Abstract
Despite the unprecedented clinical activity of the Bruton’s tyrosine kinase inhibitor ibrutinib in MCL, acquired-resistance is common. By longitudinal integrative whole-exome and whole-transcriptome sequencing and targeted sequencing, we identified the first relapse-specific C481S mutation at the ibrutinib-binding site of BTK in MCL cells at progression following a durable response. This mutation enhanced BTK and AKT activation and tissue-specific proliferation of resistant MCL cells driven by CDK4 activation. It was absent, however, in patients with primary-resistance or progression following transient response to ibrutinib, suggesting alternative mechanisms of resistance. Through synergistic induction of PIK3IP1 and inhibition of PI3K-AKT activation, prolonged early G1 arrest induced by PD 0332991 (palbociclib) inhibition of CDK4 sensitized resistant lymphoma cells to ibrutinib killing when BTK was unmutated, and to PI3K inhibitors independent of C481S mutation. These data identify a genomic basis for acquired-ibrutinib resistance in MCL and suggest a strategy to override both primary- and acquired-ibrutinib resistance.
Keywords: Ibrutinib, PD 0332991, AKT
INTRODUCTION
Mantle cell lymphoma (MCL), a non-Hodgkin lymphoma of pre-germinal center mature B cells, remains incurable due to the development of drug resistance (1). Bruton’s Tyrosine Kinase (BTK) is a TEC family cytoplasmic tyrosine kinase required for the development, activation, and differentiation of B cells (2), as shown by the development of X-linked agammaglobulinemia when BTK is genetically inactivated (3). By mediating B-cell receptor (BCR) signaling, BTK is also indispensable for the survival of B cells and lymphoma cells (4). Targeting BTK with the irreversible, orally bioavailable inhibitor ibrutinib (PCI 32765) (5) achieved an unprecedented objective response rate of 68% in a phase II single-agent clinical trial in patients with relapsed/refractory MCL (6), and was similarly efficacious in chronic lymphocytic leukemia (CLL) (7). On this basis, ibrutinib was approved by the FDA in late 2013 for treatment of patients with recurrent MCL and in 2014 for patients with CLL (8).
However, disease progression while on ibrutinib treatment is frequent in MCL. It was reported as the principal reason for discontinuation of ibrutinib in 50/65 patients in the phase II trial, and has been associated with a highly proliferative state and poor clinical outcomes (6) (data not shown). Although the mechanism is unknown, the unrestrained proliferation of MCL cells at relapse suggests that targeting the cell cycle in combination therapy may delay the expansion of resistant clones or override some mechanisms of ibrutinib resistance. Since a hallmark of MCL is cell cycle dysregulation (9) due to aberrant Cyclin D1 expression from a t(11;14)(q13;q32) chromosomal translocation and activation of CDK4, targeting Cyclin D1 or CDK4 represents a rational approach to controlling the cell cycle in MCL. Supporting this possibility, targeting CDK4/CDK6 with a selective and potent oral inhibitor PD 0332991 (palbociclib) (10) in the first single-agent clinical trial in recurrent MCL patients effectively arrested MCL cells in early G1, resulting in a durable clinical response with an excellent toxicity profile (11).
CDK4 is overexpressed or activated at a high frequency in both hematologic malignancies and solid tumors, and PD 0332991 is now a Breakthrough Therapy for breast cancer owing to its ability to more than double the progression-free survival (PFS) of patients with metastatic breast cancer when combined with letrozole (12). The clinical efficacy of PD 0332991 may stem from induction of prolonged early G1 arrest (pG1) beyond the scheduled early G1 transit time by sustained CDK4/CDK6 inhibition (13), which reprograms cancer cells for killing by diverse clinically relevant agents (14). These include dexamethasone (13) and the proteasome inhibitor bortezomib in primary myeloma cells ex vivo and in animal models (14, 15), and PI3K inhibitors in primary MCL cells ex vivo (16).
Here we demonstrate by longitudinal functional genomics and targeted sequencing a relapse-specific C481S missense mutation at the ibrutinib-binding site of BTK in both patients who progressed on ibrutinib after a durable response, but not in patients (n=6) with a transient response or primary-resistance to ibrutinib. A further analysis of one patient revealed that the C481S BTK mutation is associated with heightened BTK and AKT activation, exacerbated genomic instability, and preferential CDK4-driven proliferation of resistant MCL cells in the spleen. Induction of pG1 by selective inhibition of CDK4 reprogrammed lymphoma cells for killing by ibrutinib when BTK is unmutated, and by selective PI3K inhibitors regardless of the C481S BTK mutation, suggesting a novel strategy to override ibrutinib resistance by targeting CDK4 in genome-based combination therapy.
RESULTS
Relapse-specific C481S BTK mutation in MCL
To elucidate the mechanism of acquired resistance to ibrutinib, we investigated the dynamic tumor evolution and discerned mutations that were expressed in MCL tumors by longitudinal integrative analysis of whole-exome sequencing (WES) and whole-transcriptome sequencing (WTS) of 5 serial biopsies of a representative male MCL patient (Pt 1). This patient achieved a partial response (PR, equal or greater than 50% reduction of tumor mass) on single agent ibrutinib therapy for 14 months before progression with mild lymphadenopathy and massive splenomegaly (see Methods).
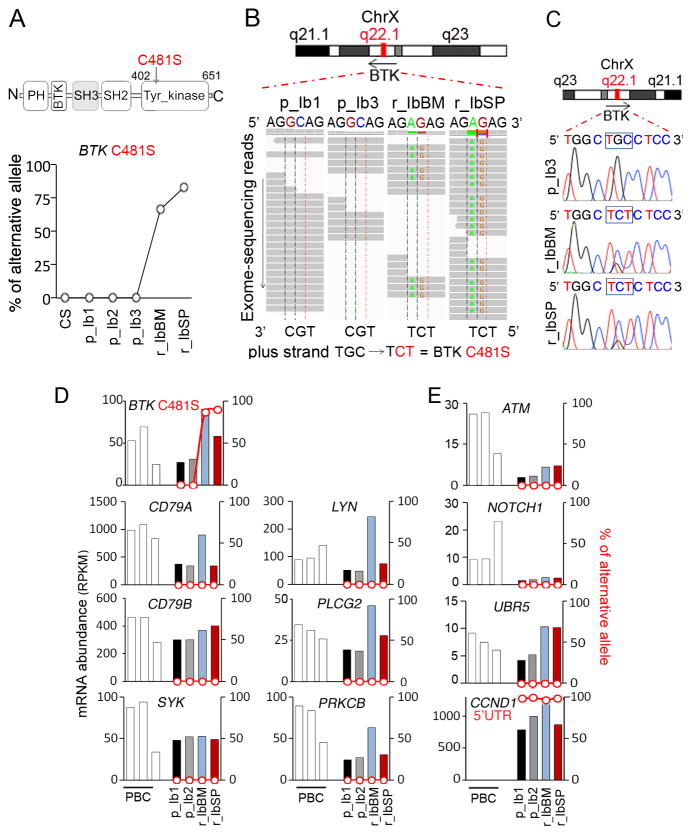
Single nucleotide variants (SNV) analysis of serial WES and Sanger sequencing detected a dinucleotide substitution of G1442C and C1443T in BTK in MCL cells at relapse in both the bone marrow (r_IbBM, 74% of the reads) and the spleen (r_IbSP, 83% of the reads). This resulted in a cysteine to serine missense mutation at residue 481 (C481S), localized in the tyrosine kinase domain of BTK (Fig. 1A–C). Importantly, the C481S mutation was not detected in any of the 3 lymph node biopsies taken 8 months (p_Ib1 and p_Ib2) or immediately (p_Ib3) before initiating ibrutinib, or in the cheek swab (CS) germline control (Fig. 1A).
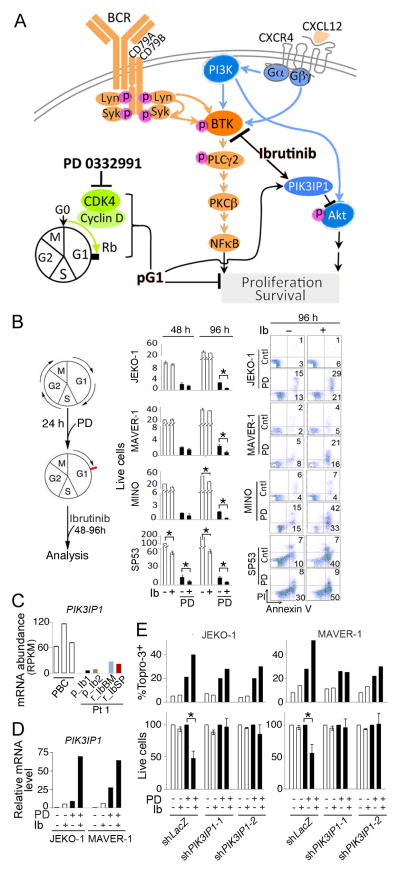
Figure 1. Identification of a relapse-specific C481S BTK mutation in MCL by longitudinal integrative WES and WTS.
A, schema for the C481S mutation in BTK and WES analysis of single nucleotide variations (SNVs; % of alternative allele) in the DNA of cheek swab (CS) and MCL cells from serial biopsies of Pt 1. The accession number for the protein sequence is NP_000052.1. B, integrative genomics viewer (IGV) visualization of nucleotide substitution and alignments of BTK on chromosome X (ChrX):100611161-100611166. C, identification by Sanger sequencing of G1442C and C1443T in BTK in bone marrow and spleen biopsies at relapse (r_IbBM, r_IbSP), and the wild type sequence in the bone marrow biopsy immediately before initiation of ibrutinib treatment (p_Ib3). D and E, WTS analysis of mRNA abundance (RPKM, reads per kilobase per million reads; bars) and non-synonymous SNVs (% of alternative allele, red line) in CDS and untranslated regions of genes commonly mutated in MCL. PBC: CD19+ B cells isolated from peripheral blood of healthy donors.
Longitudinal WTS analysis of serial biopsies corroborated the very high frequency (~80%) of C481S mutation in BTK exclusively at relapse in MCL cells in both the bone marrow (read depth = 129) and the spleen (read depth= 372) (Fig. 1D). The abundance of BTK mRNA was increased at relapse (2-fold) in bone marrow MCL cells along with elevation of mRNA expression from selective genes in the BCR signaling pathway (CD79A, LYN, PLCG2, PRKCB) (Fig. 1D). However, no SNV was identified in other genes of the BCR signaling (Fig. 1D) or genes frequently mutated in MCL such as ATM, NOTCH1, UBR5, except for SNVs in the 5′UTR of CCND1 (17, 18) (Fig. 1E). Nor has BTKC481S been detected in ibrutinib-naïve primary MCL cells by WTS or WES by us and others (16, 18–21) (data not shown). These data demonstrate the specificity of C481S BTK mutation at relapse from ibrutinib in MCL.
Further integrative WES and WTS analysis revealed 190 SNVs that were expressed in MCL cells but not present in the germline: 35 in the coding sequences (CDS) and 155 in the untranslated regions (UTR) (Fig. 2A and Supplementary Tables S1 and S2). Sixteen of the CDS SNVs were non-synonymous and predicted to be damaging at the protein level (Supplementary Table S3), of which 11 were constitutive and 5 increased in frequency with time in BRAP, RC3H1, C14orf159, BTK and TRAPPC10 (Fig. 2B–D). Only C481S in BTK and V600F in TRAPPC10 (22) were detected at a very high frequency exclusively at relapse in MCL cells in both the bone marrow and the spleen (Fig. 2B and Supplementary Table S3). The significance of the concurrent V600F TRAPPC10 mutation is unknown. But its unique association with BTKC481S in both bone marrow and splenic MCL cells at relapse implicates a clonal origin for ibrutinib resistant MCL cells.
Figure 2. Longitudinal integrative WES and WTS analysis of acquired-resistance following a durable response in MCL harboring BTKC481S.
A, SNVs (1,291,421) were identified in MCL cells from serial biopsies of Pt 1 before ibrutinib treatment (p_Ib1, p_Ib2) and after relapse from ibrutinib (r_IbBM, r_IbSP) by WTS using the Illumina platform and Genesifter (Geospiza®). After exclusion of SNVs detected in PBC libraries, 44% (n=6,060) of the 13,776 SNVs were also detected by WES analysis. A threshold of 10x coverage was applied, which reduced the number of SNVs to 2,679. After exclusion of the germline SNVs present in the cheek swab (CS), 190 SNVs were specific to the MCL cells of this patient: 155 detected in untranslated regions (UTRs) and 35 in the CDS, of which16 were predicted to be damaging at the protein level by SIFT, PROVEAN or PolyPhen-2. Among them 5 were detected at increasing frequency in serial biopsies but only C481S BTK and V600F TRAPPC10 mutations were detected at relapse exclusively (B and C), and 11 were present in all biopsies (D). The % of alternative allel (red) and mRNA abundance (RPKM) of the indicated genes were shown.
BTKC481S was identified in a second male MCL patient (Pt 2), who progressed on ibrutinib after achieving a PR that lasted for 30 months (see Methods). By targeted and Sanger sequencing of cells present in the pleural effusion, which comprised 69% CD19+/CD5+ MCL cells, G1442C was present at relapse in 31 % of the sequence reads but not before ibrutinib treatment or in the germline (Supplementary Table S4). The C481S mutation has been identified in CLL at the time of relapse following a durable response by WES as well (23). Collectively, these findings highlight the remarkable specificity of the C481S BTK mutation at ibrutinib resistance after a durable response in CLL and MCL.
Absence of C481S BTK mutation in transient ibrutinib response and primary resistance
BTKC481S was absent in serial peripheral blood or lymph node MCL cells of 6 patients with primary-resistance to ibrutinib or acquired-resistance following a transient (< 5 month) partial response, using cheek swab as controls for individual patients (Supplementary Table S5). No gain-of-function R665W mutation in the downstream PLCγ2 observed in ibrutinib relapse in CLL (23) was detected (Supplementary Table S5). Thus, mechanisms other than C481S BTK mutation or PLCγ2 activation must have contributed to the rapid resistance to ibrutinib in MCL patients who retain the wild type BTK.
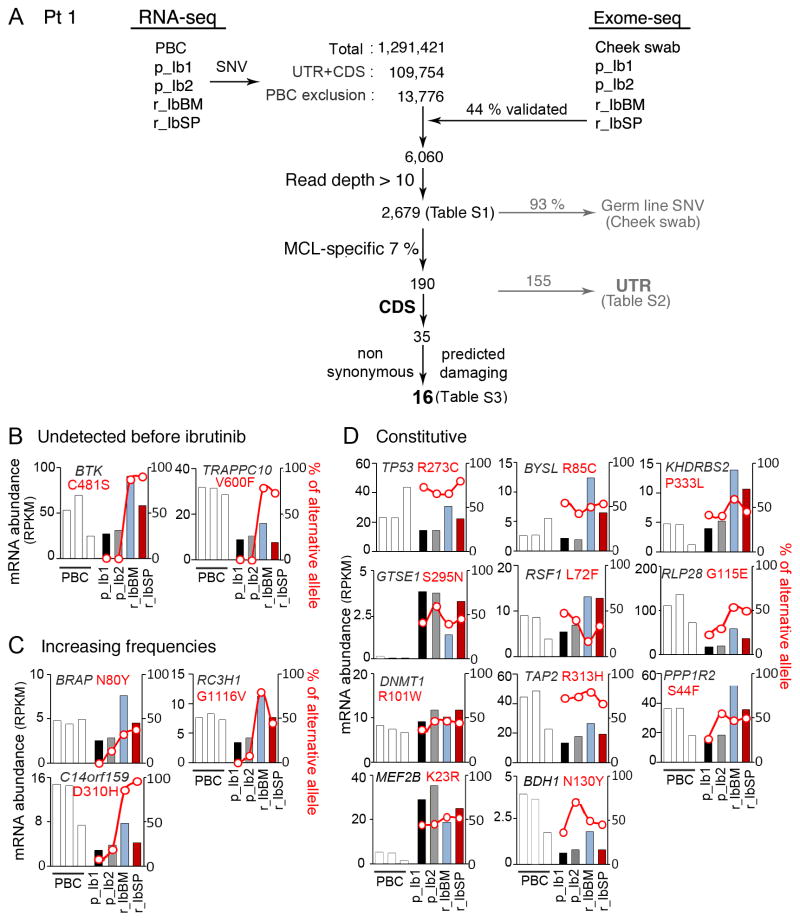
This led us to ask whether BTKWT was inhibited by ibrutinib in vivo in resistant MCL cells. BTKWT was autophosphorylated (pY223) in peripheral blood MCL cells before (p_Ib1) but not at 3 weeks of ibrutinib treatment (Ib1) in Pt 4, who was responding with a PR for over 10 months, or in Pt 8, who maintained a stable disease (SD, <50% reduction of tumor mass) for over 4 months (Fig. 3A). Concurrent with BTK inhibition was a prominent loss of ATK activation (pS473) by mTORC2 even taking into account the reduction in total AKT, while PLCγ2 activation (pY759) was diminished only modestly (Fig. 3A). Of interest, BTKWT was also inactivated in lymph node (Pt 6) and peripheral blood (Pt 9) MCL cells of primary ibrutinib resistant patients while on ibrutinib treatment, in which AKT was expressed at a high level and activated.
Figure 3. Concurrent inactivation of BTKWT and AKT by ibrutnib in responding patients and AKT activation independent of BTKWT inactivation in ibrutinib resistant patients.
A, B, immunoblotting of indicated proteins in MCL cells isolated from responding Pt 4 before (Pt 4 p_Ib1) and on 21 day of ibrutinib treatment (Pt 4_Ib1), Pt 8 on 21 day of ibrutinib treatment (Pt 8 Ib_1), primary resistant Pt 6 and Pt 9 on 21 day of ibrutinib treatment, Pt 5 and Pt 3 before ibrutinib treatment (Pt 5 p_Ib1, Pt 3 p_Ib1), on day 21 (Pt 5 Ib1, Pt 3 Ib1) of ibrutinib treatment and at relapse (Pt 3 r_Ib) after 3 months of ibrutinib response (See Supplementary Table S5 for details). N.S. denotes a non-specific signal. C, immunoblotting of MCL cells from serial lymph node biopsies of Pt 1 before ibrutinib treatment (p_Ib1, p_Ib2) and from the bone marrow at relapse from ibrutinib (r_IbBM). CD19+ B cells isolated from peripheral blood of healthy donors (PBCs) and JEKO-1 cells were used as controls. D, WTS analysis of mRNA abundance (RPKM) of indicated genes serial biopsies of Pt 1 and PBCs, as shown in Figure 1.
Primary MCL cells predominantly express PI3Kδ (PI3KCD), the hematologic lineage-restricted PI3K isoform (24), while PI3Kα (PI3KCA) is expressed at a lower level in some cases of MCLs. PI3Kβ (PIK3CB) and PI3Kγ (PIK3CG) were barely expressed, and the regulatory subunits PIK3R5 and PIK3R6 were undetectable (16). However, expression of PI3Kα was not associated with the ibrutinib response, since PI3Kα was expressed in MCL cells of Pt 4 (PR), Pt1 and Pt 3 (progression following durable PR) and all 3 primary resistant patients, but not in Pt 8 (SD) or Pt 5 (progression following transient PR) (Fig. 3A–B). Primary ibrutinib resistance or transient response therefore appears not to stem from defective ibrutinib inhibition of BTKWT in MCL cells but rather may involve sustained PI3K-AKT activation.
Activation of BTK and AKT at relapse in MCL cells harboring C481S BTK mutation
The functional consequence of C481S mutation in vivo was then characterized in MCL cells isolated from serial biopsies of Pt 1. Ibrutinib inhibits the BTK activity irreversibly by covalent binding to cysteine 481 in vitro and in cell lines (5), and the C481S mutation has been shown to markedly reduce (~25-fold) the affinity of BTK for ibrutinib in a model chicken lymphoma DT40 cell line (23). Notably, BTK was constitutively active in lymph node MCL cells before ibrutinib therapy and in peripheral blood B cells (PBCs) from healthy donors, presumably for their survival (Fig. 3C). Activation of BTK was increased in bone marrow MCL cells at relapse, concurrent with a modest increase in PI3Kα and PI3Kδ and activation of AKT (pS473) (Fig. 3C). The increase in PI3Kα and PI3Kδ proteins mirrored the selective increases in the abundance of PI3KCD and (PI3KCA) mRNA in MCL cells in the bone marrow and the spleen, suggesting that it stems in part from transcriptional activation of PI3Kα and PI3kδ (data not shown). Collectively these data suggest that the C481S mutation relieves BTK from ibrutinib inhibition, leading to heightened activation of BTK and AKT-pS473 in resistant bone marrow MCL cells in vivo.
Relapse from ibrutinib was also associated with a preferential increase in CXCR4, but not CXCR5, mRNA in bone marrow MCL cells, induction of CXCL12, but not CXCL13, mRNA in MCL cells in both the bone marrow and the spleen, and CCL3, CCL4, TNF and IL10 in the spleen (Fig. 3D). Since the expression of these chemokines and cytokines has been shown to promote proliferation and survival of MCL cells and inhibited by ibrutinib ex vivo (25), the tissue-specific upregulation at relapse provides additional functional evidence for ibrutinib resistance in vivo.
Tissue-specific cell cycle control of proliferation of BTKC481S MCL cells at relapse
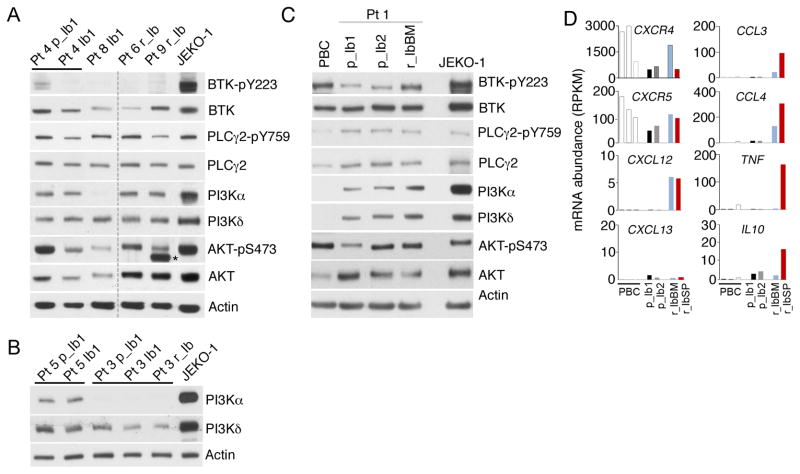
Unrestrained tumor cell proliferation is characteristic of ibrutinib relapse in MCL. Progression of Pt 1 was associated with massive splenomegaly and mild lymphadenopathy, suggesting a tissue-specific response. Longitudinal WTS analysis of cell cycle genes showed that as expected, most cell cycle genes were expressed at a higher level in MCL cells than in resting PBCs from normal donors (Fig. 4A). However, MKI67 (Ki67) mRNA was markedly repressed in the bone marrow at relapse, concurrent with selective elevation of CDK4 mRNA among genes scheduled for early G1 in both bone marrow and splenic MCL cells, and CCNA2, CCNB1 and CDK1 mRNAs (S and G2/M) in splenic MCL cells (Fig. 4A). These genes were expressed correspondingly at the protein level in bone marrow and splenic MCL cells, along with a reduction of p27 protein and increases in Cyclin A and Cyclin B in the splenic MCL cells (pre-therapy MCL cells were no longer available for protein analysis) (Fig. 4B). Surprisingly, the MYC mRNA level was lower in MCL cells of Pt 1 compared with PBCs, and the c-Myc protein was undetectable despite a striking (>200-fold) increase in MYC mRNA in splenic MCL cells (Fig. 4B). Collectively, these data indicate that preferential proliferation of MCL cells in the spleen at relapse in this patient is independent of c-Myc, in part driven by selective upregulation of CDK4 which cooperates with Cyclin A, Cyclin B and CDK1 to accelerate the cell cycle in MCL cells.
Figure 4. Tissue-specific cell cycle control of proliferation of BTKC481S MCL cells at relapse.
A, WTS analysis of mRNA abundance (RPKM) of indicated genes in in serial biopsies of Pt 1 before ibrutinib treatment (p_Ib1, p_Ib2) and at relapse (r_IbBM, r_IbSP) and PBCs. B, immunoblotting of indicated proteins in serial biopsies of Pt 1 at relapse from ibrutinib (r_IbBM, r_IbSP) and PBCs. JEKO-1 cells treated with PD 0332991 (PD, 0.3 μmol/L) for 72 hours or left untreated were used as a control. C, FISH was performed on a spleen section at relapse using a green-labeled LSI 13 (13q14) probe spanning the RB1 region, and an orange-labeled LSI TP53 probe as a control. D, copy number variation (CNV) analysis of chromosome 13 (Chr_13) of the WES data before ibrutinib treatment (p_Ib1) (upper panel) and at ibrutinib relapse (r_IbBM) samples (lower panel) of Pt. 1. CS: Cheek swab. E, representative images of IHC analysis of Ki67, pSRb and Rb in MCL cells (PAX5+) cells in the lymph node before ibrutinib treatment (p-Ib1) and at relapse in the bone marrow (r_IbBM) and spleen (r_ibSP) (left), and quantification (right).
Cytogenetic and FISH analyses further revealed a complex karyotype in proliferating splenic MCL cells at relapse. Multiple numerical and structural abnormalities were observed, most notably the hemizygous deletion of chromosome 13q that encompasses the RB1 gene (Fig. 4C). Copy number variation (CNV) analysis of serial WES of purified MCL cells independently identified the hemizygous 13q deletion at relapse in bone marrow MCL cells (Fig. 4D), and discovered two additional relapse-specific hemizygous deletions in 21q that included the TRAPPC10 gene (data not shown). However, Rb expression was unabated at the mRNA in MCL cells at relapse (Fig. 4A) presumably through a dosage compensation mechanism to ameliorate the haploinsufficiency. Relapse from ibrutinib in Pt 1 is thus associated with exacerbated genomic instability of MCL cells in the presence of sustained Rb expression.
Further evaluation of proliferation of MCL cells in situ in serial biopsies by immunohistochemistry (IHC) demonstrated that more MCL cells (Pax 5+) were cycling (Ki67+) in the spleen at relapse (r_IbSP) than in the lymph nodes before ibrutinib therapy (p_Ib1) (Fig. 4E). This was apparently driven by accelerated progression through early G1 given the >3-fold increase in CDK4/CDK6-specific phosphorylation of Rb (pSRb) (Fig. 4E). By contrast, few bone marrow MCL cells cycled or expressed pSRb at relapse despite the common C481S BTK mutation (Fig. 4E). These results confirm that in Pt 1, MCL cells harboring the C481S mutation preferentially proliferate in the spleen due to CDK4 activation but rarely in the bone marrow at relapse.
pG1 sensitizes resistant BTKWT MCL cells to ibrutinib via synergic induction of PIK3IP1
Previously, we have shown that induction of prolonged early G1 arrest (pG1) by sustained inhibition of CDK4 with a selective inhibitor PD 0332991 reprograms cancer cells for killing by diverse cytotoxic partners (14, 16). Given that BTKWT was inhibited by ibrutinib in highly proliferative primary resistant MCL cells in vivo (Fig. 3A), induction of pG1 may lower the threshold of ibrutinib killing in addition to inhibiting the cell cycle (Fig. 5A).
Figure 5. pG1 sensitizes resistant BTKWT MCL cells to ibrutinib via synergic induction of PIK3IP1.
A, schema for dual targeting of BTK with ibrutinib and CDK4 with PD 0332991 in MCL cells expressing BTKWT. pG1: prolonged early G1 arrest that exceeds the schedule early G1 transit time (16–20 hours in MCL cells) by selective and sustained inhibition of CDK4. B, schema for sequential incubation with PD 0332991 (0.3 μmol/L) and ibrutinib (left), Total viable cells (x 20,000 cells/mL) at 48 and 96 hours of ibrutinib treatment (1 μmol/L for JEKO-1, MAVER-1 and MINO and 0.1 μmol/L for SP53) (middle), FACS analysis of apoptotic (Annexin V+/PI+) MCL cells at 96 hours of ibrutinib treatment (right). C, WTS analysis of PIK3IP1 mRNA abundance in serial biopsies of Pt 1 and PBCs. D, q-RT-PCR analysis of relative PIK3IP1 mRNA levels in MCL cells cultured with PD for 72 hours and ibrutinib for 48 hours as indicated. E, live cells (percentage of untreated cells) and cell death (Topro-3+ cells) in MCL cell lines infected with PIK3IP1 shRNA or LacZ shRNA lentivirus and treated with PD and ibrutinib as in D. Error bars represent SD. *, P <0.05, calculated using Student t test. Data are representative of 4 independent experiments.
We tested this hypothesis in four Rb+ MCL cell lines harboring no mutations in BTK or PLCG2 (WES data not shown), by first confirming that PD 0332991 induced pG1, which maintained Cyclin D1 and CDK4 expression, elevated the p27 protein and prevented the expression Cyclin A, Cyclin B, CDK1 and c-Myc protein as expected for pG1 (Fig. 4B). Ibrutinib (0.01– 1 μmoles/L) was not toxic to JEKO-1, MAVER-1 or Mino MCL cells, although it moderately inhibited S phase entry in Mino cells and potently induced apoptosis in SP53 cells during G1-S transition by 48 hours, as shown by BrdU/PI analysis (Supplementary Fig. S1). pG1 did not augment ibrutinib killing of SP53 cells, but profoundly inhibited the expansion of live cells through cell cycle control (Fig. 5B). Importantly, induction of pG1 both prevented the ibrutinib resistant JEKO-1, MAVER-1 and MINO cells from replicating and sensitized them to ibrutinib-induced apoptosis (AnnexinV+/PI+), resulting in a 50–100 fold reduction of live cells by 96 hours of ibrutinib treatment (Fig. 5B).
We have recently uncovered that synergistic induction of PIK3IP1, a negative regulator of PI3K (26), mediates pG1 reprogramming MCL cells for durable and enhanced killing by selective PI3K inhibition (16). PIK3IP1 mRNA expression in MCL cells from all serial biopsies of Pt 1 was 10–30-fold lower than in PBCs (Fig. 5C), consistent with previous observations in primary MCL cells (16). It was induced by pG1 and by ibrutinib modestly, but by ibrutinib and pG1 in synergy (~50 fold) in JEKO-1 and MAVER-1 cells (Fig. 5D). Knocking down PIK3IP1 by two independent shRNA-lentivirus constructs blunted the enhancement of ibrutinib killing in pG1, but not the death induced by pG1 alone in JEKO-1 or MAVER-1 cells (Fig. 5E). Thus, induction of pG1 by selective CDK4 inhibition not only prevents refractory MCL cells that are expressing the wild type BTK from proliferating, but also reprograms them for ibrutinib killing through synergistic induction of PIK3IP1.
pG1 and ibrutinib cooperatively inactivate BTK, AKT and NF-κB in BCR signaling
BTK and AKT were coordinately inactivated on ibrutinib treatment in MCL cells of a responding patient (Pt 4). However, AKT activation (pS473) was maintained despite inactivation of the BTKWT in MCL cells of primary resistant patients (Pt 6 and Pt 9) (Fig. 3A). Since pG1 inactivates AKT in primary MCL cells ex vivo (16), it may sensitize BTKWT resistant MCL cells to ibrutinib through inactivation of AKT. Indeed, activation of BTK (pY223) and AKT (pS473) in BCR signaling stimulated by anti-IgM was partially neutralized by ibrutinib and in pG1, but completely abolished by ibrutinib in pG1 reprogrammed JEKO-1 cells (Fig. 6A). This finding was reminiscent of synergistic and durable inhibition of AKT activation by a PI3K inhibitor in pG1, which led to enhanced killing of primary MCL cells and MCL cell lines by PI3K inhibitors (16), suggesting that inactivation of PI3K-AKT mediates pG1 reprogramming of MCL cells for killing by both ibrutinib and PI3K inhibitors.
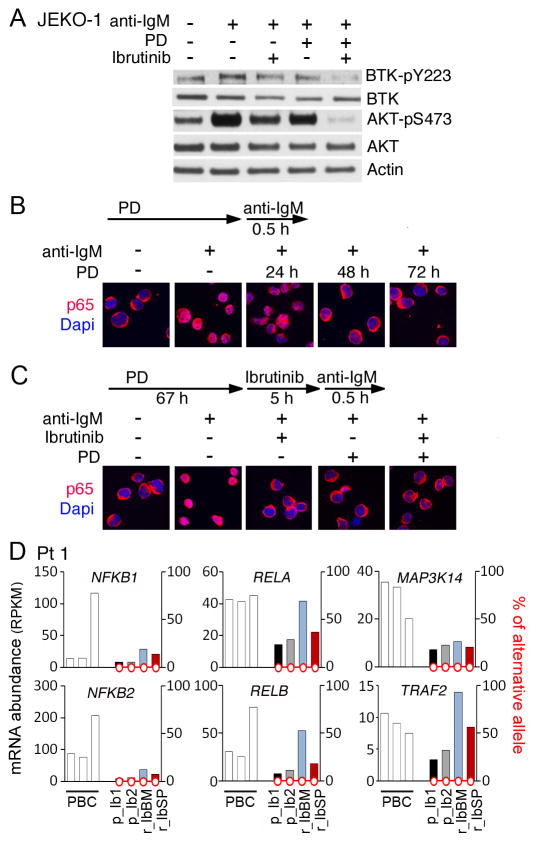
Figure 6. pG1 and ibrutinib cooperatively inhibit BTK, AKT and NF-κB activation in BCR signaling.
A, immunoblotting of activated BTK (pY223) and total BTK, and activated AKT (pS473) and total AKT proteins in JEKO-1 cells cultured in the presence or absence of PD 0332991 (0.3 μmol/L) for 24 hours before addition of ibrutinib (1 μmol/L) and goat anti-human IgM (α-IgM, 5 μg/mL) for 24 hours. B and C, immunofluorescence staining of p65 subcellular localization in JEKO-1 cells cultured with PD 0332991 (PD) for time indicated and α-IgM (5 μg/mL) for 0.5 hour in B or cultured with PD 0332991 for 67 hours before addition of Ibrutinib for 5 hours and anti-IgM for 0.5 hour. Data are representative of 3 independent experiments. D, WTS analysis of mRNA abundance and non-synonymous SNVs in the CDS of genes indicated in serial biopsies of Pt 1 before ibrutinib treatment (p_Ib1, p_Ib2) and at relapse from ibrutinib (r_IbBM, r_IbSP).
Primary resistance to ibrutinib despite inactivation of BTKWT suggests that activation of distal BCR signaling may contribute to ibrutinib resistance. The classical (p50/RELA) NF-κB pathway mediates BCR signaling for B cell proliferation and survival (4), further implicating that pG1 may reprogram MCL cells by attenuating NF-κB activation. To address this possibility, we showed by immunofluorescence staining (IMF) that nuclear localization of RELA (p65) indicative of NF-κB activation in response to anti-IgM was undetectable in some JEKO-1 cells by 24 hours of PD 0332991 treatment and completely absent by 48 hours (Fig. 6B). Thus, in a time-dependent manner pG1 reconstitutes the rapid (within 5 hours) inactivation of the classical NF-κB pathway by ibrutinib in BCR signaling (Fig. 6B–C). The alternative (p52/RELB) NF-κB pathway has been shown recently to contribute to ibrutinib resistance in MCL cell lines (20). p52 was expressed in the ibrutinib-resistant JEKO-1 cells used in this study but not appreciably inhibited by ibrutinib in pG1, along with invariable expression of NIK (MAP3K14) required for the processing of p100 to p52 (Supplementary Fig. S2).
Longitudinal WES/WTS analysis of serial biopsies of Pt 1 further revealed an association of ibrutinib relapse with upregulation of selective genes of the classical NF-κB pathway such as NFKB1 and RELA as well as NFKB2 and RELB of the alternative NF-κB pathway in bone marrow MCL cells, although MAP3K14 expression did not vary (Fig. 6D). Taken together, these data suggest tissue-specific up-regulation of genes in the NF-κB pathways at relapse in a patient with C481S BTK mutation, and that pG1 reprograms MCL cells for cytotoxic killing in part by selective inactivation of the classical, but not the alternative NF-κB pathway.
pG1 sensitizes resistant lymphoma cells independent of C481S BTK mutation to PI3K inhibitors
PI3K is required for B cell development and proliferation (27), and acts upstream of BTK for BCR-dependent survival in mature B cells (28). Expression of PI3K and AKT was sustained in all MCL patients, whereas activation of ATK (pS473) was reduced in an ibrutinib-responding patient, sustained in BTKWT MCL cells of primary resistant patients and enhanced in BTKC481S MCL cells in a patient at relapse from a durable ibrutinib response (Fig. 3). Since pG1 reprograms primary MCL cells for PI3K inhibitor killing ex vivo through durable inhibition of AKT activation (16), it may sensitize ibrutinib resistant lymphoma cells to PI3K inhibitors independent of the C481S BTK mutation (Fig. 7A).
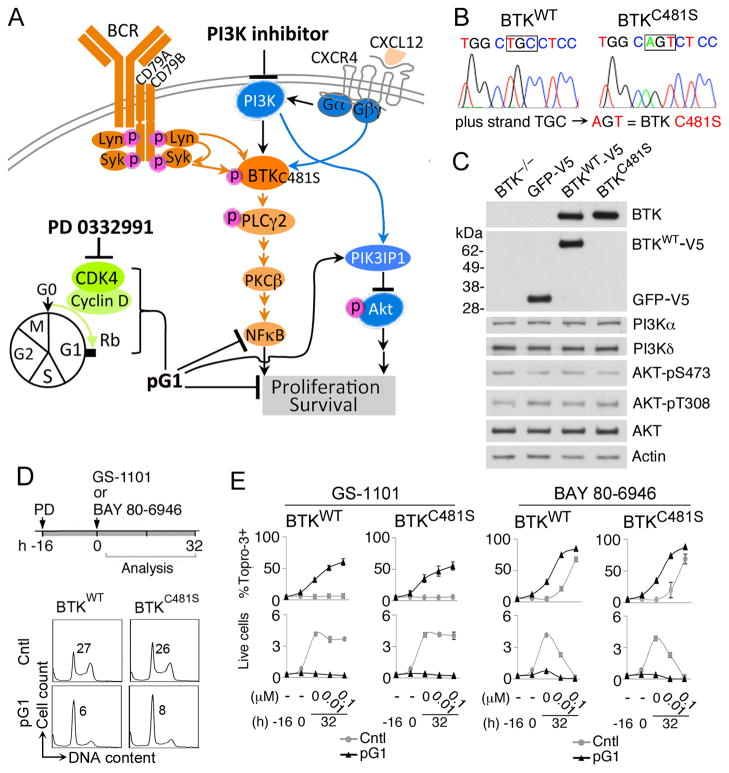
Figure 7. pG1 sensitizes resistant lymphoma cells to inhibition of PI3K independent of C481S BTK mutation.
A, schema for overriding the BTKC481S mutation by dual inhibition of CDK4 and PI3K. B, confirmation of the BTK sequence (WT or C481S) reconstituted in DT40 BTK−/− cells by Sanger sequencing. C, immunoblotting of indicated proteins in DT40 BTK−/− cells stably infected with a lentivirus expressing human BTKWT-V5, BTKC481S-6xHis or the GFP-V5 control vector as described in the Supplementary Methods. D, Cell cycle analysis by propidium iodide staining of DT40_BTKWT and DT40_BTKC481S cells cultured with PD 0332991 (PD) (0.3 μmol/L) for 16 hours. Number in the FACS profile indicates the percentage of cells in S phase as analyzed by FlowJo. E, DT40_ BTKWT and DT40_ BTKC481S cells were cultured in the absence (Cntl) or presence of PD 0332991 (pG1) as in D before addition of GS-1101 or BAY 80-6946 for 32 hours at concentrations indicated in the continuous presence of PD 0332991. Cell death was determined by ToPro-3 staining and FACS analysis. The total number of live cells was determined by Trypan Blue staining (x106 cells/ml). Error bars represent SD. Data are representative of 3 independent experiments.
To test this hypothesis, we reconstituted the expression of a V5-tagged BTKWT and BTKC481S in equal molar in the DT40 BTK−/− chicken lymphoma cell line, in which C481S BTK mutation has been shown to attenuate ibrutinib inhibition of BTK (23) (Fig. 7B–C). The expression of p110δ p110α and AKT, and AKT activation in DT40 BTK−/− cells were not altered by stable expression of BTKWT, BTKC481S or the GFP-V5 vector (Fig. 7C). Sustained inhibition of CDK4 by PD 0332991 led to pG1 in DT40 cells expressing either BTKWT or BTKC481S (Fig. 7D), which were then reprogramed for killing by three clinically-relevant selective PI3K inhibitors independent of C481S BTK mutation. pG1 conferred sensitivity to the PI3Kδ-specific inhibitor GS-1101 (idelalisib) (29–31), which alone did not kill DT40 cells expressing BTKWT or BTKC481S presumably due to functional compensation by the PI3Kα expressed in these cells Thus, it appears that when combined with inhibition of PI3Kδ and ATK activation, pG1 bypasses PI3Kα expression. pG1 also profoundly enhanced the killing by the dual PI3Kα/δ inhibitors BAY 80-6946 (copanlisib) (32) and GDC-0941 (pictilisib) (33) (Fig. 7E) (Supplementary Fig. S3). Sensitization by pG1 led to virtual eradication of live cells by all PI3K inhibitors characterized, even when BTKC481S was expressed at a 5-fold excess of BTKWT (Fig. 7E and Supplementary Fig. S3). Induction of pG1 by sustained CDK4 inhibition thus profoundly reprograms ibrutinib resistant lymphoma cells for PI3K inhibitor killing independent of C481S BTK mutation.
DISCUSSION
Acquired-resistance to therapy remains a formidable challenge in human cancer. It is particularly urgent in MCL, which remains incurable due to the development of drug resistance even to the novel BTK inhibitor ibrutinib, despite its exceptional single-agent activity. By longitudinal integrative WES and WTS analysis of MCL cells from serial biopsies of a MCL patient who progressed following a durable partial response, we have now identified the first relapse-specific C481S missense mutation at the ibrutinib binding site of BTK in MCL. The heightened activation of BTK and AKT, induction of the CXCR4-CXCL12 autocrine loop and de-repression of growth promoting CCL3 and CCL4, TNFα and IL-10, in BTKC481S MCL cells at relapse (Fig. 1 and 3) provided the first functional evidence in vivo for impaired BTK inhibition caused by a BTK mutation in acquired ibrutinib resistance.
The acquired-resistance to ibrutinib could be treatment-related or due to clonal evolution, which are not mutually exclusive. Although no mutations in BTK have been identified by WES or WTS in ibrutinib-naive MCL patients so far, the existence of a C481S BTK mutant clone at a very low frequency before treatment can’t be excluded, as demonstrated by <0.01% of cells becoming the predominant clone in relapsed leukemia (34). In addition, the C481S BTK mutation is likely to confer a selective advantage over MCL cells expressing BTKWT and responding to ibrutinib-mediated killing. Consistent with a clonal origin, ibrutinib resistant MCL cells at relapse in bone marrow and spleen of Pt 1 have in common: 1) identical dinucleotide substitution in BTK; 2) unique concurrent high frequency V600F TRAPPC10 mutation; 3) exacerbated genomic instability in the absence of mutations in ATM, the most frequently mutated gene in MCL (35) and hemizygous 13q deletion (Fig. 4).
This raises the question of whether other non-synonymous potentially damaging mutations, identified by WES and WTS analysis, contribute to the development of acquired ibrutinib resistance in MCL. Of particular interest are the increasing N80Y in BRAP, a BRCA-2-binding protein; the constitutive R273C in TP53 and S295N in GTSE1, a p53-binding protein and G2/M checkpoint; and K23R in MEF2B, a transcriptional activator frequently mutated in MCL (19) and in diffuse large B cell lymphoma, which led to deregulation of the target oncogene BCL-6 (36) (Fig. 2).
Detection of BTKC481S in a second MCL patient who progressed following an even more durable PR (30 months), but not in any of the 6 MCL patients who progressed on ibrutinib within 5 months or without a response suggest that the C481S mutation in MCL is specific to acquired ibrutinib resistance after a durable response. While this possibility is being addressed in a larger MCL patient population, alternative mechanisms must have contributed to primary or rapid resistance to ibrutinib.
The C481S BTK mutation has been identified by WES in 5 of 13 CLL patients relapsed from ibrutinib (23). Apart from this common genomic aberration, ibrutinib resistance in MCL is distinguishable from that in CLL in other aspects. First, disease progression on ibrutinib is much more frequent in MCL (50/115 with a median follow up of 15.3 months) (6) than in the more indolent CLL (11/85 with a median follow up of 20.9 months) (7), suggesting that more proliferative lymphomas may be less well controlled by ibrutinib. Second, the gain-of-function R665W mutation in PLCγ2 identified in CLL (23) has not been detected in MCL (Supplementary Table S5). Third, BTK was inactivated by ibrutinib in BTKWT MCL cells of primary resistant patients (Fig. 3), suggesting that dysregulation of distal BCR signaling other than activating PLCγ2 mutation contributes to rapid ibrutinib resistance. Fourth, the enhanced BTK and Pi3K-AKT activation at relapse in BTKC481S MCL cells provided the first evidence for divergent functional consequences caused by C481S BTK mutation in vivo and reinforces the critical importance of developing genome-based combination therapy in MCL.
Relapse from ibrutinib is often characterized by aggressive proliferation of resistant MCL cells and poor clinical outcomes. Here we show that it was also associated with sustained AKT activation (pS473) in primary resistant BTKWT MCL cells and enhanced AKT activation (pS473) in BTKC481S MCL cells at relapse (Fig. 3). These findings demonstrate a pivotal role of PI3K-AKT activation in ibrutinib resistance and provide a strong rationale to override ibrutinib resistance by targeting the cell cycle in combination with genome-based inhibition of BTK or PI3K. Indeed, induction of pG1 by PD 0332991 inhibition of CDK4 reprograms MCL cells expressing BTKWT for killing by ibrutinib, which together with inhibition of cell proliferation, resulted in a dramatic reduction of live cells (Fig. 5). Furthermore, pG1 sensitizes ibrutinib resistant MCL cell lines (16) and DT40 lymphoma cells independent of C481S BTK mutation to killing by clinically relevant PI3K inhibitors (Fig. 7), which has important mechanistic and clinical implications.
Previously, we have shown that induction of pG1 by selective CDK4/CDK6 inhibition leads to restricted expression of genes scheduled for early G1 only, thereby forcing an imbalance in gene expression that reprograms cancer cells for killing by diverse cytotoxic partners (14). PIK3IP1 was synergistically induced by a PI3K inhibitor (GS-1101 or GDC-0941) and pG1 in MCL cells for enhanced killing through sustained inhibition of pAKT (16). Here we demonstrated that PIK3IP1 was also synergistically induced by ibrutinib and pG1 in MCL cells to mediate pG1 enhancement of ibrutinib killing, most likely by abrogating the activation of BTK and AKT in BCR signaling (Fig. 5–6). PIK3IP1 thus plays a pivotal role in pG1 sensitization of MCL cells to killing by either PI3K or BTK inhibitor. As such, it represents a novel molecular therapeutic biomarker for pG1 combination therapy.
In addition, we have now discovered that pG1 selectively antagonizes the activation of the classical NF-κB pathway in BCR signaling, but not the alternative NF-κB pathway (Fig. 6). Inactivation of NF-κB in pG1 is redundant with inhibition of BTK by ibrutinib, but it may cooperate with inactivation of PI3K-AKT in pG1 reprogrammed MCL cells to enhance PI3K inhibitor killing independent of BTK C481S mutation. By inactivating NF-κB, pG1 antagonizes BCR signaling downstream of PKCβ, and could also bypass various gain-of-function upstream mutations in the BCR signaling pathway (Fig. 5A). Given the importance of NF-κB in transcriptional regulation, inactivation of NF-κB may in fact mediate the imbalance in gene expression in pG1, thereby playing a broader role in pG1 reprogramming.
Targeting CDK4 with PD 0332991 in genome-based combination with PI3K or BTK inhibition is poised for clinical validation in MCL as well. PD 0332991 had already achieved a durable clinical response with a favorable toxicity profile in the first single agent clinical trial in recurrent MCL patients (11). It is now a Breakthrough Therapy for metastatic breast cancer owing to its exceptional clinical activity when combined with letrozole. Although pG1 reprogramming by PD 0332991 requires Rb, the substrate of CDK4 and CDK6 (14, 16), it is independent of ATM and TP53, the most frequently mutated genes in MCL (17) and many other human cancers.
As for targeting PI3K, the novel PI3Kδ-specific inhibitor GS-1101 is a Breakthrough Therapy for CLL, and exhibited transient clinical activity in MCL. We showed that pG1 reprogrammed primary MCL cells for sustained and enhanced inhibition by GS-1101 via inhibition of AKT activation, and by the dual PI3Kα/δ inhibitor GDC-0941 (16) and BAY 80-6946 (Fig. 7). CDK4 and PI3K are dysregulated at a high frequency in human cancers that develop resistance to therapy (37). To overcome drug resistance by targeting the cell cycle, one of the critical next steps is to define the mechanism by which pG1 inactivates PI3K-AKT. Given that the carboxyl-terminus of AKT is directly phosphorylated by CDK2/Cyclin A (38) and that induction of pG1 suppresses Cyclin A synthesis (14) it seems likely that pG1 may inactivate AKT via Cyclin A suppression (Fig. 4). This exciting possibility is amenable to investigation in targeting CDK4 in clinical studies by longitudinal integrative WTS.
Our unbiased, longitudinal integrative WES and WTS analysis of individual patients represents the first such undertaking in targeted lymphoma therapy. It has provided new insight into the genomic basis and mechanism for acquired resistance in MCL. In conjunction with functional genomics, the availability of exciting new targeting agents offers a unique opportunity to overcome acquired resistance by selective targeting of CDK4 in genome-based combination therapy in lymphoma, which has implications for other human cancers as well.
METHODS
(Additional methods are detailed in the supplemental data).
Patients and isolation of primary MCL cells
Tissue biopsies from lymph node, bone marrow and spleen and peripheral blood samples (Supplementary Table S5) were obtained from 9 mantle cell lymphoma (MCL) patients at the New York-Presbyterian Hospital. Patient 2 (Pt 2) samples were obtained at the Willamette Valley Cancer Institute and Research Center. All samples were collected after informed consent as part of a study approved by the local Institutional Review Boards. All patients were treated with ibrutinib after prior therapies. Pt 1 had received four therapies before starting single-agent ibrutinib and achieved a partial response that lasted 14 months before disease progression marked by a rapidly enlarging spleen and spontaneous splenic hematoma requiring urgent surgery. Pt 2 achieved a partial response lasting 30 months before progression marked by progressive adenopathy and a pleural effusion. Time to progression and best response of other patients were indicated in Supplementary Table S5. Primary MCL cells were purified using MACS CD19 MicroBeads (Miltenyi Biotec) at 4°C, and the percentage of MCL tumor cells (CD19+/CD5+) was determined to be > 90% by flow cytometry. Peripheral blood B cells (PBC)s were isolated from healthy volunteers using the same protocol.
MCL Cell lines
JEKO-1 cells were obtained from DSMZ, and MAVER-1 and MINO from ATCC. SP53 cells were kindly provided by Dr. Jiangao Tao (Moffit Cancer Center, Tampa FL) and the chicken DT40 lymphoid cells deficient in Btk (BTK−/−) (39) were described previously (3). No authentication of these cell lines was done by the authors. Cell lines were cultured in the presence of PD 0332991, GS-1101, GDC-0941 and BAY 80-6946 (all from Sellek Chemical) or ibrutinib (Pharmacyclics) at concentrations and for times indicated.
Whole transcriptome sequencing (WTS)
For each experimental condition, 100 ng of high quality total RNA (RIN > 8 on the Agilent BioAnalyzer 2100) was isolated using the RNeasy kit according to the manufacturer’s instructions (QIAGEN). All RNAs were converted to cDNA with the SuperScript® III First-Strand Synthesis kit (Life Technologies), isolated with the TruSeq mRNA prep kit (v2) (Illumina), and then ligated to Illumina adapters, as per the standard TruSeq Illumina protocol. Using these multi-plexed cDNA libraries, we generated clusters on the Illumina cBot station and paired-end sequenced each sample to 50×50 bp on the Illumina HiSeq2000 at the Weill Cornell Medical College (WCMC) Genomics Core. Cluster generation, sequencing, and processing of the images were done using the Real-Time Analysis (RTA) software on the HiSeq2000 and postprocessing with CASAVA (v.1.8.2). To optimize library preparation we used a TACON high-throughput RNA prep-station. Raw data were filtered for high median quality (Q-value > 20) and then sent to Cornell’s High Performance Computing (HPC) cluster, to be run through our RNA-Seq analysis pipeline. The RNA-seq data presented in this study, which include libraries from serial biopsies of MCL tumors before ibrutinib treatment (p_Ib1, p_Ib2, p_Ib3) and after ibrutinib relapse (r_IbBM, r_IbSP) and PBCs from 3 healthy volunteers have been deposited in the Gene Expression Omnibus (GEO).
Whole exome sequencing (WES)
DNA were isolated from purified MCL cells of 5 serial biopsies before ibrutinib treatment (p_Ib1, p_Ib2, p_Ib3) and after ibrutinib relapse (r_IbBM, r_IbSP) and a cheek swab (CS) of Pt1, captured with Nextera Rapid Exome Capture Kit (62 MB) and libraries were created according to the manufacturer’s protocol. Briefly, purified DNA was fragmented and a sequencing library was created with the Illumina TruSeq (v3) DNA Preparation kits (FC-121-1031). Following isothermal cluster generation (PE-401-3001) and 75×75 paired-end (PE) sequencing on the HiSeq2500 (FC-401-3001), the samples underwent primary analysis with the Illumina base calling and primary analysis software (HCS 1.4, CASAVA 1.8.2, and RTA 1.2).
Cell cycle analysis and cell viability assays were performed as previously described (16).
Supplementary Material
SIGNIFICANCE.
We have discovered the first relapse-specific BTK mutation in MCL patients with acquired-resistance but not primary-resistance to ibrutinib, and demonstrated a rationale for targeting the proliferative resistant MCL cells by inhibiting CDK4 and the cell cycle in combination with ibrutinib in the presence of BTKWT or a PI3K inhibitor independent of BTK mutation. As drug resistance remains a major challenge and CDK4 and PI3K are dysregulated at a high frequency in human cancers, targeting CDK4 in genome-based combination therapy represents a novel approach to lymphoma and cancer therapy.
Acknowledgments
Financial Support
This study was supported in part by a Cancer Research and Treatment Fund postdoctoral fellowship (D.C), grants from The Lymphoma Research Foundation (S. C-K, P. M. and J.P. L.), The Lymphoma Foundation (J.P.L.), The Leukemia and Lymphoma Society (S. C-K), and R21 CA176362 from National Cancer Institute (S. C-K and C.E.M).
We thank Drs. Jihye Paik, John Byrd and Lewis Cantley for helpful discussions, Tricia Ellis for technical support, Dr. Jenny Zhang and the Weill-Cornell Genomics Facility for RNA and DNA sequencing and Pharmacyclics for providing ibrutinib.
Footnotes
Potential Conflicts of Interest
P. Martin has received honorarium from Janssen Pharmaceutica. J. Sharman has received research funding from Pharmacyclics. J. P. Leonard is a consultant for Pharmacyclics. S. Ali is an employee of Foundation Medicine. B. Chang is an employee of and holds stock options at Pharmacyclics. All other authors have no conflicts of interest.
Authors’ Contributions
Conception and design: D. Chiron, M. Di Liberto, P. Martin, J. Leonard. S. Chen-Kiang
Development of methodology: D. Chiron, M. Di Liberto, P. Martin, X. Huang, P. Blecua, J. Sharman. O. Elemento, C. E. Mason, J. Leonard. S. Chen-Kiang,
Acquisition of data: D. Chiron, M. Di Liberto, P. Martin, X, Huang, P. Blecua, J. Sharman, S. Mathew, P. Vijay, K. Eng, S. Ali. S. Ely, O. Elemento, C. E. Mason, J. P. Leonard, S. Chen-Kiang
Analysis and interpretation of data: D. Chiron, M. Di Liberto, P. Martin, P. Blecua, J. Sharman. S. Mathew, P. Vijay, X. Huang, S. Ely, O. Elemento, C. E. Mason, J. P. Leonard, S. Chen-Kiang
Writing, review and/or revision of manuscript:
D. Chiron, M. Di Liberto, P. Martin, X, Huang, J. Sharman, A. Johnson, O. Elemento, C. E. Mason, J. P. Leonard, S. Chen-Kiang
Administrative, technical or material support: D. Chiron, M. Di Liberto, P. Martin, P. Blecua, S Mathew, P. Vijay, X. Huang, K. Eng, B. Chang. O. Elemento, C. E. Mason, S. Chen-Kiang
Study supervision: D. Chiron, M. Di Liberto. P. Martin and S. Chen-Kiang
References
- 1.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, et al. Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8. [PubMed] [Google Scholar]
- 4.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–43. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrutinib approved for mantle cell lymphoma. Cancer discovery. 2014;4:OF1. doi: 10.1158/2159-8290.CD-NB2013-172. [DOI] [PubMed] [Google Scholar]
- 9.Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, Leo Hansmann M, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111:2385–7. doi: 10.1182/blood-2007-10-117010. [DOI] [PubMed] [Google Scholar]
- 10.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- 11.Leonard JP, Lacasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 12.Palbociclib Ups PFS in HER2−/ER+ Breast Cancer. Cancer discovery. 4:624–5. doi: 10.1158/2159-8290.CD-NB2014-053. [DOI] [PubMed] [Google Scholar]
- 13.Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66:7661–7. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Di Liberto M, Jayabalan D, Liang J, Ely S, Bretz J, et al. Prolonged early G(1) arrest by selective CDK4/CDK6 inhibition sensitizes myeloma cells to cytotoxic killing through cell cycle-coupled loss of IRF4. Blood. 2012;120:1095–106. doi: 10.1182/blood-2012-03-415984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menu E, Garcia J, Huang X, Di Liberto M, Toogood PL, Chen I, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 2008;68:5519–23. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- 16.Chiron D, Martin P, Di Liberto M, Huang X, Ely S, Lannutti BJ, et al. Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition reprograms lymphoma cells for durable PI3Kdelta inhibition through PIK3IP1. Cell Cycle. 2013;12:1892–900. doi: 10.4161/cc.24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiner TC, Dasgupta C, Ho VV, Weisenburger DD, Smith LM, Lynch JC, et al. Mutation and genomic deletion status of ataxia telangiectasia mutated (ATM) and p53 confer specific gene expression profiles in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2006;103:2352–7. doi: 10.1073/pnas.0510441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meissner B, Kridel R, Lim RS, Rogic S, Tse K, Scott DW, et al. The E3 ubiquitin ligase UBR5 is recurrently mutated in mantle cell lymphoma. Blood. 2013;121:3161–4. doi: 10.1182/blood-2013-01-478834. [DOI] [PubMed] [Google Scholar]
- 19.Bea S, Valdes-Mas R, Navarro A, Salaverria I, Martin-Garcia D, Jares P, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1314608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chun Chan F, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med. 2013 doi: 10.1038/nm.3435. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Jima D, Moffitt AB, Liu Q, Czader M, Hsi ED, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood. 2014;123:2988–96. doi: 10.1182/blood-2013-07-517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, et al. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–15. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N Engl J Med. 2014 doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci U S A. 1997;94:4330–5. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang BY, Francesco M, De Rooij MF, Magadala P, Steggerda SM, Huang MM, et al. Egress of CD19+CD5+ cells into peripheral blood following treatment with the BTK inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122:2412–24. doi: 10.1182/blood-2013-02-482125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, He X, Johnson C, Stoops J, Eaker AE, Stoffer DS, et al. PI3K is negatively regulated by PIK3IP1, a novel p110 interacting protein. Biochem Biophys Res Commun. 2007;358:66–72. doi: 10.1016/j.bbrc.2007.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85alpha. Science. 1999;283:393–7. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–86. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12:2319–30. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 33.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 34.Meyer JA, Wang J, Hogan LE, Yang JJ, Dandekar S, Patel JP, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet. 2013;45:290–4. doi: 10.1038/ng.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bea S, Salaverria I, Armengol L, Pinyol M, Fernandez V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009;113:3059–69. doi: 10.1182/blood-2008-07-170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying CY, Dominguez-Sola D, Fabi M, Lorenz IC, Hussein S, Bansal M, et al. MEF2B mutations lead to deregulated expression of the oncogene BCL6 in diffuse large B cell lymphoma. Nat Immunol. 2013;14:1084–92. doi: 10.1038/ni.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klempner SJ, Myers AP, Cantley LC. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer discovery. 2013;3:1345–54. doi: 10.1158/2159-8290.CD-13-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–5. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takata M, Kurosaki T. A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.