Abstract
The development of non-invasive methods, particularly fecal determination, has made possible the assessment of hormone concentrations in wild animal populations. However, measuring fecal metabolites needs careful validation for each species and for each sex. We investigated whether radioimmunoassays (RIAs) previously used to measure fecal testosterone (fT) in male baboons and fecal estrogens (fE) in female baboons were well suited to measure these hormones in the opposite sex. We compared fE and fT concentrations determined by RIA to those measured by liquid chromatography combined with triple quadropole mass spectrometry (LC/MS/MS), a highly specific method. Additionally, we conducted a biological validation to assure that the measurements of fecal concentrations reflected physiological levels of the hormone of interest. Several tests produced expected results that led us to conclude that our RIAs can reliably measure fT and fE in both sexes, and that within-sex comparisons of these measures are valid: (i) fTRIA were significantly correlated to fTLC/MS/MS for both sexes; (ii) fTRIA were higher in adult than in immature males; (iii) fTRIA were higher in pregnant than non-pregnant females; (iv) fERIA were correlated with 17β-estradiol (fE2) and with estrone (fE1) determined by LC/MS/MS in pregnant females; (v) fERIA were significantly correlated with fE2 in non-pregnant females and nearly significantly correlated in males; (vi) fERIA were higher in adult males than in immature males. fERIA were higher in females than in males, as predicted, but unexpectedly, fTRIA were higher in females than in males, suggesting a difference in steroid metabolism in the two sexes; consequently, we conclude that while within-sex comparisons are valid, fTRIA should not be used for intersexual comparisons. Our results should open the field to important additional studies, as to date the roles of testosterone in females and estrogens in males have been little investigated.
Keywords: fecal, estrogens, testosterone, baboon, method validation, LC/MS/MS
1. Introduction
Testosterone (T) has traditionally been viewed as a male hormone and estrogens (E) as female hormones, even though T and E are present in both sexes. However, interest has been building in recent years about possible roles of T in females and E in males. For example, T has been postulated to play an important role in female sexual behavior (Davis et al., 1995; Sherwin and Gelfand, 1987; Shifren et al., 2000), as well as in aggression in a variety of animal species (Beehner et al., 2005; Dabbs et al., 1988; Gill et al., 2007; Glickman et al., 1992; Rosvall, 2013; von Engelhardt et al., 2000; Woodley and Moore, 1999). The presence of androgen receptors in female neural and peripheral tissues suggests that the effect of T on female behavior extend beyond the conversion of T into E (Staub and DeBeer, 1997). T may also have an important role in women’s health, particularly in bone formation and bone mineral density (Davis et al., 1995; Raisz et al., 1996). Similarly, E has been shown to have an important role in male sexual function and behavior in several vertebrate species (Kacker et al., 2012). E may also have important health consequences in men, especially for bone density and for lipid and glucose metabolism (Barrett-Connor et al., 2000; Cohen, 2008; Gillberg et al., 1999; Khosla et al., 2001; Oettel, 2002; Tomaszewski et al., 2009).
Measuring hormone concentrations in wild animal populations has become increasingly feasible in recent decades with the development of non-invasive methods such as fecal determination (Touma and Palme, 2005; Whitten et al., 1998). This method presents the advantage of not interfering with the animal’s behavior and allowing for repeated sampling of the same animal. Due to its integrative nature, fecal determination provides a measurement of hormone concentrations over a longer period of time than samples from blood, which vary considerably with time of day (Touma and Palme, 2005). However, one pitfall of steroid hormone determination in feces is that the circulating hormone is degraded to several metabolites, and usually only a small quantity is present in its original form (Heistermann et al., 2006). Therefore, when using fecal hormone determination it is essential to validate the immunoassay, and verify that the chosen antibody only cross-reacts with metabolites of the original hormone and not with metabolites of other hormones (Goymann, 2005; Heistermann et al., 2006).
Traditionally, three methods have been used to verify that the level of the hormone measured is biologically relevant. The first one consists of injecting the animal with radiolabelled hormone, measuring its metabolic products in feces by high-performance liquid chromatography, and verifying that the major radioactive peaks found by this method exhibit strong immunoreactivity with the antibody used (Goymann et al., 2002; Möhle et al., 2002; Möstl et al., 2005; Palme et al., 2005; Touma et al., 2003; Wasser et al., 1994, 2000). The second method involves stimulating or suppressing the circulating hormone pharmacologically (using for example adrenocorticotropic hormone or gonadotropin-releasing hormone to stimulate glucocorticoid or testosterone production respectively), and then verifying that the metabolites measured in feces reflect that stimulation or suppression (Möstl et al., 2005; Touma and Palme, 2005; Wasser et al., 2000). The third method consists of verifying that fecal hormone concentrations follow physiological patterns previously determined in blood (e.g. elevated T concentrations during the breeding season, cyclic variation of E and Progesterone (P) during the menstrual cycle, elevated E and P concentrations during pregnancy) (Goymann, 2005; Hirschenhauser et al., 2005).
The metabolism and route of excretion of fecal steroids vary considerably among species, and previous studies have emphasized the necessity of a separate validation for each species (Goymann, 2005; Heistermann et al., 2006; Hirschenhauser et al., 2012; Möhle et al., 2002). A recent review by Goymann (2012) also underlines that excretion route and metabolite types may vary between the sexes (see also: Goymann, 2005; Hirschenhauser et al., 2012; Palme et al., 2005; Touma and Palme, 2005; Touma et al., 2003). For example, Touma et al. (2003) reported that in mice, the percentage of GC metabolites recovered in feces was greater for males than females (73% and 53% respectively). In contrast another study by Dantzer et al. (2011a, b) reported that in red squirrels, while the percentage of T metabolites excreted in feces was slightly lower for males than females (44% and 56% respectively), these differences did not reach significance. The type of GC and T metabolites excreted in feces was also shown to be different for each sex (Dantzer et al., 2011a, b; Goymann, 2005; Touma et al., 2003). Because the antibody used in an immunoassay will only recognize some of these metabolites, this could lead to a disconnect between concentrations of excreted hormones and serum levels (Goymann, 2012). Also, Möhle et al. (2002) showed that two hormones of different origin, T (gonadal) and DHEA (adrenal), can be degraded in similar metabolites when excreted in feces and urine. When a T antibody cross-reacts with metabolites that are common to both hormones, this could lead to an apparent absence of difference between male and female T levels, as shown for several species of primates and birds (Dittami et al, 2008; Goymann, 2005; Möhle et al., 2002).
In this study, we investigated whether hormone specific radioimmunoassays (RIAs) previously validated in baboons (Khan et al., 2002; Lynch et al, 2003), and used to measure fecal testosterone (fT) in males (Beehner et al., 2009; Gesquiere et al., 2011) and fecal estrogens (fE) in females (Gesquiere et al., 2005, 2007) are well suited to measure fT in females and fE in males. We compared fE and fT concentrations determined by RIA to those determined by high-pressure liquid chromatography combined with triple quadropole mass spectrometry (LC/MS/MS). In contrast to RIA, where the antibody can cross-react with several metabolites, LC/MS/MS is a highly specific method that measures the mass to charge ratio of individual steroids (Hauser et al., 2008). We also compared fE and fT concentrations between the sexes, and across reproductive state (females) or age (males) to verify that the hormone concentrations measured in the feces reflect physiological levels.
2. Methods
2.1. Field site and subjects
The males and females in the present study were the individually identified members of five social groups in the Amboseli baboon population that have been monitored for reproductive, demographic, and behavioral events on a near-daily basis for over four decades (e.g. Alberts and Altmann, 2012; Gesquiere et al., 2007; see www.amboselibaboons.nd.edu for a complete bibliography and the Baboon Project Monitoring Guide, which outlines the data collection protocols). Since December 1999, physiological data have been obtained through non-invasive collection of freshly deposited feces from known individuals and subsequent analysis of steroid hormone metabolites extracted from the feces.
All data collection procedures were non-invasive, and adhered to the laws and guidelines of Kenya (Research Permit MOEST 13/001/C351 Vol. II) and were approved by the Animal Care and Use Committee at Princeton University (IACUC 1821) and at Duke University (IACUC A028-12-02).
2.2. Demographic and reproductive data
Age was known for all females and immature males and for all adult males born in study groups. For immigrant males, age was estimated based on coat condition, degree of scarring, body carriage (e.g., degree of straightness versus curvature of the spine, how the head is carried, etc), and canine tooth condition when they first joined the study population (see Alberts and Altmann, 1995 for details).
Female sexual swelling state (turgescent or deturgescent) and size, presence of menstrual blood, and the color of the paracallosal skin, were recorded for all adult females in a group each time an observer was with that group (Altmann, 1970; Gesquiere et al., 2007; see also the Amboseli Baboon Research Project (ABRP) Monitoring Guide at www.amboselibaboons.nd.edu for details on data collection protocols). Female reproductive state was assigned subsequently based on these records. Menstrual cycles in female baboon are easy to identify by the successive turgescence (follicular phase) and deturgescence (luteal phase) of the sexual skin. Failure to cycle after 40 days and the absence of menstrual blood usually indicates that the female is pregnant (Beehner et al., 2006). Pregnancy is then confirmed by the change of color of the paracollosal skin from black to pink, approximately two months after conception (Altmann, 1970). The average gestation length for female baboons is 177 days (Altmann, 1980). After birth the female remains in a state of post-partum amenorrhea for an average of one year unless her infant dies, in which case the female usually resumes cycling about three weeks after the infant’s death (Altmann et al., 1978). Baboons are non-seasonal breeders and can reproduce all year long (Altmann, 1980).
2.3. Fecal collection and extraction
We have previously described in detail and validated our fecal sample collection, storage, and extraction methods, including studies of the effects of storage time on metabolite concentrations (Khan et al., 2002; Lynch et al., 2003). In brief, immediately after collection of freshly deposited fecal samples from known individuals, these samples were mixed and placed in 95% ethanol, and kept refrigerated until they were shipped to University of Nairobi (every two weeks). In Nairobi, samples were freeze-dried and sifted to remove vegetation. Samples were then stored at −20°C until they were shipped to Princeton University, where 0.2 g of fecal powder was extracted into 2 ml 90% methanol using a multi-pulse vortexer for 30 minutes. Following extraction, samples were further purified using a prepped Oasis cartridge (Waters, Milford, MA) and stored at −20°C.
2.4. Hormone analysis
Wasser and colleagues (1994, 2000) have shown that in baboons the large majority of steroid metabolites excreted in feces are in a non-conjugated form, with only about 20% conjugated for estrogens, progesterone and cortisol. Similarly, Möhle et al. (2002) showed that in macaques and chimpanzees fecal T metabolites were at 75% unconjugated. Therefore, we did not judge it necessary to do a solvolysis prior to assaying our samples.
2.4.1. Radioimmunoassays
fT concentrations were determined in males and females using a testosterone specific antibody, the commercially available Pantex Testosterone direct 125I kit (Pantex, Santa Monica, CA). In our laboratory, this kit replaced the Diagnostics Systems Laboratories (DSL) 125I Testosterone kit (Beckman Coulter, Webster, TX), which we used and validated previously, and which was discontinued in 2009; parallelism, accuracy, precision and physiological validation were reported in Beehner et al. (2009). We used the standards, the primary antibody, the labeled testosterone, and the precipitant solution provided by the Pantex T kit. Working buffer was the serum-based buffer in which the standards were diluted. According to the manufacturer, the primary antibody cross-reacts 100% with T, 6.9% with 5α-dihydrotestosterone (DHT), 0.52% with androsterone, 0.15% with anadrol and <0.1% with aldosterone, androstenedione, cholesterol, corticosterone, cortisol, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate, desoxycorticosterone, 11-desoxycortisol, α-estradiol, 17-β-estradiol (E2), estriol, estrone (E1), etiocholanolone, 17-hydroxypregnenolone, 17α-hydroxyprogesterone, 20-hydroxyprogesterone, pregnenolone, pregnenolone sulfate, and progesterone. All samples were run in duplicate, and the results are expressed as ng/g dry fecal matter.
The slope of a serial dilution of a mixed male-female fecal pool showed great parallelism with the slope of the standard curve (t=−0.008, p=0.994). The assay accuracy, assessed by spiking each standard with an aliquot of the male-female fecal pool, was 124% ± 15 (Mean± SE, N=16). The intra-assay coefficients of variation (CV) were 6.8% and 2.3% respectively for a 50pg/tube and a 100pg/tube of male-female fecal pool (N=10). The inter-assay CVs were 9.7% and 8.9% respectively for the 50pg/tube and 100pg/tube male-female fecal pool (N=90). In addition, we re-ran with the Pantex kit a subset of our samples that had been previously assayed with the DSL kit, in order to check if the two kits gave comparable T concentrations. Our results showed a strong correlation between the T values obtained by the two different kits (R 2=0.960, n=143, p<0.001), but the T concentrations obtained with the DSL kit were higher than those with the Pantex kit. Using the empirically derived linear regression equation (TDSL=1.399*TPantex-2.552), we transformed the T values obtained with the Pantex kit so that T concentrations determined by either method could be combined in the same analysis (see Gesquiere et al., 2011).
fE concentrations were assayed for males and females using the Total Estrogen kit (MP Biomedicals, Costa Mesa, CA), a radioimmunoassay previously validated for female baboons (see Khan et al., 2002 for parallelism, accuracy, and precision of the assay; see also Altmann et al., 2004; Beehner et al., 2006; Gesquiere et al., 2005 for physiological validation of the assay). According to the manufacturer, the primary antibody cross-reacts 100% with E1 and E2, 9% with estriol, 7% with estradiol-17α, and 2.5% with equilin. All samples were run in duplicate, and the results are expressed as ng/g dry fecal matter.
2. 4.2. LC/MS/MS
A total of 90 fecal samples (from 74 individuals) collected from Jan 2010-Jun 2011 for which we had 0.2g of extra fecal powder were used in this analysis. We had 30 samples from 29 non-pregnant females (20 from cycling females and 10 from post-partum females), 30 samples from 20 pregnant females and 30 samples from 22 adult males (each individual was represented only once in the final dataset because we took the mean of values for individuals represented by multiple samples). Prior to the methanol extraction, 50 μl of 1ng/μl of deuterated internal standards (d4-E1, d5-E2, d5-T and d9-P) were added to the fecal powder. This allowed us to adjust for procedural loss and to accurately determine the steroids of interest in the complex fecal media. The d5-T was used as internal standard for all the androgen analytes. The deuterated internal standards were obtained from CDN Isotopes (Point-Claire, Quebec, Canada).
Samples were separated with high-pressure liquid chromatography using a Shimadzu system (the system included two Shimadzu LC20ADXR pumps and a Shimadzu SIL20ACXR autosampler). The detection occurred using a QTRAP 5500 triple quadrupole mass spectrometer (AB Sciex, Concord, ON, Canada) equipped with an atmospheric pressure chemical ionization source operated at 500°C. The temperatures of the column oven and of the autosampler were 40°C and 15°C, respectively. Quantitative data were obtained in Multiple Reaction Monitoring (MRM) after determination of the response factor for each compound and internal standard. All data were acquired and processed with Analyst software, Ver 1.5.1 (AB Sciex).
The chromatographic separation was performed on a Phenomenex Kinetex 2.6u C18 100A, 150 × 4.6 mm column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 2 solvents: water (A) and methanol (B) delivered at a flow rate of 0.25 mL/min. The sample (10 μL) was injected into the LC/MS/MS after which the injector was washed with 3 post-injection washes with 20:80 methanol:water. The total run time was 40 min. During the first 2 min, the initial conditions of 3% B were held. This was followed by fast linear gradient to 50% B over the next 0.10 min. These system conditions were maintained for the next 2.9 min. The gradient was increased to 67% B over the next 15 min, then 100% B over the next 3 min. This was held for 7 min before the system was returned to initial conditions of 3% B over 0.1 min and held for the final 9.9 min of each run. Once ion pairs were selected for each of the compounds and the internal standards, the instrument settings were optimized for maximal signal using the autotune feature of the instrument software. Results were generated in positive-ion mode with the following optimized voltages: nebulizer current, 3 volts; entrance potential, 10 volts. The source temperature was 500°C. The gas settings were: curtain gas, 20 psi; nebulizing gas, 40 psi; desolvation gas, 0; collisionally activated dissociation gas, medium.
We measured T, androstenedione, DHT, DHEA, androsterone, E1 and E2. All steroid reference standards (>99 % purity) were obtained from Sigma-Aldrich (St Louis, MO). Mass labeled internal standards listed previously were obtained from CDN isotopes (Pointe Claire, Quebec Canada). Calibration standards were analyzed in the range of 10 to 250 ng/mL. The resulting correlation coefficients for the standard curve, in a linear regression through zero with no weighting, ranged from 0.9916 to 0.9999 for the steroids listed. The limits of detection, based upon a 3 to 1 signal-to-noise ratio were as follows: 0.05 ng/sample for T, androstenedione, and E2; 0.3 ng/sample for androsterone; 0.5 ng/sample for DHT and DHEA; and 1.0 ng/sample for E1. The %CV for comparison of quantifier and qualifier MRMs in the study sample results ranged from 4.6 to 13 % among all of the steroid analytes. The mean internal standard response of extracted samples compared well with the mean internal standard response of solvent-diluted calibrators (88.5 % to 106 %). Percent recoveries of standard additions (10, 15, 25, and 40 ng/sample) made to pooled fecal extract ranged from 82.2 to 106 % among all steroid analytes. Intra-day precision of detected steroids from replicate samples (n=3) ranged from 89.3 to 110 %, and inter-day precision of detected steroids from replicate samples (n=3) ranged from 92.6 to 111 %.
2.5. Biological validation
2.5.1. Fecal testosterone
We first assessed whether fT concentrations measured using the Pantex RIA reflected biological levels in males as shown previously with the Equate RIA (Lynch et al., 2003) and with the DSL RIA (Beehner et al., 2009). A total of 776 fecal samples from 96 males (55 immature and 43 adult males), collected from September 2009 through June 2013, were used in this analysis. We determined whether fT concentrations in adult males (8-10 years old) were higher than those of immature males (3-5 years).
We also assessed whether fT concentrations determined with the Pantex T RIA reflected physiological concentrations in females. We used data on 1366 fecal samples from 144 adult females, most of whom transitioned from pregnant to non-pregnant over the course of sample collection (September2009 through June 2013). Specifically, 350 fecal samples were collected from 130 cycling females, and 1016 samples were collected from 123 pregnant females. We evaluated the prediction that fT concentrations in pregnant females should be above non-pregnancy levels starting around day 16 after ovulation (Castracane et al., 1998), and that they should peak in the first trimester of pregnancy before decreasing to non-pregnant levels (Castracane and Goldzieher, 1983).
2.5.2. Fecal estrogens
Wasser et al. (1994) found that in female baboons, 10% of radio-labeled injected E2 was excreted in the feces with the remainder excreted in urine. In feces, E2 was excreted in larger portion as non-conjugates: 36% as E2, 44% as E1, and 20% as E1 sulfate.
We have previously validated the ICN Total Estrogens antibody in baboon females, showing that fE concentrations in immature females (2–4 years of age) were lower than in mature non-pregnant females > 7 years of age (Lynch et al., 2003); see also (Gesquiere et al., 2005). We have also shown that cycling females present an fE peak in the 5-day period before the onset of deturgescence, when ovulation is estimated to occur (Gesquiere et al., 2007). Moreover, fE concentrations increase progressively during pregnancy (Beehner et al., 2006) and a sudden drop is observed post-partum (Altmann et al., 2004). Determination of E in feces is therefore a good reflection of E concentrations in serum for female baboons.
In this paper, we assessed whether fE concentrations determined with the ICN Total Estrogens kit were also physiologically meaningful in males by checking that fE concentrations in adult male baboons (8-10 years of age) were higher than in immature males (3-5 years) as shown in humans (Klein et al., 1996). We had a total of 776 fecal samples collected from September 2009 through June 2013 for this analysis.
2.6. Statistical Analysis
Because our hormone concentrations were not normally distributed, we logarithm-transformed them. However, transformation did not produce normal distributions for the smaller datasets that included the 30 samples from males, 30 samples from non-pregnant females and 30 samples from pregnant females (i.e., the samples assayed via LC/MS/MS). Therefore we used non-parametric statistics for the analyses pertaining to Table 1 and Table 2. Significance levels for all the tests were set at p<0.05.
Table 1.
| a- Androgen concentrations in baboon feces | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Androgens (ng/g feces) |
Males (N=26) |
Non-pregnant females (N=29) |
Pregnant females (N=25) |
|||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | ||
| RIA | fT | 89.00 | 40.96 | 26 | 133.43 | 79.82 | 29 | 187.40 | 72.70 | 25 |
| LC/MS/MS | fT | 15.70 | 13.47 | 26 | 26.13 | 25.71 | 29 | 36.37 | 28.46 | 25 |
| Androstenedione | 6.97 | 4.90 | 26 | 9.57 | 7.19 | 29 | 13.01 | 6.43 | 25 | |
| DHEA | 418.46 | 321.87 | 26 | 439.04 | 327.20 | 29 | 518.46 | 465.49 | 25 | |
| DHT | 81.26 | 64.03 | 26 | 83.05 | 39.89 | 29 | 91.80 | 90.94 | 25 | |
| Androsterone | 57.40 | 77.01 | 26 | 134.08 | 226.39 | 29 | 172.96 | 216.10 | 25 | |
| b- Estrogen concentrations in baboon feces | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estrogens (ng/g feces) |
Males (N=26) |
Non-pregnant females (N=29) |
Pregnant females (N=25) |
|||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | ||
| RIA | fE | 39.04 | 31.83 | 26 | 53.55 | 36.16 | 29 | 602.94 | 421.85 | 25 |
| LC/MS/MS | Estrone (fE1) | 58.63 | 41.13 | 26 | 60.55 | 60.37 | 29 | 264.29 | 202.23 | 25 |
| 17β-estradiol (fE2) | 10.40 | 7.61 | 23 | 47.17 | 82.74 | 28 | 591.45 | 452.61 | 25 | |
Table 2.
a: Spearman rank correlations between fecal androgen concentrations determined by RIA and by LC/MS/MS. Significant correlations are highlighted in bold; note that after multiple testing using the method of Benjamini and Hochberg, the thresholds for significant p-values are lower than 0.05 (see Supplemental Material).
b: Spearman rank correlations between fecal estrogen concentrations determined by RIA and by LC/MS/MS. Significant correlations are highlighted in bold; note that after multiple testing using the method of Benjamini and Hochberg, the thresholds for significant p-values are lower than 0.05 (see Supplemental Material).
| Androgens | Adult males (N=26) | ||||
|---|---|---|---|---|---|
| fTRIA | fTLC/MS/MS | ||||
| r | p | r | P | ||
| LC/MS/MS | fT | 0.509 | 0.008 | ||
| DHT | 0.254 | 0.210 | 0.374 | 0.059 | |
| Androstenedione | 0.329 | 0.101 | 0.659 | <0.001 | |
| Androsterone | 0.312 | 0.121 | 0.131 | 0.524 | |
| DHEA | 0.149 | 0.466 | 0.294 | 0.145 | |
| Androgens | Non-pregnant females (N=29) | Pregnant females (N=25) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| fTRIA | fTLC/MS/MS | fTRIA | fTLC/MS/MS | ||||||
| r | p | r | P | r | p | r | P | ||
| LC/MS/MS | fT | 0.800 | <0.001 | 0.632 | 0.001 | ||||
| DHT | 0.237 | 0.215 | 0.333 | 0.077 | −0.056 | 0.790 | −0.092 | 0.662 | |
| Androstenedione | 0.688 | <0.001 | 0.696 | <0.001 | 0.647 | <0.001 | 0.850 | <0.001 | |
| Androsterone | 0.005 | 0.980 | 0.136 | 0.481 | −0.145 | 0.489 | −0.002 | 0.991 | |
| DHEA | 0.131 | 0.498 | 0.129 | 0.506 | 0.022 | 0.916 | 0.369 | 0.070 | |
| Estrogens | Adult males (N=26) |
Non-pregnant females (N=29) |
Pregnant females (N=25) |
||||
|---|---|---|---|---|---|---|---|
| fERIA | fERIA | fERIA | |||||
| r | p | r | p | r | p | ||
| LC/MS/MS | Estrone (fE1) | 0.149 | 0.466 | −0.012 | 0.951 | 0.822 | <0.001 |
| 17β-estradiol (fE2) | 0.442 | 0.035 | 0.531 | 0.004 | 0.943 | <0.001 | |
To compare male and female hormone concentrations (Table 1), we used Mann-Whitney tests. To compare fT concentrations determined with RIA (fTRIA) to those measured with LC/MS/MS (fTLC/MS/MS) we used Spearman rank correlations. We also calculated Spearman rank correlations between fTRIA and several fecal androgens measured by LC/MS/MS, in order to determine possible cross-reactivity of the T antibody with other androgens. Finally, we used Spearman rank correlations between fTLC/MS/MS and other fecal androgens within the LC/MS/MS measurements to gain insight about whether these different androgens might have a common metabolic origin with T (Preis et al., 2011). We conducted a similar analysis with fE, comparing concentrations determined with RIA (fERIA) to fE1 and fE2 concentrations given by LC/MS/MS using Spearman rank correlations. Three fE2 values for males and one fE2 value for non-pregnant females were considered outliers, because they were more than 2 SD above the mean for that category and we judged that they were disproportionately affecting the distribution of the dataset; these outliers were removed from our dataset before running our correlations. To correct for repeated correlations we used the method of Benjamini and Hochberg that corrects for multiple testing (Verhoeven et al. 2005; see Supplementary Tables S1-S3).
Finally, we used Generalized Linear Mixed-effect Models (GLMMs) on the logarithm-transformed hormone concentrations as the residuals from the raw hormone values were not normally distributed. We used a linear regression with identity link and had baboon identity as a random factor (with random intercept), for our four physiological validations: (i) across maturation stages in males, (ii) across time from the onset of deturgescence in pregnant and (iii) non-pregnant females, and (iv) between pregnant and non-pregnant females.
3. Results
3.1. Hormone concentrations determined by RIA and by LC/MS/MS
3.1.1. Fecal testosterone and other androgens
fT concentrations determined by RIA were higher than those determined by LC/MS/MS for both sexes (5.7 times higher for males, and 5.1 and 5.2 times higher for non-pregnant females and pregnant females, respectively; see Table 1a). T represented only a small percentage of the total androgens in the feces (2.7% in males, 3.8% in non-pregnant females and 4.4% in pregnant females). Of the five androgens determined, DHEA was by far the dominant androgen in feces for both males and females (72.17% in males, 63.46% in non-pregnant females and 62.27% in pregnant females).
Comparing fT measured via RIA for males and females, we found, surprisingly, that both non-pregnant (Z=−2.158, p=0.031) and pregnant females (Z=−4.447, p<0.001) had higher levels of fT than males (see Table 1a). Comparable patterns were obtained with LC/MS/MS (Table 1a). However, fTLC/MS/MS concentrations were significantly higher than males only in the case of pregnant females (Z=−3.533, p<0.001); non-pregnant females had levels that were not significantly different than males (Z=−1.371, p=0.169). Pregnant females also had higher levels of androstenedione and androsterone than males (androstenedione: Z=−3.448, p=0.001, androsterone: Z=−2.112, p=0.035), while non-pregnant female levels did not significantly differ from males (androstenedione: Z=−1.416, p=0.157, androsterone: Z=−0.230, p=0.818). DHT and DHEA showed similar concentrations in males and females (Table 1a).
3.1.2. Fecal estrogens
To compare fERIA with estrogen concentrations obtained via LC/MS/MS, we took the sum of E1 and E2 concentrations obtained by LC/MS/MS, because the MP Biomedical Total estrogens antibody cross-reacts 100% with both E1 and E2. In contrast to fT, fE concentrations determined by RIA were lower for both sexes than the sum of fE1 and fE2 determined by LC/MS/MS (1.9 times lower for males, and 2 and 1.4 times lower for non-pregnant and pregnant females, respectively). In females, fERIA were comparable to the concentrations of fE2 determined by LC/MS/MS, but in males fERIA concentrations were 3.8 times higher than fE2 determined by LC/MS/MS (Table 1b).
Comparing fE measured via RIA for males and females, we found that both non-pregnant and pregnant females had higher fE concentrations than males (Z=−2.293, p=0.022 and Z=−5.634, p<0.001, respectively). Comparable data were obtained with LC/MS/MS for fE2 (Z=−2.272, p=0.023 and Z=−5.541, p<0.001, respectively). In contrast, fE1 concentrations in males were lower than those of pregnant females (Z=−4.202, p<0.001) but not different from concentrations of non-pregnant females (Z=−0.152, p=0.879). In males, fE1 concentrations were 5.6 times higher than fE2 concentrations, in non-pregnant females fE1 and fE2 occurred in similar concentrations (fE1/fE2=1.3), and in pregnant females fE2 concentrations were 2.2 times higher than fE1 concentrations (Table 1b).
3.2. Correlations between hormone concentrations measured by RIA and by LC/MS/MS
3.2.1. Fecal Testosterone
fTRIA and fTLC/MS/MS were positively correlated in males (r=0.509, p=0.008) and even more so in females (non-pregnant: r=0.800, p<0.001 and pregnant: r= 0.632, p=0.001; see Table 2a). fTRIA and androstenedione were positively correlated in females (non-pregnant: r=0.688, p<0.001 and pregnant: r=0.647, p<0.001) but not in males (r=0.329, p=0.101). fTRIA was not correlated with androsterone, DHEA or DHT in either sex. We examined the correlations between fT and other fecal androgens within the LC/MS/MS, to help determine the extent to which fT and the other fecal androgens might have a common metabolic origin. We found a strong correlation between fTLC/MS/MS and fecal androstenedione for both males (r=0.659, p<0.001) and females (non-pregnant: r=0.696, p<0.001 and pregnant: r=0.850, p<0.001). fTLC/MS/MS concentrations were not correlated with androsterone, DHT or DHEA for either sex (Table 2a). When correcting for multiple testing using the method of Benjamini and Hochberg, the results retained their significance values (See Supplementary Table S2).
3.2.2. Fecal Estrogens
fERIA and fE2 were positively correlated in both pregnant and non-pregnant females (pregnant: r=0.943, p<0.001; non-pregnant: r=0.531, p=0.004), and in males (r=0.442, p=0.035) (Table 2b). fERIA and fE1 were positively correlated only for pregnant females (r=0.822, p<0.001), not for non-pregnant females (r=−0.012, p=0.951) or for males (r=0.149, p=0.466). Similarly, fE1 and fE2 were significantly positively correlated in pregnant females (r=0.828, p<0.001), but the correlation was not significant in non-pregnant females (r=0.194, p=0.323) or in males (r=0.164, p=0.455). Further, when correcting for multiple testing using the method of Benjamini and Hochberg, we found that in males, the correlation between fERIA and fE2 was no longer significant (the corrected significance threshold was set at 0.025). The rest of the results remained the same after multiple testing (See Supplementary Table S3).
3.3. Physiological validation
In order to confirm that the hormone concentrations measured by our RIAs reflected plasma concentrations we conducted a series of physiological validations by comparing female fTRIA concentrations in different reproductive states and male fTRIA and fERIA concentrations at different age stages.
3.3.1. Fecal testosterone in males
fTRIA concentrations were significantly higher in adult males than in immature males (F=26.636, p<0.001).
3.3.2. Fecal testosterone in females
In non-pregnant female baboons, fTRIA concentrations remained relatively constant during the luteal phase of the cycle (F=3.460, p=0.064), while in pregnant females, fTRIA concentrations increased across time (F=50.054, p<0.001; Fig. 1). Specifically, fTRIA concentrations in pregnant females became significantly higher than non-pregnant levels starting on day 21st of pregnancy (F=10.612, p=0.001) and peeked around week 5-6 (days 29-41) of pregnancy. fTRIA concentrations then dropped to early pregnancy concentrations around week 8 (days 49-55) (Fig. 2).
Fig. 1.
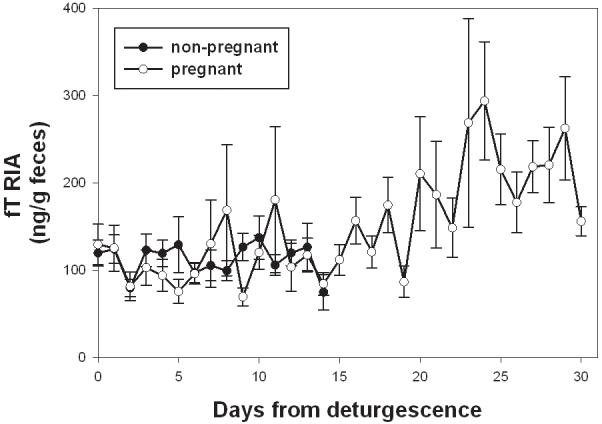
Comparison of fT concentrations determined by RIA between ‘pregnant’ and ‘non-pregnant’ cycles. Each value represents the mean ± SE across female’s cycles, for each cycle day indexed from the onset of sex skin deturgescence, i.e., the first day that the sex swelling begins to diminish in size (Gesquiere et al., 2007). Data for non-pregnant females stop at day 17 because day 18 would be the first day of a new cycle (see Gesquiere et al. 2007 for further information).
Fig. 2.

Profile across the pregnancy (mixed longitudinal and cross-sectional samples) of fT concentration. Each value represents the weekly mean ± SE across females.
3.3.3. Fecal estrogens in males
Male baboons had concentrations of fE2 that were one fifth of non-pregnant female levels and one sixtieth of pregnant female levels (Table 1b). Males also had significantly lower concentrations of fERIA than pregnant and non-pregnant females (70% of non-pregnant levels and one fifteenth of pregnant levels), but the difference was not as dramatic as that observed with fE2. Immature males had significantly lower fERIA concentrations than adult males (F=7.981, p=0.006).
4. Discussion
4.1. Fecal testosterone
fT concentrations measured using the Pantex T RIA were higher than those measured by LC/MS/MS, as has been reported in other studies (Preis et al., 2011). This result suggests that the Pantex antibody may cross-react with other androgens, in both male and female baboons. However, our antibody didn’t appear to cross-react with DHEA or DHT, as we didn’t find a correlation between fTRIA and these androgens in either males or females. Moreover, the significant positive correlation between fTRIA and androstenedione in females seems to result from the metabolic association between T and androstenedione rather than a cross-reactivity of androstenedione with the antibody, as fTLC/MS/MS was also correlated with androstenedione. The strong correlation between T with androstenedione (an androgen of adrenal origin) in females but not in males, may be explain by the fact that in females, serum T is produced by both ovaries and adrenals while in males T is nearly exclusively produced by testes. But even if serum T in females is of both adrenal and ovarian origin, T concentrations can still be important for understanding female reproduction, because the contribution of the adrenals seems to remain relatively constant over time (Abraham, 1974). The fact that fTRIA concentrations were highly correlated with fTLC/MS/MS in both sexes suggests that the metabolites we measured reflected T concentrations in both males and females.
However, our study also revealed some puzzling data. When determined by RIA, fT concentrations in females were higher than in males, while the reverse pattern has been observed in yellow baboon serum (Castracane et al., 1986; Castracane and Goldzieher, 1983). Other studies have reported similar data to ours for fecal metabolites of T, and the authors of these other studies have suggested that these elevated fT concentrations in females were the result of cross-reaction of the antibody with hormone metabolites of different origins, such as DHEA, which is of adrenal origin (Dittami et al., 2008; Goymann, 2005; Hauser et al. 2011; Möhle et al., 2002; Preis et al., 2011). However, our results do not support the idea that elevated fT in female baboons resulted from cross-reaction of our antibody with metabolites issued from a different hormone than T in females, for two reasons. First, as mentioned in the paragraph above, our T antibody did not cross-react with DHEA. Secondly, even when we measured fT with high specificity, using LC/MS/MS, we found that fT concentrations were similar (for non-pregnant females) or higher (for pregnant females) than for males.
So what could explain the elevated fT concentrations in females? One possibility is that T metabolites in females could come from the progesterone metabolic pathway, but this hypothesis is not supported by our data, as we did not find a significant correlation between fTRIA and fPLC/MS/MS for either males or females after correcting for multiple testing (data not shown). Another possibility is that male and female baboons metabolize testosterone following different pathways (i.e. urine vs. feces); female baboons may be excreting a higher proportion of T metabolites in feces than males. While we cannot verify if it is the case with our baboons, Touma et al. (2003) showed a sex difference in steroid hormone excretion in mice, in which males excreted GC metabolites in feces at a higher rate than females.
Finally, we found that fT concentrations measured with the Pantex RIA were physiologically relevant in both male and female baboons. Adult males had higher fT concentrations than immature males, as shown in our previous studies with two different RIAs (Beehner et al., 2009; Lynch et al., 2003). In pregnant females, fT concentrations increased above non-pregnant levels, starting on day 21 after onset of sex skin deturgescence (ovulation in baboons is estimated to occur in the 5-day period prior to onset of deturgescence of the sex skin). Similarly Castracane and colleagues reported that serum and plasma T concentrations increased in pregnant women (Castracane et al., 1998) and in pregnant baboons (Castracane and Goldzieher, 1983). T concentrations were higher than non-pregnant levels starting on day 15 after ovulation (for women) or day 16 (for baboons). Using fecal T determination, we did not detect a significant difference until day 21. This may result from the fact that our dataset is cross-sectional rather than longitudinal, adding some variance to our fT measure. The elevation of T concentrations in early pregnancy is thought to be related to the onset of chorionic gonadotropin production (Fortman et al., 1993; Hodges et al., 1984; Kuehl et al., 1992). Castracane and Goldzieher (1983) report that fT concentration in baboons continues increasing until day 33-40 of pregnancy when it reaches a peak and then decreases to reach luteal levels by day 50. Our fecal T profile during pregnancy was strikingly similar to that published by Castracane and Goldzieger (1983), where T was determined in the plasma of yellow baboons.
4.2. Fecal estrogens
fE concentrations measured via RIA (which captured total estrogens) were 70-80% lower than the sum of E1 and E2 determined by LC/MS/MS for both males and females. While most studies found higher steroid hormone concentrations with RIA than with LC/MS/MS, resulting from cross-reactivity of the antibody (reviewed in Preis et al., 2011), Dorgan et al. (2002) also reported that the E concentrations in human serum were lower when determined by RIA than by LC/MS/MS. LC/MS/MS may be more sensitive than RIA to E concentrations in baboon feces.
In pregnant females, fE concentrations measured via RIA were significantly positively correlated with both fE1 and fE2 measured by LC/MS/MS. These results indicate that our antibody is well suited to measure estrogens in feces of pregnant baboons. Our results in non-pregnant females showed that fERIA concentrations were correlated with fE2 but not with fE1, suggesting that the MP antibody reliably measured fE2 but not fE1 in feces of non-pregnant female baboons. Similar results were found for males, even though the fERIA correlation with fE2 became statistically non-significant after correcting for multiple testing. The lack of correlation of fERIA with fE1 may seem somewhat surprising, as E1 and E2 can be converted into one another; however they generally originate from different sources in humans. In men and non-pregnant women, E2 is produced primarily in testes and ovaries by aromatization of T (Leach et al., 1956; Longcope et al., 1972). Non-negligible amounts of E2 are also produced in the adrenal glands and in peripheral tissues, most notably fat tissues (Nelson and Bulun, 2001). By contrast, most circulating E1 is derived from peripheral aromatization of androstenedione (which is largely produced by the adrenals) (Longcope et al., 1972). In fact, we found that in males and non-pregnant females, fE1 and fE2 concentrations measured by LC/MS/MS were not correlated with each other, suggesting that E1 and E2 may also have different origins in baboons, as they do in humans. During pregnancy in baboons, E2 switches from an ovarian origin (by corpus luteum) at the beginning of pregnancy to a placental origin around day 20-25 of gestation (Fortman et al., 1993). The placenta becomes the primary source of E2 by day 60 of baboon pregnancy. E1 is also largely produced by the placenta during pregnancy in cercopithecine primates (Walsh et al., 1979). The common placental origin of E1 and E2 during pregnancy may explain why fE1 and fE2 concentrations are strongly correlated in pregnant baboon females, and therefore why fERIA concentrations in our study were highly correlated with both fE1 and fE2.
We found that male baboons had significantly lower concentrations of fERIA than females, suggesting that our RIA can reliably detect physiological difference between males and females. Our data also showed that adult males had higher fE than immature males, in agreement with other studies (Klein et al., 1996).
5. Conclusions
Taken together, our results suggested that the Pantex T RIA gave a reliable measure of T in feces of both male and female baboons and that fecal T conformed to expected physiological concentrations in both sexes. However, fT levels in females were higher than in males. The most likely reason for this is a difference in metabolism of the steroid in the two sexes; because the basis of this difference is not yet understood, comparisons between females and males should not be made. As with fecal T in females, fecal E in males conformed to expected physiological concentrations. Further, our results showed that the MP Biomedical Total Estrogens kit seemed to give a good estimate of fE2 (but not fE1) concentrations in males, even though the significance of that result was diminished by our multiple testing. The possibility of measuring T in females and E in males non-invasively should open the field to interesting investigations in the wild as to date little has been done on these hormones in the opposite sex.
Supplementary Material
-
-
Fecal testosterone levels measured by RIA and LC/MS/MS were correlated for both sexes
-
-
Testosterone concentration was higher in pregnant than non-pregnant females
-
-
Fecal estrogens (RIA) were correlated to 17β-estradiol (LC/MS/MS) in both sexes
-
-
Estrogen concentrations were higher in adult males than in immature males
-
-
RIAs can reliably evaluate testosterone in females and estradiol in males
Acknowledgements
This work was supported by the National Science Foundation (grants IOB-0322613, IOB-0322781, BCS-0323553 and BCS-0323596), National Institute of Mental Health (R03 MH65294), and National Institute on Aging (grants P30-AG024361, P01-AG031719, and R01-AG034513).Thanks to the Office of the President, Republic of Kenya, the Kenya Wildlife Services, its Amboseli staff and Wardens, the Institute of Primate Research, the National Museums of Kenya, the Department of Veterinary Anatomy and Physiology of the University of Nairobi, and the members of the Amboseli-Longido pastoralist communities. Particular thanks go to the Amboseli field team who contributed to sample and data collection (R.S. Mututua, S. Sayialel, and J.K. Warutere). Thanks to T. Wango, V.K. Oudu, C. Simao for the fecal samples processing and analyses. The NIH base grant to the Wisconsin National Primate Research, RR000167 is acknowledged for support of the LC/MS/MS work as well as the expert mass spectrometry analyses by Dr. Curtis Hedman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. Endocrinol. Metab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Altmann J. Preparation and activation: determinants of age at reproductive maturity in male baboons. Behav. Ecol. Sociobiol. 1995;36:397–406. [Google Scholar]
- Alberts SC, Altmann J. The Amboseli Baboon Research Project: Themes of continuity and change. In: Kappeler P, Watt DP, editors. Long-term field studies of primates. Springer-Verlag; Berlin Heidelberg New York: 2012. pp. 261–288. [Google Scholar]
- Altmann J. Baboon Mothers and Infants. Harvard University Press; Cambridge, Mass.: 1980. [Google Scholar]
- Altmann J, Altmann SA, Hausfater G. Primate infant’s effects on mother’s future reproduction. Science. 1978;201:1028–1029. doi: 10.1126/science.98844. [DOI] [PubMed] [Google Scholar]
- Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. Life-history correlates of steroid concentrations in wild peripartum baboons. Am. J. Primatol. 2004;64:95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- Altmann SA. The pregnancy sign in savannah baboons. Lab. Anim. Dig. 1970;6:7–10. Note: this article was re-printed in 1973 in The Journal of Zoo Animal Medicine 4, 8-12. [Google Scholar]
- Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000;85:219–223. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Gesquiere L, Seyfarth RM, Cheney DL, Alberts SC, Altmann J. Testosterone related to age and life-history stages in male baboons and geladas. Horm. Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Horm. Behav. 2006;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Phillips-Conroy JE, Whitten PL. Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am. J. Primatol. 2005;67:101–119. doi: 10.1002/ajp.20172. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Copeland KC, Reyes P, Kuehl TJ. Pubertal endocrinology of yellow baboon (Papio cynocephalus): plasma testosterone, testis size, body weight, and crown-rump length in males. Am. J. Primatol. 1986;11:263–270. doi: 10.1002/ajp.1350110308. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Goldzieher JW. Plasma androgens during early pregnancy in the baboons (Papio cynocephalus) Fertil. Steril. 1983;39:553–559. doi: 10.1016/s0015-0282(16)46950-5. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum. Reprod. 1998;13:460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- Cohen PG. Obesity in men: the hypogonadal-estrogen receptor relationship and its effect on glucose homeostasis. Med. Hypotheses. 2008;70:358–360. doi: 10.1016/j.mehy.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Ruback RB, Frady RL, Hopper CH, Sgoutas DS. Saliva testosterone and criminal violence among women. Pers. Indiv. Differ. 1988;9:269–275. [Google Scholar]
- Dantzer B, McAdam AG, Palme R, Boutin S, Boonstra R. How does diet affect fecal steroid hormone metabolite concentrations? An experimental examination in red squirrels. Gen. Comp. Endocrinol. 2011a;174:124–131. doi: 10.1016/j.ygcen.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Dantzer B, McAdam AG, Palme R, Humphries MM, Boutin S, Boonstra R. Maternal androgens and behaviour in free-ranging North American red squirrels. Anim. Behav. 2011b;81:469–479. [Google Scholar]
- Davis SR, Mccloud P, Strauss BJG, Burger H. Testosterone enhances estradiols effects on postmenopausal bone density and sexuality. Maturitas. 1995;21:227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- Dittami J, Katina S, Möstl E, Eriksson J, Machatschke IH, Hohmann G. Urinary androgens and cortisol metabolites in field-sampled bonobos (Pan paniscus) Gen. Comp. Endocrinol. 2008;155:552–557. doi: 10.1016/j.ygcen.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Fears TR, McMahon RP, Friedman LA, Patterson BH, Greenhut SF. Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids. 2002;67:151–158. doi: 10.1016/s0039-128x(01)00147-7. [DOI] [PubMed] [Google Scholar]
- Fortman JD, Herring JM, Miller JB, Hess DL, Verhage HG, Fazleabas AT. Chorionic-gonadotropin, estradiol, and progesterone levels in baboons (Papio anubis) during early pregnancy and spontaneous abortion. Biol. Reprod. 1993;49:737–742. doi: 10.1095/biolreprod49.4.737. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Khan MZ, Couret J, Yu JC, Endres CS, Lynch JW, Ogola P, Fox EA, Alberts SC, Wango EO. Coming of age: steroid hormones of wild immature baboons (Papio cynocephalus) Am. J. Primatol. 2005;67:83–100. doi: 10.1002/ajp.20171. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Onyango PO, Alberts SC, Altmann J. Endocrinology of year-round reproduction in a highly seasonal habitat: environmental variability in testosterone and glucocorticoids in baboon males. Am. J. Phys. Anthropol. 2011;144:169–176. doi: 10.1002/ajpa.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm. Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Gill SA, Alfson ED, Hau M. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis) Proc. R. Soc. B. 2007;274:2187–2194. doi: 10.1098/rspb.2007.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg P, Johansson AG, Ljunghall S. Decreased estradiol levels and free androgen index and elevated sex hormone-binding globulin levels in male idiopathic osteoporosis. Calcif. Tissue Int. 1999;64:209–213. doi: 10.1007/s002239900604. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Frank LG, Pavgi S, Licht P. Hormonal correlates of masculinization in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J. Reprod. Fertil. 1992;95:451–462. doi: 10.1530/jrf.0.0950451. [DOI] [PubMed] [Google Scholar]
- Goymann W. Noninvasive monitoring of hormones in bird droppings - Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N.Y. Acad. Sci. 2005;1046:35–53. doi: 10.1196/annals.1343.005. [DOI] [PubMed] [Google Scholar]
- Goymann W. On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol. Evol. 2012;3:757–765. [Google Scholar]
- Goymann W, Möstl E, Gwinner E. Non-invasive methods to measure androgen metabolites in excrements of European stonechats, Saxicola torquata rubicola. Gen. Comp. Endocrinol. 2002;129:80–87. doi: 10.1016/s0016-6480(02)00520-8. [DOI] [PubMed] [Google Scholar]
- Hauser B, Deschner T, Boesch C. Development of a liquid chromatography-tandem mass spectometry method for the determination of 23 endogenous steroids in small quantities of primate urine. J. Chromatogr. B. 2008;862:100–112. doi: 10.1016/j.jchromb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Hauser B, Mugisha L, Preis A, Deschner T. LC-MS analysis of androgen metabolites in serum and urine from east African chimpanzees (Pan troglodytes schweinfurthii) Gen. Comp. Endocrinol. 2011;170:92–98. doi: 10.1016/j.ygcen.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Palme R, Ganswindt A. Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am. J. Primatol. 2006;68:257–273. doi: 10.1002/ajp.20222. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Kotrschal K, Möstl E. Synthesis of measuring steroid metabolites in goose feces. Ann. N.Y. Acad. Sci. 2005;1046:138–153. doi: 10.1196/annals.1343.011. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Spreitzer K, Lepschy M, Kotrschal K, Möstl E. Excreted corticosterone metabolites differ between two galliform species, Japanese quail and chicken, between sexes and between urine and faecal parts of droppings. J. Ornithol. 2012;153:1179–1188. [Google Scholar]
- Hodges JK, Tarara R, Wangula C. Circulating steroids and the relationship between ovarian and placental secretion during early and mid pregnancy in the baboon. Am. J. Primatol. 1984;7:357–366. doi: 10.1002/ajp.1350070404. [DOI] [PubMed] [Google Scholar]
- Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and the treatment of testosterone deficiency. J. Sex Med. 2012;9:1681–1696. doi: 10.1111/j.1743-6109.2012.02726.x. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen. Comp. Endocrinol. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, Riggs BL. Estrogens and bone health in men. Calcif. Tissue Int. 2001;69:189–192. doi: 10.1007/s00223-001-1044-8. [DOI] [PubMed] [Google Scholar]
- Klein KO, Martha PM, Blizzard RM, Herbst T, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. 2. Estrogen levels as determined by an ultrasensitive bioassay. J. Clin. Endocrinol. Metab. 1996;81:3203–3207. doi: 10.1210/jcem.81.9.8784070. [DOI] [PubMed] [Google Scholar]
- Kuehl TJ, Kang IS, Silerkhodr TM. Pregnancy and early reproductive failure in the baboon. Am. J. Primatol. 1992;28:41–48. doi: 10.1002/ajp.1350280104. [DOI] [PubMed] [Google Scholar]
- Leach RB, Maddock WO, Tokuyama I, Paulsen CA. Clinical studies of testicular hormone production. Recent Prog. Horm. Res. 1956;12:377–403. [PubMed] [Google Scholar]
- Longcope C, Widrich W, Sawin CT. Secretion of estrone and estradiol-17 by human testis. Steroids. 1972;20:439–448. doi: 10.1016/0039-128x(72)90042-6. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Khan MZ, Altmann J, Njahira MN, Rubenstein N. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen. Comp. Endocrinol. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Möhle U, Heistermann M, Palme R, Hodges JK. Characterization of urinary and fecal metabolites of testosterone and their measurement for assessing gonadal endocrine function in male nonhuman primates. Gen. Comp. Endocrinol. 2002;129:135–145. doi: 10.1016/s0016-6480(02)00525-7. [DOI] [PubMed] [Google Scholar]
- Möstl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann. N.Y. Acad. Sci. 2005;1046:17–34. doi: 10.1196/annals.1343.004. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Bulun SE. Estrogen production and action. J. Am. Acad. Dermatol. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- Oettel M. Is there a role for estrogens in the maintenance of men’s health? Aging Male. 2002;5:248–257. [PubMed] [Google Scholar]
- Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl Stress hormones in mammals and birds. Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Annals NY Acad. Sci. 2005;1040:162–171. doi: 10.1196/annals.1327.021. [DOI] [PubMed] [Google Scholar]
- Preis A, Mugisha L, Hauser B, Weltring A, Deschner T. Androgen and androgen metabolite levels in serum and urine of East African chimpanzees (Pan troglodytes schweinfurthii): Comparison of EIA and LC-MS analyses. Gen. Comp. Endocrinol. 2011;174:335–343. doi: 10.1016/j.ygcen.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Raisz LG, Wiita B, Artis A, Bowen A, Schwartz S, Trahiotis M, Shoukri K, Smith J. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J. Clin. Endocrinol. Metab. 1996;81:37–43. doi: 10.1210/jcem.81.1.8550780. [DOI] [PubMed] [Google Scholar]
- Rosvall KA. Life history trade-offs and behavioral sensitivity to testosterone: an experimental test when female aggression and maternal care co-occur. PloS One. 2013;8:1–9. doi: 10.1371/journal.pone.0054120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. The role of androgen in the maintenance of sexual functioning in oophorectomized women. Psychosom. Med. 1987;49:397–409. doi: 10.1097/00006842-198707000-00009. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA, Jones KP, Daugherty CA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. New Engl. J. Med. 2000;343:682–688. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- Staub NL, DeBeer M. The role of androgens in female vertebrates. Gen. Comp. Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. [DOI] [PubMed] [Google Scholar]
- Tomaszewski M, Charchar FJ, Maric C, Kuzniewicz R, Gola M, Grzeszczak W, Samani NJ, Zukowska-Szczechowska E. Association between lipid profile and circulating concentrations of estrogens in young men. Atherosclerosis. 2009;203:257–262. doi: 10.1016/j.atherosclerosis.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann. N.Y. Acad. Sci. 2005;1046:54–74. doi: 10.1196/annals.1343.006. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Möstl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. OIKOS. 2005;108:643–647. [Google Scholar]
- von Engelhardt N, Kappeler PM, Heistermann M. Androgen levels and female social dominance in Lemur catta. Proc. Biol. Sci. 2000;267:1533–1539. doi: 10.1098/rspb.2000.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SW, Wolf RC, Meyer RK, Robinson JA. Estrogens in the utero-ovarian, uterine and peripheral plasma in pregnant rhesus monkeys. Biol. Reprod. 1979;20:606–610. doi: 10.1095/biolreprod20.3.606. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Monfort SL, Southers J, Wildt DE. Excretion rates and metabolites of estradiol and progesterone in baboon (Papio cynocephalus) feces. J. Reprod. Fertil. 1994;101:213–220. doi: 10.1530/jrf.0.1010213. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Brockman DK, Stavisky RC. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Yearb. Phys. Anthropol. 1998;41:1–23. doi: 10.1002/(sici)1096-8644(1998)107:27+<1::aid-ajpa2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Moore MC. Female territorial aggression and steroid hormones in mountain spiny lizards. Anim. Behav. 1999;57:1083–1089. doi: 10.1006/anbe.1998.1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


