseeded in the presence of test antigens, including: VenusMVA, a library of overlapping p53 peptides, or positive (pp65) or negative (BKV-VP1) control peptides at a concentration of 10μg/ml. Peptides were synthesized in the laboratory as previously described (15). After 24 hours, T cells were analysed for the expression of the activation marker CD137 by flow cytometry (see below) or IFN-γ secretion by ELISPOT assay (see below). Blood draws 3 and 4 from Patient 6 were unevaluable in functional cellular assays due to poor viability.
Flow Cytometry
Immunophenotyping
Flow cytometry analysis of cell surface molecules on PBMC was conducted using antibodies from Becton Dickinson or eBioscience. Cells were washed and stained with antibody for 30 minutes at room temperature, in the dark, in the presence of 1% FBS. For T reg intra-nuclear FOXP3 analysis, permeabilization was performed using the eBioscience anti-FOXP3 staining set, according to the manufacturer’s instructions. Regulatory T cells (Tregs) were identified as CD3+CD4+CD25+CD127low/−FOXP3+ and expressed as a percentage of CD4+ cells. Myeloid-derived suppressor cells (MDSC) were identified as HLADR−LIN1low/−CD33+CD11b+. T cell markers CD4, CD8, CD27, CD28 CD57 and PD-1 were used to assess T cell differentiation status. Blood samples from age and sex matched healthy donor controls were obtained through the City of Hope Blood Donation Clinic.
Measurement of T cell activation
Cells from each in vitro stimulation were co-stained with anti-CD4, anti-CD8 and anti CD137-PE or a PE-labelled isotype control. CD137 positivity was assessed relative to the isotype control PE signal. Flow cytometric analyses were carried out using a FACSCanto (BD Biosciences) or Gallios™ (Beckman Coulter) flow cytometer. All data was analysed with Flowjo7.5.6.
MVA Neutralisation Assays
Plasma was obtained by centrifugation of whole blood for 10 minutes, at room temperature for 1500 rpm. Aliquots were frozen and stored at −80°C until analysis. A modified version of the protocol described in Cosma et al 2004 (21) was used to assess the ability of patient plasma to neutralize MVA. Plasma samples were heat treated for 30 minutes at 56°C to inactivate complement, then cooled. Serial dilutions of plasma from 1:10-1:1000 were prepared in RPMI 1640 (Cellgro) with 10% fetal calf serum, to which was added 1 × 106 pfu of VenusMVA (previously described (22)). After 90 minutes incubation at 37°C, the different infection media were added to Hela cell monolayers and incubated for a further 2 hours. Infection media was then removed and replaced with RPMI, 10% FCS and the plates incubated overnight at 37°C, 5% C02. Infected cells were trypsinised from plates, washed with PBS, and fixed in 1% paraformaldehyde. The percentage of MVA infected Hela cells was evaluated by measuring green fluorescent protein analogue (Venus) expression by flow cytometry. Intact cells were discriminated from cellular debris according to FSSC/SSC. The percentage neutralization was calculated in relation to the % Venus+ cells after infection with VenusMVA incubated with media alone. Plasma from MVA naïve individuals and purified anti-vaccinia rabbit sera (a kind gift from Dr. Mary Marovich, Henry Jackson Foundation, Bethesda, MD) were included in each assay as negative and positive controls respectively. Plasma samples from Patient 3 were found to be unevaluable by this assay.
IFN-γ ELISPOT assays
Whole thawed PBMC or stimulated effector cells, were washed and seeded at 2 × 105 per well into ELISPOT plates coated with IFN-γ capture antibody as per the manufacturer’s instructions (BD Biosciences). VenusMVA at an MOI of 0.2 was added to triplicate wells. Additional control wells; media alone and PMA + Ionomycin or PHA were included to assess the background stimulation and viability of T cells respectively. Plates were incubated for 24 hours at 37°C, 5% CO2, after which cells were removed and the wells washed with distilled water. IFN-γ spots were visualized according to the manufacturer’s instructions and counted by computer-assisted video image analysis using an AID ELISPOT reader (Autoimmun Diagnostka GmbH, Germany).
Statistical Analysis
The comparison of experimental values before and after immunization was evaluated using paired t-test. A two group t-test was used to compare the endpoint values between patients and healthy controls. The correlation between two normally distributed endpoints was evaluated using Pearson correlation analysis. Since all study endpoints consistently showed normality patterns, the use of parametric methods was considered more appropriate than non-parametric tests. P values less than 0.05 were considered significant and less than 0.005 as highly significant.
Results
p53MVA Vaccination is Well Tolerated
p53MVA immunization was well tolerated, with no adverse events exceeding grade 2 attributable to the vaccine. Table 1 details the adverse events that were related to p53MVA vaccination. Injection site reaction (ISR) was the most commonly reported side-effect. Other commonly reported adverse events included fatigue, fever and nausea. Two patients did not complete the course of three immunizations and left the study early due to events not related to the vaccine injection.
p53MVA Vaccination Stimulates anti-p53 Immune Responses
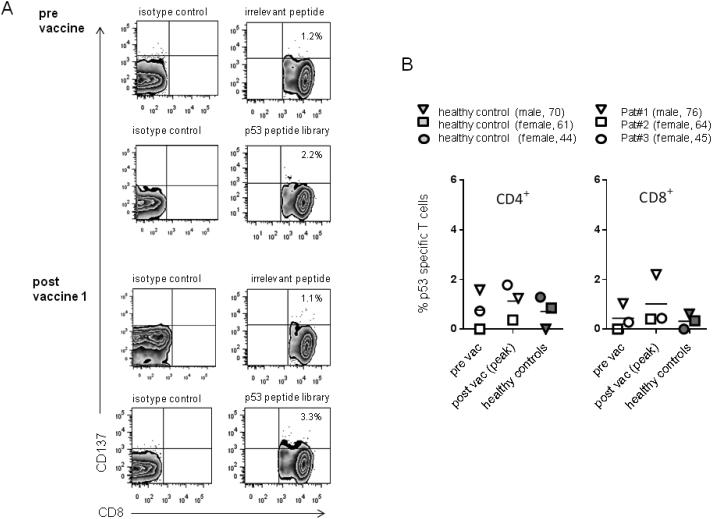
p53-specific T cell responses were initially evaluated in the low dose patient cohort by quantification of CD137+ T cells after stimulation with p53 peptides. The CD137 marker has been validated for measuring both CD8+ and CD4+ responses (23-25), with the suggestion that this assay may detect a broader repertoire of antigen-specific T cells than measurement of IFN-γ (23). Figure 2A shows a representative flow cytometry plot from Patient 1. An isotype control for the CD137 antibody was used to set the negative gates. To control for non specific stimulation the % CD137+ T cells after stimulation with an irrelevant peptide was subtracted from the % CD137+ T cells after stimulation with the p53 peptide library (details in Supplementary Table 1). Figure 2B shows the peak response detected after vaccination compared to the pre-vaccine value in the CD4+ and CD8+ compartments. The initial dose of 1.0 × 108 pfu was not predicted to be therapeutic but some enhanced p53-immune reactivity were detected in these three patients, most notably in the CD8+ T cells from Patient 1. However, in most cases, the frequency of p53-reactive T cells was comparable to that detected using PBMC from unvaccinated, healthy controls.
Figure 2. T cells collected from healthy donors and GI cancer patients receiving a subtherapeutic dose of p53MVA show low levels of p53 reactivity, despite in vitro stimulation.
Patient T cells were co-cultured for 5 days with p53 expressing APC and 10U/ml IL-2. Stimulated cells were then washed and reseeded in fresh media for 2 days. After a final, overnight stimulation with a p53-peptide library, the percentage of CD4+ or CD8+ T cells expressing the activation marker CD137 was determined. Comparative analysis using PBMC from three unvaccinated, matched healthy controls were carried out in parallel. Panel A shows representative flow cytometry plots of the CD8+ population, pre and post vaccination 1 from Patient#1. Panel B shows the peak post-vaccine p53 response compared to pre-vaccine for both CD4+ and CD8+ T cells in patients 1-3. To control for non-specific stimulation % CD137+ T cells in response to p53 peptide - % CD137+ T cells in response to an irrelevant peptide were plotted. Each patient (open symbols) and the corresponding matched control (closed symbols) is represented by a different symbol.
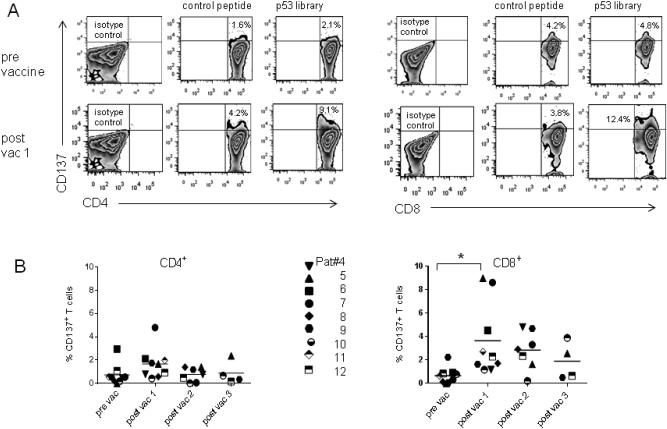
Vaccine enhanced p53-immune reactivity was higher in the patient cohort receiving the 5.6 × 108 pfu dose of p53MVA. This was most striking in the CD8+ T cell compartment. Figure 3A shows a representative flow cytometry plot from Patient 7. As with the low dose patient cohort, background stimulation was apparent, but even after subtraction of the CD137 signal from an irrelevant peptide, an enhancement of CD8+ p53 reactivity was significant after the first vaccination. This was seen in the majority of the high dose patients, and reached statistical significance (p=0.03). Furthermore, when the highest p53-specific responses detected post vaccination (peak response) were compared to pre vaccine levels, significance was even higher (p=0.002) in the CD8+ compartment. Despite the p53 peptide library containing both CD4 and CD8 epitopes, the increased p53 reactivity in the CD4+ compartment was less apparent in the CD8+ population and did not reach statistical significance (Fig 3B). The expansion of p53-reactive CD8+ T cells was greatest after the first vaccination, and subsequent immunizations did not further amplify the response in the majority of patients.
Figure 3. Vaccination with high dose p53MVA transiently increases the frequency of p53-reactive T cells in the peripheral blood of GI cancer patients.
Blood samples were collected pre-vaccination and two weeks after each p53MVA immunization. Purified CD3+ cells were expanded in vitro for 7 days prior to an overnight stimulation with a p53 peptide library or control peptides. The T cell activation marker, CD137, was used as a measure of T cell responsiveness and quantified by flow cytometry. Panel A shows representative flow cytometry plots from Patient#7. Panel B shows the % CD137+ T cells in response to p53 peptide - %CD137+ T cells in response to an irrelevant peptide in the CD4+ or CD8+ populations. Patients 4-12 are each represented by a different symbol. The increase in % of p53 responsive CD8+ T cells post-vaccine 1 compared to pre-vaccine values was statistically significant (p =0.03 ).
p53MVA Vaccination Stimulates Strong anti Vector Immune Responses
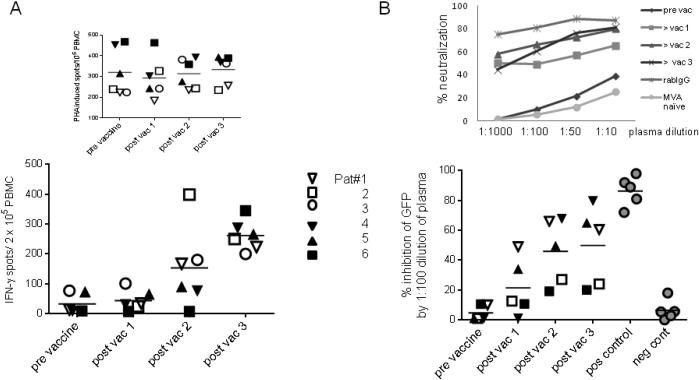
A VenusMVA vector was used to assess the humoral and T cell response to the MVA backbone pre and post-vaccine in the low dose cohort and the first three patients of the high dose cohort. Figure 4 shows the anti-MVA T cell response as measured by IFN-γ ELISPOT (panel A) and the MVA antibody response measured by neutralization assay (Panel B). It was not known which patients had a history of smallpox immunization, but due to the age of all trial participants, it is likely that they had been vaccinated. All tested patients showed low pre-exisiting anti-MVA responses. After vaccination with p53MVA all patients showed increased T cell reactivity and neutralizing activity against MVA. The highest induction of anti-MVA T cell response seen was a 54 fold increase (Patient 1). The strongest increase in neutralizing antibodies against MVA observed was a 79.7 fold increase (Patient 4). Both the low and high dose of p53MVA stimulated comparable levels of anti-MVA T cell and antibody responses. Unlike T cell responses against p53, which required in vitro expansion to reach measurable levels, MVA responses were detected in unstimulated PBMC. This suggests that MVA-reactive T cells had greater proliferative capacity and hence reached higher frequencies than p53-specific T cells post vaccination. This is an expected finding since anti-viral T cell responses are less tolerized than those against self antigens.
Figure 4. p53MVA immunization induces anti-MVA neutralizing antibody and T cell responses in advanced GI cancer patients.
Panel A shows the T cell response from 3 low dose patients (open symbols) and 3 high dose patients (closed symbols) against VenusMVA. PBMC tested IFN-γ ELISPOT assays for reactivity to media alone, VenusMVA or PHA. Values plotted represent the mean number of spots from PBMC incubated with VenusMVA – the mean number of spots from PBMC seeded in media alone (background). The number of IFN-γ spots from PHA stimulated PBMC are shown separately above. Panel B shows the % neutralization of the Venus signal by plasma collected from the same patients. This was assessed by incubating the VenusMVA virus with patient or control plasma prior to infection of a permissive cell line. Venus expression was determined by flow cytometry. Data is shown for only 5 patients (Pat#3 plasma samples were unevaluable in this assay). Plasma from MVA naïve individuals and purified IgG from vaccinia vaccinated rabbits were included as negative and positive controls respectively. % neutralization obtained at 1:100 dilution are shown, with a representative dilution curve for Pat#1 shown above.
MDSC Decline Transiently after p53MVA in a Subset of Patients
MDSC were characterized by flow cytometry as LIN−/lowHLADR−CD11b+CD33+.gated populations as shown in Supplementary Fig 1A. Monocytic MDSC and granulocytic MDSC populations (26) were not differentiated from each other. The frequency of MDSC as a % of the live PBMC did not differ significantly between patients and healthy controls. Interestingly, in 5 out of 9 of the high dose cohort the frequency of MDSC in the peripheral blood declined after vaccination, however in many cases this was a transient effect. Supplementary Figure 1C shows a representative example of the MDSC decline in Patient 6. However, when analyzed as a group, the difference in MDSC frequency pre- and post-immunization did not reach statistical significance (Supplementary Fig 1B).
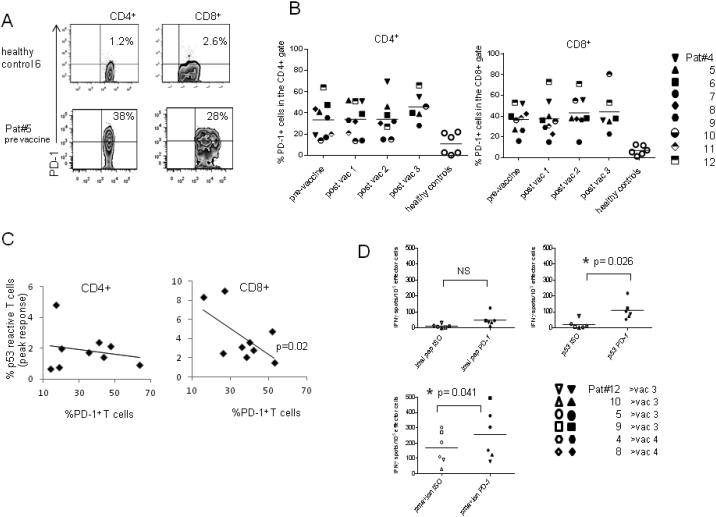
The Frequency of PD-1+ CD8+ T cells is Greater in Patients than Healthy Controls and Shows a Correlation with p53 Response
The negative T cell co-stimulatory molecule PD-1, is exploited by tumor cells to evade immune clearance. The percentages of PD-1+ T cells detected in trial participants PBMC were significantly higher than in healthy controls (Fig 5A and B). This was statistically significant in the CD4+ T cells (p=0.01) and highly significant in the CD8+ population (p=0.001). Further analysis of the CD8+ compartment revealed a significant correlation between the pre-vaccine percentage of PD-1+ T cells and the peak response to p53 peptides post-immunization (correlation r = 0.74, p=0.02, Fig 5C-right plot). To clarify, patients with lower frequencies of PD-1+ CD8+ T cells pre-vaccine showed greater induction of CD8+ p53-reactivity after immunization. However this is a small data set, which requires confirmation in a larger patient cohort.
Figure 5. Advanced GI cancer patients show higher frequency of PD-1+ T cells than healthy controls both pre and post-immunization with high dose p53MVA.
Peripheral blood T cells collected pre- and post-immunization were assessed by flow cytometry for the expression of the programmed cell death receptor PD-1. Panel A shows representative plots from a patient and healthy control. Quadrant gates were set according to isotype control staining. Panel B shows the % PD-1+ T cells in the CD4+ and CD8+ populations at different times in the immunization schedule. Different patients are represented by different symbols. Panel C shows an inverse relationship between the frequency of PD-1+ T cells pre-immunization and the peak p53-reactive T cells in the CD4+ and CD8+ populations. Correlation in the CD8+ population reached statistical significance (p=0.02). Figure 5D shows the reactivity of effector cells after in vitro expansion in the presence of αPD-1 (closed symbols) or isotype control (open symbols). Stimulated cells were tested in IFN-γ ELISPOT assays against control peptide, p53 library, VenusMVA and PMA + Ionomycin. Data shown is from lymphocytes obtained after 2 or 3 vaccinations.
In vitro PD-1 Blockade can Increase T cell Responses to p53 Peptides
Since p53-reactivity showed a relationship with frequency of PD-1+ T cells, PD-1 blockade was investigated. Since the p53-reactivity detected after the second or third vaccinations were lower than those detected after the first vaccination, we examined if these responses could be enhanced by PD-1 antibody blockade. A single round of In vitro expansions were carried with 6 patient samples as previously described but with the addition of anti PD-1 antibody or an isotype control at the start of the culture period. Figure 5D shows the IFN-γ responses of the expanded cells as measured by IFN-γ ELISPOT assay. PD-1 blockade modestly increased the T cell response to the p53 library, reaching statistical significance (p=0.026). These results support future clinical studies in which PD-1 antibody blockade is combined with the p53MVA vaccine.
The Frequency of Early, Intermediate and Late Differentiated T Cells or T regulatory Cells Does not Significantly Change after p53MVA Immunization
T regulatory cells (Tregs) were defined as the % of CD3+CD25+CD127low/−FOXP3+ gated populations within the CD4+ T cell compartment. The frequency of Tregs in patients PBMCs did not differ significantly from those detected in a set of healthy control PBMC samples. A slight downward trend in the % Tregs within the CD4+ T cell population was apparent post-immunization, but this did not reach statistical significance (Supplementary data).
Malignant disease is thought to cause chronic antigenic stimulation and drive T cell progression. This results in an expansion of late-differentiated, senescent lymphocytes which can impair anti-tumor immunity. When the differentiation stage of patients T cells were evaluated, some showed a higher frequency of terminal effector T cells (CD28+CD27+) and senescent T cells (CD8+CD27−CD28−CD57+PD-1+) than healthy controls, but this did not reach statistical significance (S1). In addition, the frequency of early, intermediate or late stage effector T cells did not change significantly after p53MVA immunization.
Discussion
In this report we describe the first in human clinical trial of p53 delivered by an MVA vector in patients with advanced, refractory GI malignancies. Clinical and immunological responses were assessed, but the primary endpoint was safety. p53MVA was well tolerated in all 12 patients, causing only low grade side-effects (Table 1). The p53MVA tolerability findings presented here are in line with previous reports of MVA clinical studies (17, 27, 28). No clinical responses were detectable at the post vaccine CT scan in any of the 12 patients by RECIST criteria, however immunological responses were transiently robust. Stimulating objective clinical responses is challenging in patients with high morbidity due to advanced disease and high levels of pre-treatment. Since chemotherapy naive patients can respond better to immunotherapy (20), p53MVA therapy in less heavily pre-treated patients is an attractive approach.
The role of MDSC and regulatory T cells in tumor immune evasion and cancer progression is well accepted (29, 30). Human MDSC are a heterogeneous population which unlike murine MDSC, are still being characterized, but the currently accepted definition for human MSDC is CD33+CD11b+HLA-DRlow/−. Within this population, the CD14+CD15low/− and CD14−CD15+ subsets are considered equivalent to the murine monocytic and granulocytic populations respectively(26). Tregs also exhibit some heterogeneity, with two main subtypes being recognized, natural Tregs generated during thymic development and inducible Tregs that arise in the periphery after interaction with tolerogenic stimuli. Tregs are crucial in maintaining peripheral tolerance to self antigens, and hence dampen effector T cell responses against many tumor antigens (31) . These suppressive cell types pose an obstacle to effective anti-tumor immunity, with both Tregs (32) and MDSC (33) being shown to affect vaccine induced immune responses. Hence, we thought it valuable to assess the frequency of these suppressive cells in the participants both pre- and post-vaccine. In 4/9 of the high dose patients, the frequency of MDSC increased slightly during the vaccination, concurrent with disease progression. However in 5/9 of the high dose cohort, the frequency of MDSC fell transiently after the first immunization (Figure S1). Tregs also showed a slight downwards trend after immunization, but did not reach statistical significance (Figure S2 lower panel). In addition, the small sample size and the fact that both Treg and MDSC frequency were in the range for healthy donors, makes it difficult to attribute disease relatedness to these findings.
Detection of p53 specific T cells required an in vitro re-stimulation to expand them to detectable levels, a finding reported by other groups developing p53 targeted therapies (13, 34). In order to ensure that is not a purely in vitro effect (in vitro immunization), healthy donor controls were included for comparison. The levels of p53 reactive T cells were generally lower than the responses seen in patients from the low dose cohort (Figure 2B). Vaccine induced responses were of greater magnitude in the high dose cohort, particularly with regard to CD8+ responses. 7/9 patients in the high dose cohort showed increased p53 reactive CD4+ T cells post vaccination, the median fold increase being 3.5 (Figure 3B-left panel). All the high dose patients showed increased p53 reactive CD8+ T cells post-immunization, with a median fold increase of 5 (Figure 3B-right). Our in vitro stimulation method using endogenously expressed p53 protein may have favoured the expansion of CD8+ T cells in our cultures prior to analysis. Although, it has been demonstrated that endogenously processed antigen can enter the MHCII pathway and prime CD4+ responses (35). The lower CD4+ T cell response to the p53 peptide library could have clinical implications, since CD4+ T cells play a critical role in sustaining the anti-tumor action of CTL (36). Hence, providing a greater helper signal by combining the vaccine with IL-2 or immunological adjuvants could further expand the CD8+ T cell response. It is possible that Tregs could be expanded during the in vitro stimulation and we will address this in future studies.
The enhancement of p53 response did not show continued expansion with successive immunizations in the majority of patients. However, in the small number of high dose patients that provided an evaluable fourth blood draw, 2 out of 4 still showed a higher frequency of p53-reactive CD8+ T cells than before immunization. It is possible that primed p53 reactive T cells may have moved out of the periphery into tissues, and hence not been accessible in peripheral blood draws. It would be interesting to examine the tumor infiltrating lymphocyte (TIL) population to test this hypothesis.
Immunodominant viral epitopes present in vector backbones may hamper the priming of responses against transgene encoded epitopes (27). Despite the notable immunogenicity of the MVA backbone (Figure 4), anti-p53 T cell responses were detectable post-immunization with p53MVA. However, expansion of responses against immunodominant viral antigens could be minimized by a prime boost strategy, delivering alternate doses of p53 with different viral vectors (37).
Chronic antigen stimulation causes upregulation of the ‘exhaustion marker’ PD-1 on T cells, including TILs (38, 39) and has been associated with disease progression (40) and poor prognosis (41). The primary ligand, PDL-1, has been detected on tumor cells, hence the PD-1/PDL-1 pathway can hamper anti-tumor immune responses (42). In addition, blood DC express elevated levels of PD-L1 in cancer patients (43). This could reduce the effectiveness of viral based vaccines, which require uptake and expression within DC. Inhibition of the PD-1/PDL-1 pathway has been shown to enhance anti tumor responses both in vitro (44) and murine models (45). Furthermore, antibodies targeting PD-1 or PD-L1 exhibited impressive clinical activity in patients with a variety of solid tumors, including lung cancers. Both antibodies were able to induce tumor regression or stable disease, with objective response rates of 18–27% for α-PD-1 and 6–17% for α-PDL-1 being reported. (3, 46, 47).
We have conducted in vivo studies utilizing p53MVA combined with anti PD-1 antibody in an orthotopic murine model of pancreatic cancer and observed impressive tumor rejection (data not shown). Correspondingly, significantly higher frequencies of PD-1+ T cells were detected in our trial participants compared to healthy donors (Figure 5A and B). Furthermore, lower frequencies of pre-vaccine PD-1+ CD8+ correlated with higher CD8+ p53-reactive T cells (p=0.02). In light of these observations and recent clinical reports, we hypothesized that PD-1 blockade would enhance the vaccine induced, anti-p53 T cell responses. In vitro expansions conducted in the presence of αPD-1 showed that significant enhancement of vaccine induced responses by PD-1 antibody was indeed achievable (Figure 5D). Amplification of antigen specific responses by PD-1 antibodies in vitro have been previously reported (44, 48), but to our knowledge, this is the first report of enhanced anti-p53 T cell responses due to PD-1 blockade. The combination of viral based vaccines and chemotherapy has also shown promise in GI cancer patients (18, 49), which raises another possible combination for p53MVA vaccine therapy.
In conclusion, it is likely that the high levels of immunosuppression in this patient group were a barrier to achieving objective clinical responses from p53MVA immunization. However, the tolerability profile and the ability to elevate the p53-specific CD8+ T cell response, support the continued development of p53MVA. Furthermore the combination of p53MVA with additional agents, such as antibody based immune checkpoint inhibitors, warrants investigation.
Supplementary Material
Translational Relevance.
Mutations in the p53 gene are present in the majority of solid tumors and result in high levels of p53 protein within tumor cells. In contrast, the concentration of wild type p53 in normal tissue is low. Hence p53 is an attractive target for immunotherapy of a wide range of malignancies, and data from in vitro studies, murine models and clinical trials support the rationale of targeting this protein. We conducted a first-in-human, Phase I trial of a Modified Vaccinia Ankara vector delivering wild type human p53 (p53MVA) in patients with refractory GI cancers. p53MVA was well tolerated and elevated the p53-specific CD8+ T cell response. Furthermore, higher anti-p53 immune responses were detected in patients with lower frequencies of PD-1+ T cells and enhanced responses were achievable with antibody blockade of PD-1 in vitro. These initial findings support the continued development of p53MVA, particularly in combination with immune checkpoint inhibition.
Table 2.
Healthy Controls
| Healthy Controls |
Age | Sex | |
|---|---|---|---|
| Controls for lowdose cohort |
1 | 70 | M |
| 2 | 61 | F | |
| 3 | 44 | F | |
| Controls for highdose cohort |
4 | 51 | 51 |
| 5 | 57 | 57 | |
| 6 | 58 | 58 | |
| 7 | 52 | 52 | |
| 8 | 60 | 60 | |
| 9 | 60 | 60 |
Acknowledgements
We would like to thank the following City of Hope staff and departments; The Investigational Drug Service, David Hsu, Yasmine Shad and Larry Couture (Centre Biomedicine and Genetics), Richard Ermel (Director of Animal Resources), The Office of IND Development and Regulatory Affairs, Mario Dimacali (CRA) and Michael A Friedman ( CEO Emeritus of City of Hope). We would like to thank Bernard Moss (National Institutes of Health) for allowing access to 1974-MVA and the NIAID for agreeing to the transfer for clinical use. We acknowledge Dimitri Gabriolvich and Scott Antonia (Moffit Cancer Center) for their kind gift of Advexin™.
This work was supported by funds from the National Cancer Institute (R29CA70819 and R21CA114889), a contract from SAIC (25X5061), FAMRI, Champion Power Equipment and the City of Hope Phase I program.
Footnotes
The authors declare there are no conflicts of interests.
References
- 1.Katz SC, Donkor C, Glasgow K, Pillarisetty VG, Gonen M, Espat NJ, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2010;12:674–83. doi: 10.1111/j.1477-2574.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC immunology. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever MA, Higano C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA Approved Therapeutic Cancer Vaccine. Clin Cancer Res. 2011;17:3520–6. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS. Overcoming immunological tolerance to melanoma: Targeting CTLA-4. Asia Pac J Clin Oncol. 2010;6(Suppl 1):S16–23. doi: 10.1111/j.1743-7563.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 6.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? The Journal of clinical investigation. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 8.Chikamatsu K, Nakano K, Storkus WJ, Appella E, Lotze MT, Whiteside TL, et al. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281–8. [PubMed] [Google Scholar]
- 9.Nikitina EY, Clark JI, Van Beynen J, Chada S, Virmani AK, Carbone DP, et al. Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001;7:127–35. [PubMed] [Google Scholar]
- 10.Espenschied J, Lamont J, Longmate J, Pendas S, Wang Z, Diamond DJ, et al. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. Journal of immunology. 2003;170:3401–7. doi: 10.4049/jimmunol.170.6.3401. [DOI] [PubMed] [Google Scholar]
- 11.Zwaveling S, Vierboom MP, Ferreira Mota SC, Hendriks JA, Ooms ME, Sutmuller RP, et al. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer research. 2002;62:6187–93. [PubMed] [Google Scholar]
- 12.Leffers N, Vermeij R, Hoogeboom BN, Schulze UR, Wolf R, Hamming IE, et al. Long-term clinical and immunological effects of p53-SLP(R) vaccine in patients with ovarian cancer. International journal of cancer Journal international du cancer. 2012;130:105–12. doi: 10.1002/ijc.25980. [DOI] [PubMed] [Google Scholar]
- 13.Svane IM, Pedersen AE, Johansen JS, Johnsen HE, Nielsen D, Kamby C, et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother. 2007;56:1485–99. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 15.Song GY, Gibson G, Haq W, Huang EC, Srivasta T, Hollstein M, et al. An MVA vaccine overcomes tolerance to human p53 in mice and humans. Cancer Immunol Immunother. 2007;56:1193–205. doi: 10.1007/s00262-006-0270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song GY, Srivastava T, Ishizaki H, Lacey SF, Diamond DJ, Ellenhorn JD. Recombinant modified vaccinia virus ankara (MVA) expressing wild-type human p53 induces specific antitumor CTL expansion. Cancer Invest. 2011;29:501–10. doi: 10.3109/07357907.2011.606248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. The journal of gene medicine. 2003;5:690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 18.Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–24. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. Journal of immunology. 2001;166:3908–14. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 20.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosma A, Buhler S, Nagaraj R, Staib C, Hammarin AL, Wahren B, et al. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clinical and diagnostic laboratory immunology. 2004;11:406–10. doi: 10.1128/CDLI.11.2.406-410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Martinez J, Zhou W, La Rosa C, Srivastava T, Dasgupta A, et al. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine. 2010;28:1547–57. doi: 10.1016/j.vaccine.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehler TC, Karg M, Distler E, Konur A, Nonn M, Meyer RG, et al. Rapid identification and sorting of viable virus-reactive CD4(+) and CD8(+) T cells based on antigen-triggered CD137 expression. Journal of immunological methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Litjens NH, de Wit EA, Baan CC, Betjes MG. Activation-induced CD137 is a fast assay for identification and multi-parameter flow cytometric analysis of alloreactive T cells. Clinical and experimental immunology. 2013;174:179–91. doi: 10.1111/cei.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing L, Schiffer JT, Chong TM, Bruckner JJ, Davies DH, Felgner PL, et al. CD4 T-cell memory responses to viral infections of humans show pronounced immunodominance independent of duration or viral persistence. Journal of virology. 2013;87:2617–27. doi: 10.1128/JVI.03047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunologic research. 2013;57:172–84. doi: 10.1007/s12026-013-8455-2. [DOI] [PubMed] [Google Scholar]
- 27.Meyer RG, Britten CM, Siepmann U, Petzold B, Sagban TA, Lehr HA, et al. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr) Cancer Immunol Immunother. 2005;54:453–67. doi: 10.1007/s00262-004-0616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrop R, Chu F, Gabrail N, Srinivas S, Blount D, Ferrari A. Vaccination of castration-resistant prostate cancer patients with TroVax (MVA-5T4) in combination with docetaxel: a randomized phase II trial. Cancer Immunol Immunother. 2013;62:1511–20. doi: 10.1007/s00262-013-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clinical and experimental immunology. 2013;171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vergati M, Cereda V, Madan RA, Gulley JL, Huen NY, Rogers CJ, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60:197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Met O, Balslev E, Flyger H, Svane IM. High immunogenic potential of p53 mRNA-transfected dendritic cells in patients with primary breast cancer. Breast Cancer Res Treat. 2011;125:395–406. doi: 10.1007/s10549-010-0844-9. [DOI] [PubMed] [Google Scholar]
- 35.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. European journal of immunology. 2003;33:1250–9. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 36.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. The Journal of experimental medicine. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishizaki H, Song GY, Srivastava T, Carroll KD, Shahabi V, Manuel ER, et al. Heterologous prime/boost immunization with p53-based vaccines combined with toll-like receptor stimulation enhances tumor regression. Journal of immunotherapy. 2010;33:609–17. doi: 10.1097/CJI.0b013e3181e032c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunology letters. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 39.Ye P, Weng ZH, Zhang SL, Zhao L, Dong JH, Pang R. [Significance of PD-1 up-regulation on CD8+ T lymphocytes from patients with chronic hepatitis B virus infection] Zhonghua Gan Zang Bing Za Zhi. 2008;16:706–7. [PubMed] [Google Scholar]
- 40.Kronig H, Julia Falchner K, Odendahl M, Brackertz B, Conrad H, Muck D, et al. PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. International journal of cancer Journal international du cancer. 2012;130:2327–36. doi: 10.1002/ijc.26272. [DOI] [PubMed] [Google Scholar]
- 41.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 42.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjomsland V, Spangeus A, Sandstrom P, Borch K, Messmer D, Larsson M. Semi mature blood dendritic cells exist in patients with ductal pancreatic adenocarcinoma owing to inflammatory factors released from the tumor. PLoS One. 2010;5:e13441. doi: 10.1371/journal.pone.0013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. Journal of immunotherapy. 2011;34:409–18. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer research. 2005;65:1089–96. [PubMed] [Google Scholar]
- 46.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current opinion in immunology. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. International immunology. 2007;19:1223–34. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 49.Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, et al. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69–74. doi: 10.1097/MPA.0b013e318197a9e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.