Abstract
Epidemiologic studies suggest a reduced risk of breast cancer among women who use aspirin. A plausible mechanism is through aspirin’s effect on estrogens, possibly mediated through interference with estrogen synthesis via reduction in inflammation, which is increased in adipose tissues including breast. In a randomized placebo-controlled trial, we evaluated the effects of 6-months administration of 325 mg/day aspirin on serum estrogens (estradiol, estrone, free estradiol, bioavailable estradiol) and sex hormone binding globulin [SHBG] in 144 healthy postmenopausal women. Eligible participants, recruited 2005 - 2007, were not taking nonsteroidal anti-inflammatory medication including aspirin > 2 times/week or menopausal hormone therapy, and had a BI-RAD mammographic density classification of 2, 3, or 4. The intervention effects (intent-to-treat) were evaluated by differences in the geometric mean outcome changes at 6 months between aspirin and placebo groups using generalized estimating equations (GEE). Participants were a mean 59.4 (SD 5.4) years, with mean body mass index (BMI) of 26.4 (SD) 5.4 kg/m2. Between baseline and 6-months, none of the serum estrogens or SHBG changed substantially and there were no differences between groups. Stratifying by BMI did not change results. In conclusion, a single daily administration of 325 mg of aspirin for 6 months had no effect on serum estrogens or SHBG in postmenopausal women. Larger doses or longer duration of aspirin administration may be needed to affect circulating estrogens. Alternately, if aspirin influences breast cancer risk in postmenopausal women, it may do so through direct breast tissue effects, or through pathways other than estrogens.
Keywords: aspirin, NSAIDs, estrogen, estradiol, estrone, body mass index, breast cancer
Introduction
Inflammation may play a role in breast cancer etiology; blockade of this process has strong potential for cancer chemoprevention. Animal experimental studies have consistently shown that nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin, inhibit mammary carcinogenesis (1-5). A meta-analysis which included 38 epidemiologic studies with 2,788,715 women, found that aspirin use was associated with a 13 percent reduced risk of breast cancer (relative risk 0.87, 95% confidence interval (CI) 0.82-0.92) (6), although a large clinical trial found no effect of alternate day low-dose aspirin on breast cancer risk (7, 8).
NSAIDs may exert their effects by a number of mechanisms. Aspirin and ibuprofen NSAIDs inhibit both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), catalytic enzymes involved in prostaglandin synthesis, by irreversible acetylation and competitive inhibition (9). COX-2 is expressed in breast cancer, is associated with poor prognosis in breast cancer (10), and can up-regulate aromatase expression, leading to increased estradiol levels (11-13); NSAIDs including aspirin may thus lower circulating estradiol levels by inhibiting COX-2 (14). Supporting this, cross-sectional studies in postmenopausal women demonstrated that NSAID use is associated with lower circulating estrogen concentrations (12, 15).
NSAIDs may also affect neoplastic growth and development by reducing cell proliferation, increasing epithelial apoptosis, decreasing infiltration by inflammatory cells and subsequent diminished release of destructive enzymes and reactive oxygen species, and modulating tumor immunogenicity (16).Excess adipose tissue, including in the breast, produces excessive inflammation-related biomarkers, which in turn stimulate adipose-induced production of estrogens (17). Therefore, assessing the influence of adiposity on aspirin’s effects on serum estrogens is important.
Given the potential anti-carcinogenic properties of aspirin, and the consistent associations of increased circulating estrogen concentrations with breast cancer risk, we tested the effect of 6-months administration of 325 mg/day aspirin vs. placebo on estrogens (estradiol, estrone, free estradiol, bioavailable estradiol) and sex hormone binding globulin (the latter in order to calculate free and bioavailable estradiol fractions, [SHBG]), in postmenopausal women. We chose aspirin rather than other NSAIDs because of the low risk for cardiotoxic effects of aspirin compared with other NSAIDs. We chose the particular dose of aspirin because many studies have suggested that lower doses commonly used for cardio-protection (i.e., 100 mg/day or less) are not sufficient for reducing breast cancer risk (6), and because higher doses of aspirin are associated with increased risk for adverse events (18, 19). This study was ancillary to a trial (20) that tested aspirin’s effect on mammographic breast density in women with increased mammographic density (American College of Radiology Breast Imaging Reporting and Data System (BIRAD) 2, 3, or 4) (21). Percent density decreased from 17.6% to 16.8% in women randomized to aspirin and from 19.2% to 18.0% in women randomized to placebo (p=0.84 comparing change over time between trial arms).
Materials and Methods
Recruitment and Eligibility
In an ancillary study to a randomized placebo-controlled double-blind clinical trial (20) (ClinicalTrials.gov Identifier: NCT00470561), we evaluated the effects of 6-months of daily aspirin (325 mg) on estrogens and SHBG. Detailed methods of the parent trial have been published previously (20). Women were recruited between 2005–2007 in western Washington State through a variety of mechanisms including mass mailings and media placements (Figure 1). Women were screened for eligibility through medical history, review of prior mammography reports for radiologist-determined BIRADS mammogram density category, and physical exam.
Figure 1.
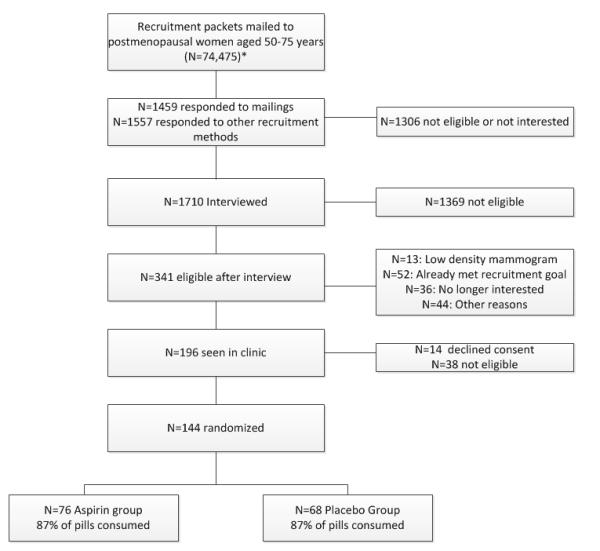
Participant Screening and Randomization
Eligible women were postmenopausal (no menstrual periods for 24 months, follicle-stimulating activity > 50 IU/L for women without a uterus), aged 50-75 years, not using menopausal hormone therapy, oral contraceptives, or selective estrogen response modulators (SERMS) for the previous six months, with BI-RADS mammogram density category on prior mammograms through their own providers of 2 (scattered fibroglandular), 3 (heterogeneously dense tissue), or 4 (extremely dense tissue present) (21), healthy with no significant co-morbidities including any cancer, not currently using any NSAIDs two or more times per week and willing to avoid NSAID use during the 6-month trial duration, not using any other anticoagulant medication, and with no contra-indications to use of aspirin. Screening blood tests included complete blood count (white blood cells, hematocrit, platelets), prothrombin time (PT), and partial thromboplastin time (PTT). Women with anemia (hematocrit < 35); abnormal bleeding tests; history of bleeding disorders, renal disease, or hemorrhagic stroke; current uncontrolled hypertension; planning extensive weight loss in next 6 months; consuming >2 alcohol drinks per day, or with current significant mental illness or alcohol or drug abuse, were also excluded. Potentially eligible women completed a 3-week daily placebo run-in trial, with taking > 80% of placebo run-in capsules (by pill count) required. No woman was excluded solely for noncompliance with placebo run-in.
Informed consent was obtained following the requirements of the Fred Hutchinson Cancer Research Center Institutional Review Board. An independent Data and Safety Monitoring Committee oversaw study protocol and procedures, and reviewed trial data biannually.
Covariate and Clinical Measures
Baseline and 6-month measures included anthropometrics (height, weight, body mass index (BMI, kg/m2), waist and hip circumferences), resting pulse and blood pressure, and clinical exam including breast exam and bilateral screening mammograms.
Randomization Assignment
A total of 144 women were assigned by simple randomization into one of two arms: 325 mg/day aspirin (N=76), or an identical-appearing placebo capsule/day (N=68). Aspirin and placebo capsules (not enteric coated) were prepared and blind-packaged by the University of Washington (UW) Pharmacy Services. Participants attended an enrollment visit where they were given their 6-month supply of study medication and instructed to take one capsule daily, and informed of potential complications such as bleeding and gastric upset, and provided with a bottle of acetaminophen pills for use for pain/fever during the trial. All study investigators, staff, and participants were blinded to study arm, with the exception of the study statisticians and the UW pharmacy services. Compliance to the aspirin intervention was assessed by pill count at the 6-month end-of-study clinic visit. Participants were called each month to inquire about safety issues and potential adverse effects (i.e., bleeding episodes, major illnesses, or hospitalizations), and problems with taking study medications.
Blood Collection and Processing
At baseline and 6-months, participants provided a 12-hour fasting blood sample, which was processed within 1 hour of collection and stored at −70°C. Samples had not been thawed prior to analysis.
Estrogen and SHBG Measurements
Laboratory assays were performed at the Reproductive Endocrine Research Laboratory (University of Southern California). Estrone and estradiol were quantified by radioimmunoassays after organic solvent extraction and Celite column partition chromatography (22, 23) SHBG was quantified via chemiluminescent immunometric assay using the Immulite Analyzer (Siemens Medical Solutions Diagnostics, Malvern, PA). Total estradiol includes SHBG-bound, albumin-bound and free hormone; bioavailable estradiol includes albumin-bound and free hormone; and free estradiol is free or unbound hormone. Free and bioavailable (non-SHBG-bound) estradiol was calculated using the measured total estradiol levels, SHBG concentrations, and an average assumed concentration for albumin (24, 25). This method has been found to have high validity, with Pearson’s correlation coefficients ≥ 0.80 between free estradiol values calculated using this method and the values measured by dialysis-based methods (26). The inter-assay and intra-assay coefficients of variation were 8.2% and 7.7% for estrone, 17.9% and 15.0% for estradiol, and 6.6% and 5.5% for SHBG, respectively.
Statistical Analysis
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence or retention status (i.e., intent-to-treat), and all participants’ data were included in the primary analyses. The main study outcomes were estrone, estradiol, free estradiol, bioavailable estradiol, and SHBG. Geometric means were used for outcome variables because logarithmically transformed data were less skewed. The geometric mean for skewed data is generally close to the median, which is less sensitive to outliers, than the sample mean. The intervention effects were evaluated by the differences in the geometric mean changes in analytes at 6 months between the aspirin and placebo groups using the generalized estimating equations (GEE) in order to account for the longitudinal nature of the data.
We also explored differential intervention effects by baseline BMI (categorized by WHO criteria <25 kg/m2, 25.0-29.9 kg/m2, and >30.0 kg/m2) (27) because of the associations of obesity with estrogens in postmenopausal women (28). This effect-modification analysis was planned a priori.
All statistical tests were two-sided. Statistical analyses were performed using SAS software (Version 9.1; SAS Institute Inc, Cary, NC). Endpoint data were available for all 144 women. Based on the means and SDs of the study data, with 13% non-compliance, we would have 80% power to detect an absolute difference of 11% for estrone, 15% for estradiol, 17% for free estradiol, 17% for bioavailable estradiol, and 14% for SHBG, between aspirin and placebo arms at the end of the study.
Results
All 144 study participants returned for end-of-study measurements including blood draw and covariates. Women were a mean (SD) 59.4 (5.4) years of age, and had a mean BMI of 26.4(5.4) kg/m2. Most were non-Hispanic white, and more than 70 percent had a college degree or higher (Table 1). Baseline characteristics did not differ significantly between aspirin and placebo arms (Table 1), including age, BMI, weight, education, baseline clinical BIRAD mammogram density classification, history of previous breast biopsy, and reproductive history. Concentrations of estrogens and SHBG did not differ by study arm at baseline
Table 1.
Baseline Characteristics by Study Arm.
| Aspirin N=76 |
Placebo N=68 |
All Participants N=144 |
||
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | P (t-test) | Mean (SD) | |
|
| ||||
| Age | 59.9 (5.5) | 59.1 (5.4) | 0.38 | 59.4 (5.4) |
|
| ||||
| Age at Menopause (years) | 49.6 (6.4) | 50.1 (5.4) | 0.51 | 49.9 (5.9) |
|
| ||||
| Years Since Menopause | 9.7 (8.0) | 8.8 (7.7) | 0.24 | 9.6 (8.1) |
|
| ||||
| BMI (kg/m2) | 26.8 (6.1) | 25.9 (4.1) | 0.28 | 26.4 (5.4) |
|
| ||||
|
Number of Pregnancies >6
Months |
1.68 (1.84) | 1.65 (1.30) | 0.53 | 1.66 (1.57) |
|
| ||||
| N (%) | N(%) | P (χ2 test) | N(%) | |
|
| ||||
| Race/Ethnicity | ||||
| Non-Hispanic White | 70 (92.1) | 65 (95.6) | 0.21 | 135 (93.7) |
| Other | 6 (7.9) | 3 (4.4) | 9 (6.3) | |
|
| ||||
| Education | ||||
| High School or Less | 2 (2.6) | 2 (2.9) | 0.87 | 4 (2.8) |
| Some College/Vocational | 17 (22.4) | 16 (23.5) | 33 (22.9) | |
| College Degree | 28 (36.8) | 23 (33.8) | 60 (41.7) | |
| Post-Graduate | 29 (38.2) | 27 (39.7) | 56 (38.9) | |
|
| ||||
|
Family History of Breast
Cancer |
||||
| Yes | 18 (23.6) | 18 (26.9) | 0.41 | 36 (25.%) |
| Missing | 1 (1.3%) | 1 (1.5%) | 2 (1.4) | |
|
| ||||
|
BI-RADS mammogram
density |
||||
| Class 2 | 7 (9.2) | 7 (10.3) | 14 (9.7) | |
| Class 3 | 40 (52.6) | 39 (57.4) | 0.74 | 79 (54.9) |
| Class 4 | 27 (35.5) | 22 (32.3) | 49 (34.0) | |
| Missing | 2 (2.6) | 0 | 2 (1.4) | |
|
| ||||
| Previous breast biopsy | ||||
| Yes | 16 (21.1) | 20 (29.4) | 0.23 | 36 (25.0) |
| Don’t Know | 2 | 0 | 2 (1.4) | |
|
| ||||
| Bilateral Oophorectomy | ||||
| Yes | 7 (9.2) | 7 (10.3) | 0.35 | 14 (9.7) |
| Don’t Know | 1 | 0 | 1 (0.7) | |
|
| ||||
| Hysterectomy | ||||
| Yes | 16 (21.1) | 12 (17.7) | 0.61 | 28 (19.4%) |
|
| ||||
|
Past Menopausal Estrogen
Use |
||||
| Yes | 46 (60.5) | 38 (55.9) | 0.64 | 84 (58.3) |
| Missing | 0 | 1 | 1 (0.7) | |
|
| ||||
|
Geometric Mean
(95% CI) |
Geometric Mean
(95% CI) |
Geometric Mean
(95% CI) |
||
|
| ||||
| Estrone (pg/mL) | 31.1 (29.0, 33.3) | 33.4 (30.8, 36.2) | 0.20 | 32.2 (30.5, 33.9) |
| Estradiol (pg/mL) | 7.2 (6.5, 8.1) | 7.6 (6.8, 8.4) | 0.56 | 7.4 (0.69, 8.0) |
| Free Estradiol (pg/mL) | 0.177 (0.16, 0.20) | 0.180 (0.16, 0.20) | 0.56 | 0.178 (0.164, 0.194) |
|
Bioavailable Estradiol
(pg/mL) |
4.53 (4.01, 5.11) | 4.56 (4.04 - 5.15) | 0.94 | 4.54 (4.17, 4.95) |
| SHBG (nmol/L) | 45.8 (41.5, 50.7) | 51.1 (46.3, 56.3) | 0.13 | 48.2 (45.0, 51.8) |
Women randomized to aspirin and placebo were similarly adherent to study medications (87% pills taken in aspirin; 87% in placebo). A small number of intervention (N=5) and placebo (N=7) reported using non-aspirin NSAIDs during the 6 months of the trial.
There were no significant differences between changes in concentrations of estradiol (total, free or bioavailable), estrone or SHBG between arms, comparing baseline to 6 months (Table 2). Stratifying intervention arms by baseline BMI (<25; 25-30; and >30 kg/m2), had no effect on the results (Table 3). Removing extreme values (estrone >200pg/mL; estradiol >30pg/mL; SHBG >=100nmol/L) did not significantly alter the results (data not shown), and therefore results include data for all participants.
Table 2.
Estrone, Estradiol, and SHBG Concentrations (Geometric Means, 95% Confidence Intervals) at Baseline and 6 Month Follow-up
| Aspirin (N=76) | Placebo (N=68) |
P- Value* |
|||||
|---|---|---|---|---|---|---|---|
| Baseline (95% CI) |
Follow-up (95% CI) |
ΔA (%) | Baseline (95% CI) |
Follow-up (95% CI) |
ΔP (%) | ||
| Estrone (pg/mL) | 31.1 (29.0, 33.3) |
31.5 (29.4, 33.9) |
0.4 (1.3) | 33.4 (30.8, 36.2) |
33.3 (30.8, 36.1) |
−0.1 (−0.3) | 0.75 |
| Estradiol (pg/mL) | 7.25 (6.49, 8.09) |
7.42 (6.72, 8.20) |
0.17 (2.3) | 7.58 (6.84, 8.40) |
7.72 (6.92, 8.62) |
0.14 (1.8) | 0.94 |
| Free estradiol (pg/mL) | 0.177 (0.156, 0.200) |
0.180 (0.161, 0.202) |
0.003 (1.7) | 0.180 (0.159, 0.203) |
0.182 (0.160, 0.207) |
0.002 (1.1) |
0.96 |
| Bioavailable estradiol (pg/mL) |
4.53 (4.01, 5.11) |
4.58 (4.08, 5.13) |
0.05 (1.1) | 4.56 (404, 5.15) |
4.62 (4.08, 5.24) |
−0.06 (− 1.3) |
0.97 |
| SHBG (nmol/L) | 45.9 (41.5, 50.7) |
47.3 (42.7, 52.4) |
1.4 (3.1) | 51.1 (46.3, 56.4) |
51.7 (46.9, 57.0) |
0.6 (1.2) | 0.46 |
ΔP: change in control (placebo) group at 6 month from baseline; ΔA: change in Aspirin group at 6 month from baseline.
P value: GEE model, comparing the change at 6 month follow-up from baseline between Placebo and Aspirin group
Table 3.
Estrone, Estradiol, and SHBG Concentrations (Geometric Means, 95% Confidence Intervals) at Baseline and 6 Month Follow-up, by Baseline BMI
| BMI <25.0 kg/m2 | |||||||
| ASPIRIN (N=37) | PLACEBO (N=36) | ||||||
| Baseline | 6 months | Change (%) | Baseline | 6 months | Change (%) | P | |
|
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
||||
| Estrone (pg/mL) |
28.2 (25.8, 30.9) | 27.4 (25.2, 29.8) | −0.9 (−3.1) | 29.8 (27.0, 33.0) | 29.9 (27.1, 32.9) | 0.0 (0.0) | 0.55 |
| Estradiol (pg/mL) |
6.0 (5.3, 6.8) | 6.2 (5.8, 6.8) | 0.2 (3.9) | 6.5 (5.6, 7.4) | 6.2 (5.7, 6.7) | −0.3 (−4.7) | 0.32 |
| Free estradiol (pg/mL) |
0.136 (0.119, 0.156) | 0.142 (0.128, 0.157) | 0.006 (4.0) | 0.144 (0.123, 0.169) | 0.135 (0.121, 0.151) | −0.009 (−5.9) | 0.26 |
| Bioavailable estradiol (pg/mL) |
3.50 (3.07, 3.99) | 3.61 (3.26, 3.99) | 0.11 (3.1) | 3.64 (3.10, 4.28) | 3.44 (3.08, 3.83) | −0.20 (−5.6) | 0.32 |
| SHBG (nmol/L) |
56.3 (50.6, 62.7) | 57.1 (50.8, 64.2) | 0.8 (1.4) | 60.4 (53.2, 68.6) | 62.2 (55.5, 69.8) | 1.8 (2.9) | 0.65 |
| BMI 25.0-29.99 kg/m2 | |||||||
| ASPIRIN (N=21) | PLACEBO (N=22) | ||||||
| Baseline | 6 months | Change (%) | Baseline | 6 months | Change (%) | P | |
|
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
||||
| Estrone (pg/mL) |
34.3 (29.4, 40.0) | 34.4 (29.5, 40.0) | 0.1 (0.3) | 35.9 (30.9, 41.7) | 36.5 (31.5, 42.2) | 0.6 (1.6) | 0.90 |
| Estradiol (pg/mL) |
8.1 (6.2, 10.5) | 7.4 (6.3, 8.6) | −0.7 (−9.2) | 8.4 (7.1, 9.9) | 9.6 (7.6, 12.2) | 1.2 (14.4) | 0.15 |
| Free estradiol (pg/mL) |
0.206 (0.156, 0.272) | 0.186 (0.154, 0.224) | −0.02 (−9.9) | 0.210 (0.178, 0.249) | 0.244 (0.192, 0.309) | 0.033 (15.9) | 0.13 |
| Bioavailable estradiol (pg/mL) |
5.26 (3.99, 6.93) | 4.72 (3.90, 5.70) | −0.54 (−10.3) | 5.35 (4.51, 6.35) | 6.19 (4.89, 7.85) | 0.85 (15.8) | 0.12 |
| SHBG (nmol/L) |
41.2 (33.9, 50.0) | 42.6 (35.1, 51.7) | 1.4 (3.4) | 44.7 (39.0, 51.2) | 43.0 (37.2, 49.7) | −1.6 (−3.7) | 0.12 |
| BMI ≥ 30 kg/m2 | |||||||
| ASPIRIN (N=17) | PLACEBO (N=10) | ||||||
| Baseline | 6 months | Change (%) | Baseline | 6 months | Change (%) | P | |
|
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
Geometric mean
(95% CI) |
||||
| Estrone (pg/mL) |
33.9 (30.3, 37.8) | 37.9 (33.7, 42.7) | 4.1 (12.0) | 42.4 (35.2, 51.1) | 40.4 (33.3, 49.0) | −2.0 (−4.7) | 0.22 |
| Estradiol (pg/mL) |
9.5 (8.1, 11.3) | 10.7 (8.0, 14.4) | 1.2 (12.5) | 10.7 (9.1, 12.7) | 10.7 (8.6, 13.4) | 0.0 (0.0) | 0.53 |
| Free estradiol (pg/mL) |
0.256 (0.213, 0.307) | 0.280 (0.210, 0.374) | 0.025 (9.6) | 0.284 (0.231, 0.348) | 0.279 (0.217, 0.359) | −0.005 (−1.7) | 0.56 |
| Bioavailable estradiol (pg/mL) |
6.50 (5.40, 7.83) | 7.14 (5.36, 9.51) | 0.63 (9.7) | 7.20 (5.86, 8.83) | 7.06 (5.51, 9.05) | −0.14 (−1.9) | 0.55 |
| SHBG (nmol/L) |
35.8 (29.5, 43.4) | 38.3 (31.1, 47.3) | 2.6 (7.2) | 37.5 (28.8, 49.0) | 39.8 (30.5, 52.0) | 2.3 (6.0) | 0.88 |
P value: GEE model, comparing the change at 6 month follow-up from baseline between Aspirin and Placebo group, stratified by baseline BMI
Discussion
We found no effect of 325 mg/day aspirin administered over 6 months on estrone, estradiol, free estradiol, bioavailable estradiol, or SHBG in a group of postmenopausal women, despite outstanding adherence and retention to the trial and sufficient statistical power to detect an absolute difference of 11% for estrone, 15% for estradiol, 17% for free estradiol, 17% for bioavailable estradiol, and 14% for SHBG, between aspirin and placebo arms at the end of the study.. This degree of change in serum estrogens is similar to that observed with weight loss in overweight/obese postmenopausal women.(29)
Stratifying by baseline BMI had no effect on the results. To our knowledge, no previous randomized controlled clinical trials have examined effects of NSAID use on estrogens in postmenopausal women. A cross-sectional study of 740 postmenopausal women not using menopausal hormone therapy found a statistically significant negative association between frequency of use of NSAIDS and concentrations of estradiol, free estradiol, and estrone sulfate (15). An earlier cross-sectional study of 260 postmenopausal women not using menopausal hormones found statistically significantly lower concentrations of estradiol in NSAID users compared with nonusers (12),
There are several plausible explanations for this lack of effect. First, if aspirin reduces risk for breast cancer, it may do so through a pathway other than reducing circulating estrogens. Second, the effect of NSAIDs on breast cancer risk may be dose-dependent (30), and it is possible that the single dose of 325 mg/day used in this study was insufficient to produce an effect. Third, 6-months’ administration may be an insufficient duration to change estrogens with this particular medication (31). Finally, NSAIDs may affect estrogen production and metabolism locally in the breast (32) which may not be reflected in serum.
Strengths of this report include the double-blind randomized design, the high quality of estrogen and SHBG assays, and the high degree of participant adherence and retention. Study limitations include the evaluation of a single dose of aspirin, the relatively short period of follow-up, and lack of tissue-specific outcomes.
In conclusion, use of aspirin for 6 months resulted in no change in serum estradiol, estrone, free estradiol, bioavailable estradiol, or SHBG in postmenopausal women. If aspirin is associated with reduced risk for breast cancer in postmenopausal women, it may do so through pathways other than change in circulating estrogens. Future studies should test different doses and durations of aspirin and other NSAID medications on circulating estrogens as well as direct breast tissue effects.
Acknowledgments
Funding: NIH U54CA116847; Pacific Ovarian Cancer Research Consortium (POCRC)/SPORE in Ovarian Cancer, (NIH/NCI P50 CA83636), Seattle, WA; Breast Cancer Research Foundation
Footnotes
ClinicalTrials.gov Identifier: NCT00470561
References
- 1.Abou-Issa H, Alshafie G, Harris R. COX-2 Blockade in Cancer Prevention and Therapy. Humana Press; Totowa, NJ: 2002. Chemoprevention of breast cancer by nonsteroidal anti-inflammatory drugs and selective COX-2 blockade in animals; pp. 95–8. [Google Scholar]
- 2.Alshafie G, Harris R, Robertson F, Parrett M, Ross M, Abou-Issa H. Comparative chemopreventive activity of ibuprofen and N-(4-hydroxyphenyl) retinamide against the development and growth of rat mammary adenocarcinomas. Anticancer Research. 1999;19:3031–6. [PubMed] [Google Scholar]
- 3.Joarder F, Abou-Issa H, Robertson F, Parrett M, Alshafie G, Harris R. Growth arrest of DMBA-induced mammary carcinogenesis with ibuprofen treatment in Sprague-Dawley rats. Oncology Reports. 1997;4:1271–3. doi: 10.3892/or.4.6.1271. [DOI] [PubMed] [Google Scholar]
- 4.Lee I-M, Sesso HD, Paffenbarger RS. Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28:620–5. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 5.Steele V, Moon R, Lubet R. Preclinical efficacy evaluation of potential chemopreventive agents in animal carcinogenesis models: methods and results from the NCI Chemoprevention Drug Development Program. Journal of Cellular Biochemistry - Supplement. 1994;20:32–54. doi: 10.1002/jcb.240560905. [DOI] [PubMed] [Google Scholar]
- 6.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Journal of the National Cancer Institute. 2008;100:1439–47. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Smith-Warner SA, Collins LC, Rosner B, Willett WC, Hankinson SE. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and postmenopausal breast cancer incidence. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3468–77. doi: 10.1200/JCO.2012.42.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Annals of internal medicine. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taketo M. Cyclooxygenase-2 inhibitors in tumorigenesis (Part 1) Journal of the National Cancer Institute. 1998;90:1529–36. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 10.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–87. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemeier R, Quinn A, Parrett M, Joarder F, Harris R, Robertson F. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Letters. 1999;140:27–35. doi: 10.1016/s0304-3835(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 12.Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, et al. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:680–7. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- 13.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer discovery. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. The Journal of clinical endocrinology and metabolism. 2005;90:2563–70. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 15.Gates MA, Tworoger SS, Eliassen AH, Missmer SA, Hankinson SE. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:1033–41. doi: 10.1158/1055-9965.EPI-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris R, Namboodiri K, Farrar W. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–5. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6074–83. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron J, Cole B, Sandler R, Haile R, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. The New England journal of medicine. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 19.Sandler R, Halabi S, Baron J, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer.[comment][erratum appears in N Engl J Med. 2003 May 8;348(19):1939]. New England Journal of Medicine. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 20.McTiernan A, Wang CY, Sorensen B, Xiao L, Buist DS, Aiello Bowles EJ, et al. No effect of aspirin on mammographic density in a randomized controlled clinical trial. Cancer Epidemiol Biomarkers Prev. 2009;18:1524–30. doi: 10.1158/1055-9965.EPI-08-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Radiology . Breast Imaging -- Reporting and Data System (BI-RADS) 3rd ed Reston, VA: 1998. [Google Scholar]
- 22.Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakmura RM, Coulson AH, et al. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R, editors. Vasectomy: Immunologic and Pathophysiologic Effect in Animals and Man. Academic Press; New York: 1979. p. 165. [Google Scholar]
- 23.Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, et al. Aromatase and breast cancer susceptibility. Endocrine-related cancer. 1999;6:165–73. doi: 10.1677/erc.0.0060165. [DOI] [PubMed] [Google Scholar]
- 24.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of clinical endocrinology and metabolism. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–71. [PubMed] [Google Scholar]
- 27.Physical status: the use and interpretation of anthropometry. (World Health Organization technical report series).Report of a WHO Expert Committee. 1995;854:1–452. [PubMed] [Google Scholar]
- 28.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring, Md) 2006;14:1662–77. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 29.Campbell KL, Foster-Schubert KE, Alfano CM, Wang CC, Wang CY, Duggan CR, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2314–26. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris R, Chlebowski R, Jackson R, Frid D, Ascenseo J, Anderson G, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096–101. [PubMed] [Google Scholar]
- 31.Terry M, Gammon M, Zhang F, Tawfik H, Teitelbaum S, Britton J, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA : the journal of the American Medical Association. 2004;291:2433–40. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 32.Hudis CA, Subbaramaiah K, Morris PG, Dannenberg AJ. Breast cancer risk reduction: no pain, no gain? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3436–8. doi: 10.1200/JCO.2012.44.8597. [DOI] [PubMed] [Google Scholar]


