Abstract
Background
Dysregulated insulin signaling is thought to contribute to cancer risk.
Methods
To determine if insulin-related serum factors are associated with colon polyps, 126 asymptomatic men (48–65yr) were recruited at colonoscopy. Blood was collected. Odds ratios were determined using polytomous logistic regression for polyp number and type.
Results
Males with serum C-peptide concentration >3.3 ng/ml were 3.8 times more likely to have an adenoma relative to no polyp than those with C-peptide ≤1.8 ng/ml. As C-peptide tertile increased, an individual was 2 times more likely to have an adenoma (p=0.01) than no polyp. There were no associations between insulin-like growth factor or its binding proteins with polyp number or type. Males with soluble receptor for advanced glycation end products (sRAGE) concentration >120.4 pg/ml were 0.25 times less likely to have ≥3 polyps relative to no polyps compared to males with sRAGE ≤94.5 pg/ml. For each increase in sRAGE tertile, a man was 0.5 times less likely to have ≥3 polyps than no polyps (p=0.03). Compared to males with a serum vascular endothelial growth factor (VEGF) concentration ≤104.7 pg/ml, males with a serum VEGF concentration >184.2 pg/ml were 3.4 times more likely to have ≥3 polyps relative to no polyps. As the VEGF tertile increased, a man was 1.9 times more likely to have ≥3 polyps than no polyps (p=0.049).
Conclusions
Serum concentrations of C-peptide, sRAGE, and VEGF may indicate which men could benefit most from colonoscopy.
Impact
Identification of biomarkers could reduce medical costs through the elimination of colonoscopies on low-risk individuals.
Keywords: colon, insulin, polyp, prevention, males
INTRODUCTION
Obesity increases the risk of colorectal cancer (1). Furthermore, there is an association between obesity and polyp formation (2). However, the mechanism(s) for this link are unknown. In the body, insulin is responsible for regulating glucose metabolism by stimulating glucose uptake by cells. Obese individuals commonly have high concentrations of insulin and its associated factors in their blood. These high insulin levels and resulting changes in glucose metabolism may be a contributing link between obesity and colorectal polyp/cancer formation (3).
The role of insulin in the development of colorectal adenomas is under active investigation. Insulin is responsible for regulating glucose metabolism by facilitating glucose uptake by cells including hepatocytes, adipocytes, and myocytes. An increase in glucose levels in the blood normally leads to an increased secretion of insulin. In healthy individuals, secreted insulin signals cells to remove the glucose from the blood. However, obese individuals have an increased risk for insulin resistance wherein their cells do not efficiently remove excess glucose from the blood. In part, this is because adipose tissue releases large amounts of inflammatory cytokines which further impair insulin’s action (4). The hyperglycemia then maintains the inflammatory state through a variety of mechanisms and provides a ready source of energy for rapidly dividing neoplastic cells (5, 6). Normoglycemia in a mouse model of diabetes has been achieved through neutralization of vascular endothelial growth factor (VEGF) linking high VEGF levels with insulin resistance (7). Additionally, pre/post bariatric surgery changes in a novel biomarker, soluble receptor for advanced glycation end products (sRAGE), showed a significant negative correlation with well-known measures of insulin resistance. After the surgery, insulin resistance decreased and sRAGE levels increased (8). Thus, insulin-related serum factors may be indicative of the failure to regulate blood glucose and the resulting increased inflammation in obese individuals at a heightened risk for colorectal cancer.
Because the insulin-signaling pathway is associated with cancer (9, 10), molecules involved in these pathways were selected as a focus for this analysis. Evidence that serum factors involved in glucose metabolism are related to colorectal polyps in otherwise healthy adult males is presented. Colonoscopies are costly and undesirable to some adults. Serum markers that could predict which individuals are more likely to have polyps, and thus benefit most from colonoscopy, are desired.
MATERIALS AND METHODS
Study Population
Between August 2009 and February 2011, healthy males ranging from 48–65 years of age were recruited from either Tri-County Gastroenterology Clinic (Macomb, MI) or Michigan State University Clinic (East Lansing, MI) at the time of colonoscopy. These individuals were undergoing routine colonoscopies and were asymptomatic. Exclusion criteria included: 1) cancer within the past two years, 2) surgery within the past two years, 3) inflammatory bowel diseases (i.e. Crohn’s, ulcerative colitis), 4) autoimmune disorders (i.e. Rheumatoid arthritis, HIV/AIDS, Lupus), 5) type I or type II diabetes, 6) chronic liver or kidney disease, 7) history of heart failure, 8) current immunosuppressant usage (i.e. Prednisone), 9) asthma, chronic obstructive pulmonary disease or other lung problems, 10) familial adenomatous polyposis, and 11) Lynch syndrome or hereditary non-polyposis colorectal cancer. 126 men (> 96% Caucasian) participated in the study (Table 1). All enrolled individuals completed the study, and no records were maintained as to the number of individuals approached but not enrolled. At the time of enrollment, immediately prior to routine colonoscopy, written informed consent was obtained and clinical metadata on subject co-morbidities, current medications, and family history were collected. Anthropometric measures were taken to calculate BMI and to record waist circumference (11). Also at the time of enrollment, venous blood was drawn, and serum was isolated by standard procedures and stored at −80°C. The study was approved by the institutional review board of Michigan State University (IRB# 08-786).
Table 1.
Participant† Characteristics
| No Polyps n=69 | Any Polyps n=57 | |
|---|---|---|
|
|
||
| Age‡ (years) | 57 (48–65) | 57 (50–65) |
| Smokers* (%) | 26.2 | 37.2 |
| BMI‡ (kg/m2) | 28.4 (21.7–39.1) | 31.3 (19.2–45.6) |
| Waist circumference‡ (inches) | 40.1 (30.0–55.0) | 42.9 (29.8–57.5) |
| sRAGE‡ (pg/ml) | 132.1 (50.2–466.0) | 119.2 (61.7–595.0) |
| C-peptide‡ (ng/ml) | 2.7 (0.7–9.9) | 3.2 (0.8–9.2) |
| VEGF‡* (pg/ml) | 180.7 (24.3–1371) | 176.1 (21.8–512) |
| IGF–1‡ (ng/ml) | 112.1 (38.0–223.9) | 105.9 (52.9–201.4) |
| IGFBP-3‡ (ng/ml) | 685.9 (542.3–957.8) | 691.3 (518.4–930.9) |
| IGFBP-7‡ (ng/ml) | 65.1 (29.6–117.0) | 69.9 (39.6–106.8) |
| IGF-1/IGFBP-3‡ | 0.17 (0.06–0.34) | 0.16 (0.08–0.38) |
All participants (n=126) were male, > 96% Caucasian
Reported as mean (range)
Does not include those with missing data
Colonoscopy Interpretation
Full colonoscopy was performed on each participant. A gastroenterologist (MSU-affiliated clinics, MI) categorized the location of each polyp during the colonoscopy. Board-certified pathologists assigned a polyp type to each specimen collected during colonoscopy (regional medical center pathology departments).
Serum Biomarker Analysis
C-peptide concentrations (ng/ml) were measured using an ELISA (Calbiotech, Spring Valley, CA, REF: CP1795) following the manufacturer’s instructions. IGF-1 was measured by ELISA following the manufacturer’s protocol (R&D Systems, DG100, Minneapolis, MN). Both ELISAs were read using the absorbance function on a Bio-Tek (Winooski, VT) plate reader. Insulin growth factor binding proteins (IGFBP) 1 through 7 were measured by multiplex kit per the manufacturer’s protocol (HIGFBP-53K, Millipore, Billerica, MA). Serum concentrations of the soluble vascular endothelial growth factor receptors (sVEGFR) including sVEGFR-1, sVEGFR-2, and sVEGFR-3, as well as the soluble receptor for advanced glycation end products (sRAGE), and the soluble endothelial growth factor receptor (sEGFR) were determined using a multiplex kit for soluble cytokine receptors (HSCR-32K, Millipore) as per the manufacturer’s instructions. Further, fibroblast growth factor (FGF)-2, endothelial growth factor (EGF), and vascular endothelial growth factor (VEGF) serum concentrations were measured using a multiplex human cytokine/chemokine kit (MPXHCYTO-60K, Millipore). Multiplex assays were analyzed on a Bio-Plex 100 using Bio-Plex 4.1 software (Bio-Rad, Hercules, CA).
Statistical Analyses
Frequencies, means, standard deviations and ranges were calculated for descriptive analysis. Both Pearson and Spearman correlations were calculated. Because some variables were non-normally distributed, the results from the Spearman correlation are presented (Table 2). All of the biological markers (C-peptide, sRAGE, IGF-1, IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6, IGFBP-7, EGF, sEGFR, FGF-2, VEGF, sVEGFR-1, sVEGFR-2, sVEGFR-3) were categorized into tertiles for polytomous logistic regression analysis. Biological cut off points were used for the outcome variables: polyp number and polyp type. Polyp number was set to four categories: 0, 1, 2, or ≥ 3 polyps. Polyp type was set to three categories: no polyps, hyperplastic polyp(s) or tubular adenoma(s). For polyp type, each individual was assigned a single score based on the colorectal polyp with the worst pathology.
Table 2.
Spearman correlation coefficients
| C-peptide | IGFBP-1 | IGFBP-3 | IGFBP-4 | IGFBP-5 | IGFBP-6 | IGFBP-7 | FGF-2 | sEGFR | sRAGE | sVEGFR-1 | sVEGFR-2 | sVEGFR-3 | VEGF | IGF-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age |
0.193 0.031 |
0.099 0.266 |
−0.093 0.300 |
−0.063 0.485 |
−0.055 0.539 |
0.179 0.044 |
0.039 0.663 |
0.023 0.797 |
−0.234 0.009 |
0.048 0.593 |
0.099 0.271 |
−0.159 0.076 |
0.016 0.861 |
0.023 0.798 |
−0.199 0.026 |
| BMI |
0.513 <.0001 |
−0.353 <.0001 |
−0.262 0.003 |
−0.094 0.293 |
−0.075 0.405 |
−0.079 0.375 |
0.252 0.004 |
0.031 0.732 |
−0.050 0.578 |
−0.205 0.021 |
0.007 0.941 |
−0.033 0.714 |
−0.098 0.275 |
0.155 0.085 |
−0.199 0.026 |
| C-peptide |
−0.423 <.0001 |
−0.059 0.514 |
−0.075 0.401 |
−0.067 0.458 |
0.013 0.889 |
0.203 0.023 |
0.155 0.084 |
−0.048 0.595 |
−0.026 0.769 |
0.049 0.580 |
0.018 0.839 |
0.059 0.512 |
0.129 0.149 |
−0.178 0.047 |
|
| IGFBP-3 | −0.159 0.075 |
0.017 0.853 |
−0.130 0.146 |
0.075 0.404 |
0.032 0.724 |
0.085 0.345 |
0.051 0.569 |
−0.042 0.639 |
0.084 0.348 |
−0.098 0.274 |
−0.068 0.449 |
0.123 0.170 |
|||
| IGFBP-4 | 0.148 0.097 |
0.078 0.388 |
−0.182 0.041 |
0.099 0.267 |
0.084 0.351 |
0.279 0.002 |
0.109 0.224 |
0.159 0.074 |
0.160 0.073 |
0.028 0.759 |
0.264 0.003 |
||||
| IGFBP-6 | 0.149 0.095 |
−0.050 0.579 |
−0.003 0.970 |
0.057 0.529 |
0.055 0.538 |
−0.127 0.156 |
0.163 0.069 |
0.111 0.218 |
−0.076 0.397 |
||||||
| EGF |
0.429 <.0001 |
0.245 0.006 |
0.122 0.175 |
0.034 0.707 |
0.192 0.032 |
0.019 0.837 |
0.313 0.0004 |
0.026 0.775 |
|||||||
| FGF-2 | 0.074 0.414 |
0.309 0.0005 |
0.207 0.021 |
0.348 <.0001 |
0.132 0.143 |
0.397 <.0001 |
0.065 0.471 |
||||||||
| sEGFR |
0.235 0.008 |
0.310 0.0004 |
0.361 <.0001 |
0.299 0.0007 |
0.089 0.319 |
0.122 0.175 |
|||||||||
| sRAGE |
0.403 <.0001 |
0.429 <.0001 |
0.475 <.0001 |
−0.006 0.945 |
0.302 0.0006 |
||||||||||
| sVEGFR-1 |
0.391 <.0001 |
0.706 <.0001 |
0.057 0.529 |
0.106 0.236 |
|||||||||||
| sVEGFR-2 |
0.366 <.0001 |
0.105 0.242 |
0.326 0.0002 |
||||||||||||
| sVEGFR-3 | 0.065 0.474 |
0.162 0.069 |
Note: The top number is the Spearman correlation coefficient. The P values are shown under the correlation coefficient.
Each individual was assigned a smoking status of “never smoked” or “ever smoked”. An individual was classified as “never smoked” if he had smoked fewer than 100 cigarettes over the course of his lifetime. Smoking status was missing for 22 individuals (17.5% missing-ness). All missing data were considered missing at random.
Multiple imputation (seed = 20121119, imputations = 7) was used to impute all missing smoking data and the one participant’s value missing for EGF, FGF-2, and VEGF (12). The factors: age, smoking, polyp type, polyp number, BMI, C-peptide, IGFBP-1, IGFBP-3, IGFBP-4, IGFBP-5, IGFBP-6, IGFBP-7, EGF, FGF-2, sEGFR, sRAGE, sVEGFR-1, sVEGFR-2, sVEGFR-3, VEGF, and IGF-1 were used in the imputation algorithm of missing values. Sensitivity analyses demonstrated that these imputations did not bias the results.
Odds ratios (OR) were determined using polytomous logistic regression models for categorical outcome data with more than two levels. A generalized logit model (also known as a, “baseline category logit model”) formed the basis of the polytomous logistic regression model for our analyses. In this model, men having ≥3 polyps, 2 polyps or 1 polyp were compared to men having no polyps. Results were obtained for each serum factor categorized into tertiles (with lowest tertile as reference) for each category of polyp number or polyp type relative to no polyp. For clarity, results from the categories featuring the most polyps (≥3) and the polyp type most likely to progress to colorectal cancer (adenoma). Otherwise odds ratios were determined using logistic regression. All models were adjusted for age and smoking status. Test for trend was carried out across tertiles for the factors of interest. Because imputation was used, multiple imputation analyze (Proc MIANALYZE) was used to determine the results from analysis of the imputed data sets. SAS version 9.3 (SAS Institute Inc.; Cary, NC) was used for all statistical analysis. p ≤ 0.05 indicates significance.
RESULTS
Participant age, BMI, waist circumference, and average values for some serum factors are given in Table 1. As previously published (11), 57 (45%) of the 126 participants had at least one polyp, and 23 (18%) had ≥ 3 polyps. 37 (29.4%) of the participants had a tubular adenoma. Age did not differ between individuals with no polyps and those with ≥ 3 polyps. Both BMI and waist circumference increased with increasing number of polyps and polyp type. In addition, 17 (13.5%) of the participants had ≥ 3 polyps with at least one tubular adenoma.
Several of the serum factors analyzed correlated with subject characteristics as well as with each other (Table 2). IGFBP-1, IGFBP-3, IGF-1, and sRAGE were inversely correlated with BMI. However, IGFBP-7 and C-peptide were positively correlated with BMI. Although it was inversely correlated with BMI, sRAGE was positively correlated with FGF-2, sEGFR, sVEGFR-1, sVEGFR-2, sVEGFR-3 and IGF-1. A significant correlation was observed between C-peptide and IGFBP-1 (−), IGF-1 (−), as well as IGFBP-7 (+). IGF-1 was also positively correlated with the soluble VEGF receptor (sVEGFR-2).
Although serum insulin-like growth factor (IGF) and the IGF binding proteins were not associated with colorectal polyp number or type, other insulin-related serum factors were correlated with these parameters. Serum concentrations of two insulin-related factors, sRAGE and VEGF, were significantly associated with the presence of ≥ 3 polyps. The odds that a participant with a serum sRAGE concentration > 120.35pg/ml would have ≥ 3 polyps relative to no polyp were 0.25 times (CI: 0.07 – 0.92) lower than those that a participant with serum sRAGE concentration ≤ 94.45 pg/ml would have ≥ 3 polyps (p=0.04, Figure 1a) relative to no polyp. Additionally, for each category increase in serum sRAGE concentration, a man was 0.5 (CI: 0.27 – 0.94) times less likely to have ≥ 3 polyps than no polyps (p=0.03). Participants with serum VEGF concentrations > 184.16 tended to be 3.4 times (CI: 0.97 – 11.74) more likely to have ≥ 3 polyps relative to no polyps compared to the likelihood that his counterpart with serum VEGF concentrations ≤ 104.67 pg/ml would have ≥ 3 polyps (p=0.055; Figure 1b) relative to no polyps. For each category increase in serum VEGF concentration tertile, a man was 1.9 (CI: 1.003 – 3.53) times more likely to have ≥ 3 polyps than no polyps (p=0.049). Non-significant relationships were observed between polyp number and all other factors analyzed, including C-peptide, IGF-1, IGFBP-3, IGFBP-7, and the IGF-1/IGFBP-3 ratio (Table 3) as well as the remaining IGFBPs, sVEGFR-1, sVEGFR-2, sVEGFR-3, sEGFR, FGF-2 and EGF (data not shown).
Figure 1. The OR of having ≥ 3 polyps relative to no polyps is negatively associated with serum sRAGE (A) and positively associated with serum VEGF (B).
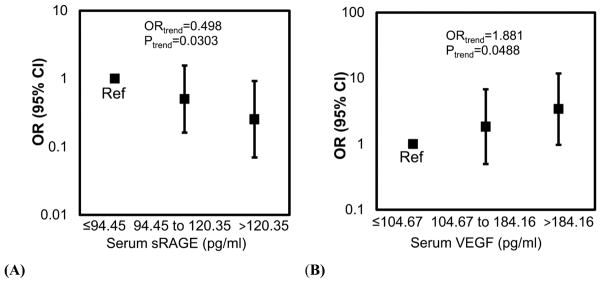
A, Compared to males with a serum sRAGE concentration ≤ 94.45 pg/ml, males with a serum sRAGE concentration > 120.35pg/ml are 0.25 times less likely to have ≥ 3 polyps (p=0.0368) relative to no polyps. In addition for each increase in serum sRAGE tertile, a man is 0.498 times less likely to have ≥ 3 polyps than no polyps (p=0.0303). Reference group (Ref) = males with sRAGE concentrations in the lowest tertile. B, Compared to males with a serum VEGF concentration ≤ 104.67 pg/ml, males with a serum VEGF concentration > 184.16 pg/ml tend to be 3.4 times more likely to have ≥ 3 polyps (p=0.0550) relative to no polyps. For each category increase in serum VEGF tertile, a man is 1.9 times more likely to have ≥ 3 polyps than no polyps (p=0.0488). The models were adjusted for age and smoking status (ever/never). Reference group (Ref) = males with VEGF concentrations in the lowest tertile.
Table 3.
Association of insulin-related factors with having ≥ 3 polyps relative to no polyps
| OR (95% CI) | Trend OR (p trend) | |
|---|---|---|
| sRAGE (pg/ml) | ||
| ≤ 94.45 | 1 | |
| > 94.45 to ≤ 120.35 | 0.501 (0.162 – 1.555) | 0.498 |
| > 120.35 | 0.253 (0.070 – 0.919) | (0.0303) |
| VEGF (pg/ml) | ||
| ≤ 104.67 | 1 | |
| > 104.67 to ≤ 184.16 | 1.827 (0.495 – 6.738) | 1.881 |
| > 184.16 | 3.382 (0.974 – 11.744) | (0.0488) |
| C-peptide (ng/ml) | ||
| ≤ 1.77 | 1 | |
| > 1.77 to ≤ 3.31 | 1.77(0.494 – 6.35) | 1.727 |
| > 3.31 | 2.965 (0.847 – 10.374) | (0.0852) |
| IGF-1 (ng/ml) | ||
| ≤ 92.28 | 1 | |
| > 92.28 to ≤ 124.02 | 0.498 (0.145 – 1.705) | 0.852 |
| > 124.02 | 0.736 (0.227 – 2.385) | (0.6074) |
| IGFBP-3 (ng/ml) | ||
| ≤ 644.45 | 1 | |
| > 644.45 to ≤ 720.24 | 0.209 (0.047 – 0.936) | 1.020 |
| > 720.24 | 0.926 (0.300 – 2.859) | (0.9499) |
| IGFBP-7 (ng/ml) | ||
| ≤ 58.74 | 1 | |
| > 58.74 to ≤ 73.51 | 4.850 (1.331 – 17.669) | 1.428 |
| > 73.51 | 2.309 (0.589 – 9.053) | (0.2430) |
| IGF-1/IGFBP-3 | ||
| ≤ 0.136 | 1 | |
| > 0.136 to ≤ 0.1716 | 0.253 (0.073 – 0.885) | 0.670 |
| > 0.1716 | 0.475 (0.149 – 1.516) | (0.1950) |
Note: Model is adjusted for age and ever/never smoked.
sRAGE was the only serum factor in this investigation which was negatively associated with the presence of a hyperplastic polyp (Supplementary Table 1). Males with a serum sRAGE concentration in the highest tertile (>120.35 pg/ml) were 0.12 (CI: 0.02 – 0.63) times less likely to have a hyperplastic polyp relative to no polyp than males with a serum sRAGE concentration in the lowest tertile (≤94.45 pg/ml) (p=0.01). For each category increase in serum sRAGE concentration, a man was 0.4 (CI: 0.2 – 0.79) times less likely to have a hyperplastic polyp (p=0.01) relative to no polyp. No other analyzed factors were associated with the presence of a hyperplastic polyp.
An insulin-related factor associated with polyp type was C-peptide. Participants with a serum C-peptide concentration in the highest tertile (>3.31 ng/ml) were 3.8 (CI: 1.31 – 11.15) times more likely to have a tubular adenoma relative to no polyp than males with a serum C-peptide concentration in the lowest tertile (≤1.77 ng/ml) (p=0.01, Figure 2). For each category increase in serum C-peptide concentration, a man was 1.97 (CI: 1.16 – 3.37) times more likely to have a tubular adenoma than no polyp (p=0.01). Non-significant associations were found between the presence of a tubular adenoma and all other serum factors analyzed, including sRAGE, VEGF, IGF-1, IGFBP-3, IGFBP-7, and the ratio between IGF-1 and IGFBP-3 (Table 4).
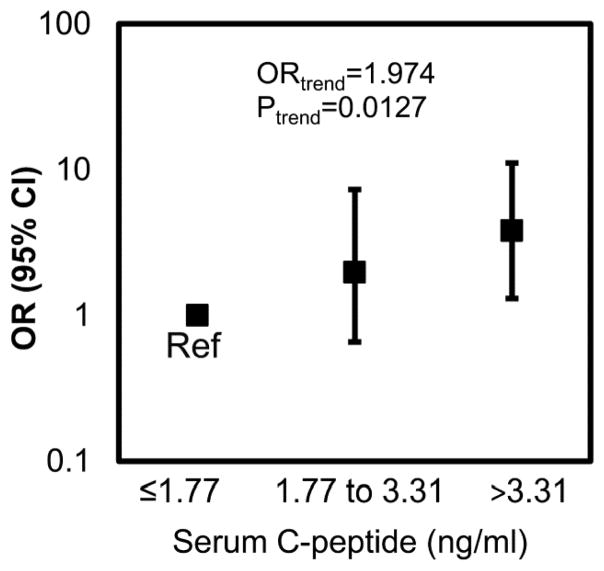
Figure 2. Serum C-peptide concentration is associated with the presence of adenomas.
Compared to males with a serum C-peptide concentration ≤ 1.77 ng/ml, males with a serum C-peptide concentration > 3.31 ng/ml are 3.8 times more likely to have an adenoma (p=0.0144) relative to no polyps. Further, for each increase in serum C-peptide tertile, an individual is 1.97 times more likely to have an adenoma than no polyp (p=0.0127). The model was adjusted for age and smoking status (ever/never). Reference group (Ref) = males with C-peptide concentrations in the lowest tertile.
Table 4.
Association of insulin-related factors with the presence of a tubular adenoma relative to no polyps
| OR (95% CI) | Trend OR (p trend) | |
|---|---|---|
| sRAGE (pg/ml) | ||
| ≤ 94.45 | 1 | |
| > 94.45 to ≤ 120.35 | 0.753 (0.278 – 2.041) | 0.761 |
| > 120.35 | 0.587 (0.216 – 1.592) | (0.2882) |
| VEGF (pg/ml) | ||
| ≤ 104.67 | 1 | |
| > 104.67 to ≤ 184.16 | 1.170 (0.425 – 3.220) | 1.306 |
| > 184.16 | 1.670 (0.623 – 4.477) | (0.301) |
| C-peptide (ng/ml) | ||
| ≤ 1.77 | 1 | |
| > 1.77 to ≤ 3.31 | 1.982 (0.656 – 5.994) | 1.974 |
| > 3.31 | 3.814 (1.305 – 11.145) | (0.0127) |
| IGF-1 (ng/ml) | ||
| ≤ 92.28 | 1 | |
| > 92.28 to ≤ 124.02 | 0.677 (0.251 – 1.828) | 0.802 |
| > 124.02 | 0.644 (0.233 – 1.782) | (0.3966) |
| IGFBP-3 (ng/ml) | ||
| ≤ 644.45 | 1 | |
| > 644.45 to ≤ 720.24 | 0.662 (0.233 – 1.881) | 1.005 |
| > 720.24 | 0.987 (0.359 – 2.716) | (0.9855) |
| IGFBP-7 (ng/ml) | ||
| ≤ 58.74 | 1 | |
| > 58.74 to ≤ 73.51 | 2.812 (0.974 – 8.121) | 1.567 |
| > 73.51 | 2.487 (0.883 – 7.005) | (0.0841) |
| IGF-1/IGFBP-3 | ||
| ≤ 0.136 | 1 | |
| > 0.136 to ≤ 0.1716 | 0.344 (0.121 – 0.976) | 0.809 |
| > 0.1716 | 0.648 (0.242 – 1.739) | (0.4080) |
Note: Model is adjusted for age and ever/never smoked.
DISCUSSION
Herein we describe associations between insulin-related serum factors with polyp number or polyp type. Serum concentrations of C-peptide were positively associated with the presence of a tubular adenoma. IGF and its binding proteins were not associated with colorectal polyp number or type. Two other proteins related to insulin and glucose regulation were associated with polyp number and severity. Serum concentrations of sRAGE were negatively associated with the presence of ≥ 3 polyps while serum VEGF concentrations were positively associated with the presence of ≥ 3 polyps. Because polyps are considered precursors to colon cancer (13), the associations demonstrated herein indicate that several insulin-related factors are associated with the risk of colorectal cancer in adult, Caucasian males.
This study had several strengths. The design of this study, cross-sectional with consecutive enrollment, reduced enrollment bias. Additionally, only asymptomatic individuals undergoing a complete screening were enrolled. Anthropometric measurements were taken by trained staff instead of being given by self-report. Participants provided information about relevant confounding variables, thus allowing for control of these confounding variables in our analysis. Because our population is well-defined, the results are likely typical for men of an ethnicity and age similar to that of the study population.
The study also had several limitations. Thus, caution must be used when interpreting the results of this study. Because this study was conducted in a modest-sized sample of primarily Caucasian, adult males, the results may not apply to individuals of other ethnicities or females. Additionally, although sensitivity tests did not show an effect of imputation on the conclusions drawn from the analysis, it is important to note that some data was imputed and that these imputations may have minor effects on the results. The associations described in this study were based on cross-sectional data thus cause cannot be assigned to any factors identified as associated with polyp number or type. Additionally, only a single time point is analyzed, but effects of some serum proteins may be related to the duration of exposure. In addition, local production rather than serum concentrations of the analyzed proteins may be relevant to colorectal polyp formation. However, local production is not easily measured, and serum measurements are more useful in a clinical setting, for instance as a potential pre-colonoscopy screening tool. A larger sample size would decrease the width of the reported 95% confidence intervals, thus, this research should be repeated with a larger sample size.
It was surprising that IGF-1 levels and the IGF-1 to IGFBP-3 ratios were not associated with colorectal polyp number or type in our population. Several other publications report a link between IGF and colorectal cancer or polyps (14–16). However, a lack of such an association has been demonstrated in prostate cancer (17). We also did not observe associations between the IGF binding proteins and colorectal polyps despite evidence that these binding proteins can block IGF-signaling (18). IGF-1 is important for cellular proliferation, differentiation and apoptosis (19). IGF-1 levels in our study were similar to levels in at least one other study (20). In addition, the inverse relationship between BMI and IGF-1 levels that we observed has also been observed by others (21). Given the outcomes of recent clinical trials (10) showing that anti-IGF-1 receptor treatments are ineffective in treating cancer, our results demonstrating the IGF-1 and its binding proteins are not associated with early steps in the progression to colon cancer are less surprising. Additionally, IGF signaling in the colon rather than blood may be more predictive of colon cancer risk (22).
A study population consisting only of men may also explain the lack of association between IGF and polyps. In women, fasting IGFBP-1 was inversely associated with risk of colon cancer with a relative risk of 0.28. The IGF-I to IGFBP-3 molar ratio was associated with colon cancer risk with a relative risk of 2.82. Further, women with low levels of both IGF-I/IGFBP-3 and C-peptide (or high IGFBP-1) were at low risk; however, elevation of either was sufficient to increase risk (23). Colon cancer risk showed a nonsignificant positive trend with IGF-1 levels and was significantly increased for women with the highest IGFBP-3 levels (24). Thus, the association between IGF-1 and colorectal cancer may be sex-specific.
Alternatively, glucose regulation rather than insulin signaling itself may be most important in cancer. Anti-diabetic drugs can inhibit carcinogenesis in mice and rats. For instance, Metformin decreases the risk of cancer in individuals with diabetes (25). Significant alterations in tumor glucose levels were identified when metabolomics were used to identify metabolic changes resulting from colorectal neoplasia in a mouse model (26). Furthermore, actual changes in insulin receptor signaling may not become important in colorectal cancer pathogenesis until cancer progression or metastases have occurred. Therefore, we would not be able to detect these changes in our study of colorectal polyp formation.
However, in support of the potential importance of insulin and its related signaling pathways in the development of colon cancer, we found that C-peptide serum concentrations were positively associated with the presence of colonic tubular adenomas. Insulin levels in serum can be indirectly assessed by measuring the C-peptide concentrations in samples. The latter protein is formed in equimolar amounts with insulin when proinsulin is secreted by pancreatic beta cells and cleaved into the two proteins. Insulin is known to have a shorter half-life than C-peptide, which makes C-peptide concentration a better indicator of insulin concentration (reviewed in (27)). Furthermore, serum C-peptide levels are more likely to represent long-term insulin levels since they are not as influenced by disease status in non-diabetic individuals (28).
The increased odds of tubular adenoma with high serum C-peptide concentrations may be due to effects of C-peptide or insulin on inflammatory signaling pathways as C-peptide affects cardiovascular disease risk. For instance, when C-peptide levels were analyzed in 5153 non-diabetic adults between 40 and 74 years of age, individuals with the highest C-peptide measurements (≥0.984 nmol/L = 2.95 ng/ml, (27)) had a 60% increase in the adjusted hazards of cardiovascular death compared to those with the lowest (≤0.418 nmol/L = 1.25 ng/ml). This trend persisted in risk for cerebrovascular disease, ischemic heart disease, myocardial infarction, and overall mortality (29). C-peptide induces proliferation of smooth muscle cells potentially leading to the development of atherosclerotic lesions (30).
Likewise, high C-peptide levels were associated with increased colorectal adenoma risk in some studies. In a 2012 study by Vidal et al., individuals with C-peptide levels in the fourth quartile had a 2-fold increased risk of adenomas compared to individuals with C-peptide levels in the lowest quartile (31). Furthermore, C-peptide levels were associated with increased cancer risk in the colon, but less so in the rectum (32). Additionally, a meta-analysis reported that subjects with the highest levels of circulating C-peptide were at an increased risk for colon but not rectal cancer (33). The relationship between C-peptide and colon cancer has been reported to be stronger in men than women (33), but the positive association has also been reported in women (23, 24). Other studies have shown no link between C-peptide and colon cancer (34).
Until recently, C-peptide was not thought to have any physiological function. However, in a 2012 study using BLAST searches and comparative biology, C-peptide was suggested to be a receptor ligand which behaves similarly to a cytokine. Specifically, a highly-conserved portion located around leucine-86 and glutamine-87 of the C-peptide gene sequence is associated with an active receptor binding spot (35). The development of colorectal adenomas was attributed to inflammatory cytokines. For example, levels of TNF-α in patients with hyperplastic polyps were significantly increased compared to those in patients with healthy mucosa (36). These results suggest that C-peptide may behave like a cytokine, inducing inflammatory responses in tissues. Thus, this inflammatory action of C-peptide is potentially important in the development of colon polyps, colorectal cancer or other intestinal inflammatory diseases. Alternatively, C-peptide could lead to adenomas through its anti-apoptotic properties (31).
Notably, individuals who consume high levels of meat, fish, and sweetened beverages and low levels of dairy and whole grains have higher serum C-peptide concentrations (37, 38). This type of diet is positively associated with colorectal cancer risk (39). Depending on the methods of preparation, for instance roasting, broiling or frying, this high C-peptide dietary pattern is also likely to be associated with high intake of advanced glycation end products (AGE) (40). AGE are formed by the nonenzymatic glycation of proteins, lipids, and nucleic acids. Two major contributors of AGE are endogenous AGE that form during normal metabolism and exogenous AGE from tobacco smoke or food processing. AGE accumulation is associated with insulin resistance (41).
The inverse association between serum sRAGE concentrations and the presence of three or more polyps may be due to the anti-inflammatory effects of sRAGE. It is the interaction between AGE and RAGE that leads to inflammation since RAGE signaling mediates NF-kB transcription. sRAGE neutralizes the oxidative stress and inflammatory effects mediated by the AGE/RAGE complex because it competes with RAGE to bind AGE (reviewed in (42)). Therefore, when serum concentrations of sRAGE are high, inflammation may be prevented through inhibition of the AGE/RAGE interaction.
In our subjects, as reported by others (43), serum sRAGE concentrations were negatively correlated with BMI. sRAGE was not correlated with C-peptide. We hypothesize that the increased levels of sRAGE in our subjects are an effective response to chronic low-grade inflammation. Rising levels of serum sRAGE, would, in turn, inhibit the inflammatory response that initiates abnormal colonic cell proliferation. Thus, when sRAGE is present and the inflammatory response is inhibited, fewer complications would arise. Since high levels of sRAGE may prevent progression to adenoma or other inflammatory complications in individuals with colorectal polyps, future research should address strategies to increase serum concentrations of sRAGE. For instance, physical activity can increase sRAGE concentrations in patients with Type 2 diabetes (44). Thus, increased physical activity may be one potential intervention strategy to prevent polyps. Vitamin D supplementation is another intervention that could potentially affect the progression of polyps through modulation of serum sRAGE concentrations as increased levels of serum calcitriol have been associated with increased sRAGE (45).
Others have reported inverse associations between sRAGE and cancer. One of the first studies to relate sRAGE levels with a decreased risk of colon cancer found that higher prediagnostic levels of serum sRAGE were even associated with a decreased risk in smokers (46). The same group later reported that individuals with colorectal adenomas had higher levels of serum EGF and VEGF and tended to have lower levels of sRAGE than individuals with no adenomas (47). Thus, we are the second group to associate serum sRAGE with colorectal polyps.
A more expected observation from our study was the positive association between VEGF and the presence of three or more polyps. VEGF is a well-known promoter of angiogenesis and, therefore, cancer. But, VEGF is less well known for its association with insulin resistance, where neutralization of VEGF restores insulin sensitivity and normal glucose levels in a mouse model of diabetes (7). Others have reported positive relationships between serum VEGF and colorectal cancer (48) as well as colonic VEGF and cancer (49). In fact anti-VEGF monoclonal antibodies are being used as a therapy in metastatic colorectal cancer (50).
Our research suggests that high serum concentrations of VEGF (> 184.16 pg/ml), high serum concentrations of C-peptide (>3.31 ng/ml), and low serum concentrations of sRAGE (≤94.45 pg/ml) could be used to identify individuals at increased risk for colorectal polyp formation. Since it is known that removal of polyps decreases the incidence of colon cancer (13), this research provides additional evidence that colonoscopy should be recommended for Caucasian males who have inappropriate concentrations of these serum factors. Colonoscopy screening of individuals with these risk factors could potentially decrease colorectal cancer rates. Prior to any policy changes, studies verifying the usefulness of these proteins as predictors of colorectal polyps are needed.
Supplementary Material
Acknowledgments
Research supported in part by the National Cancer Institute 1R03CA142000 to J.I. Fenton and the Clinical and Translational Sciences Institute at Michigan State University to J.I. Fenton, K. Hortos and B. Kovan.
We thank the Michigan State University Center for Statistical Training and Consulting, specifically, our consultant, David Reyes-Gastelum for valuable insight into methods of multiple imputation. We thank Lori Houghton-Rahrig for IRB protocol and standard operating procedure preparation assistance and anthropometric measurement training of support staff. We thank Dr. Sharon Hoerr for providing anthropometric measurement consultation, initial training, and training tools.
Footnotes
No authors have conflict of interests to declare.
References
- 1.Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PloS one. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omata F, Deshpande GA, Ohde S, Mine T, Fukui T. The association between obesity and colorectal adenoma: systematic review and meta-analysis. Scandinavian journal of gastroenterology. 2013;48:136–46. doi: 10.3109/00365521.2012.737364. [DOI] [PubMed] [Google Scholar]
- 3.Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5:1260–72. doi: 10.1158/1940-6207.CAPR-12-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology. 2013;57:2338–45. doi: 10.1002/hep.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Sato K, Niedzwiecki D, Ye C, Saltz LB, Mayer RJ, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. Journal of the National Cancer Institute. 2012;104:1702–11. doi: 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Archives of physiology and biochemistry. 2009;115:86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsater H, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–30. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 8.Brix JM, Hollerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes (Lond) 2012;36:1412–7. doi: 10.1038/ijo.2012.107. [DOI] [PubMed] [Google Scholar]
- 9.Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. The Journal of nutrition. 2014;144:109–13. doi: 10.3945/jn.113.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature reviews Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 11.Comstock SS, Hortos K, Kovan B, McCaskey S, Pathak DR, Fenton JI. Adipokines and Obesity Are Associated with Colorectal Polyps in Adult Males: A Cross-Sectional Study. PloS one. 2014;9:e85939-e. doi: 10.1371/journal.pone.0085939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y. Multiple Imputation Using SAS Software. Journal of Statistical Software. 2011;45 doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sridhar SS, Goodwin PJ. Insulin-insulin-like growth factor axis and colon cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:165–7. doi: 10.1200/JCO.2008.19.8937. [DOI] [PubMed] [Google Scholar]
- 15.Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoen RE, Weissfeld JL, Kuller LH, Thaete FL, Evans RW, Hayes RB, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:464–75. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 17.Neuhouser ML, Platz EA, Till C, Tangen CM, Goodman PJ, Kristal A, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Prev Res (Phila) 2013;6:91–9. doi: 10.1158/1940-6207.CAPR-12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, et al. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Science signaling. 2012;5:ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- 19.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nature reviews Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 20.Erarslan E, Coskun Y, Turkay C, Koktener A, Aydogan T. IGF-I levels and visceral fat accumulation in colonic neoplasia. Clinics and research in hepatology and gastroenterology. 2013 doi: 10.1016/j.clinre.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Faupel-Badger JM, Berrigan D, Ballard-Barbash R, Potischman N. Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Annals of epidemiology. 2009;19:841–9. doi: 10.1016/j.annepidem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Ivanov I, Dougherty ER, Hartman TJ, Lanza E, Bobe G, et al. Noninvasive detection of candidate molecular biomarkers in subjects with a history of insulin resistance and colorectal adenomas. Cancer Prev Res (Phila) 2009;2:590–7. doi: 10.1158/1940-6207.CAPR-08-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14:850–5. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 24.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. Journal of the National Cancer Institute. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 25.Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Critical reviews in oncology/hematology. 2013;87:201–23. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montrose DC, Zhou XK, Kopelovich L, Yantiss RK, Karoly ED, Subbaramaiah K, et al. Metabolic profiling, a noninvasive approach for the detection of experimental colorectal neoplasia. Cancer Prev Res (Phila) 2012;5:1358–67. doi: 10.1158/1940-6207.CAPR-12-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2013;30:803–17. doi: 10.1111/dme.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Giovannucci EL, Wu K, Smith-Warner SA, Fuchs CS, Pollak M, et al. Magnesium intake, plasma C-peptide, and colorectal cancer incidence in US women: a 28-year follow-up study. British journal of cancer. 2012;106:1335–41. doi: 10.1038/bjc.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel N, Taveira TH, Choudhary G, Whitlatch H, Wu WC. Fasting serum C-Peptide levels predict cardiovascular and overall death in nondiabetic adults. Journal of the American Heart Association. 2012;1:e003152. doi: 10.1161/JAHA.112.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasic D, Walcher D. C-peptide: a new mediator of atherosclerosis in diabetes. Mediators of inflammation. 2012;2012:858692. doi: 10.1155/2012/858692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal AC, Lund PK, Hoyo C, Galanko J, Burcal L, Holston R, et al. Elevated C-peptide and insulin predict increased risk of colorectal adenomas in normal mucosa. BMC cancer. 2012;12:389. doi: 10.1186/1471-2407-12-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenab M, Riboli E, Cleveland RJ, Norat T, Rinaldi S, Nieters A, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer Journal international du cancer. 2007;121:368–76. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Li L, Wang Y, Li P, Luo L, Yang B, et al. Circulating C-peptide level is a predictive factor for colorectal neoplasia: evidence from the meta-analysis of prospective studies. Cancer causes & control: CCC. 2013;24:1837–47. doi: 10.1007/s10552-013-0261-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Smith-Warner SA, Chan AT, Wu K, Spiegelman D, Fuchs CS, et al. Aspirin use, body mass index, physical activity, plasma C-peptide, and colon cancer risk in US health professionals. American journal of epidemiology. 2011;174:459–67. doi: 10.1093/aje/kwr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Wei W, Zheng Y, Hou J, Dou Y, Zhang S, et al. The role of insulin C-Peptide in the coevolution analyses of the insulin signaling pathway: a hint for its functions. PloS one. 2012;7:e52847. doi: 10.1371/journal.pone.0052847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marszalek A, Szylberg L, Wisniewska E, Janiczek M. Impact of COX-2, IL-1beta, TNF-alpha, IL-4 and IL-10 on the process of carcinogenesisin the large bowel. Polish journal of pathology: official journal of the Polish Society of Pathologists. 2012;63:221–7. doi: 10.5114/pjp.2012.32768. [DOI] [PubMed] [Google Scholar]
- 37.Bobe G, Murphy G, Rogers CJ, Hance KW, Albert PS, Laiyemo AO, et al. Serum Adiponectin, Leptin, C-Peptide, Homocysteine, and Colorectal Adenoma Recurrence in the Polyp Prevention Trial. Cancer Epidemiology Biomarkers & Prevention. 2010;19:1441–52. doi: 10.1158/1055-9965.EPI-09-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung TT, Hu FB, Schulze M, Pollak M, Wu T, Fuchs CS, et al. A dietary pattern that is associated with C-peptide and risk of colorectal cancer in women. Cancer causes & control: CCC. 2012;23:959–65. doi: 10.1007/s10552-012-9969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theodoratou E, Farrington SM, Tenesa A, McNeill G, Cetnarskyj R, Korakakis E, et al. Associations between dietary and lifestyle risk factors and colorectal cancer in the Scottish population. Eur J Cancer Prev. 2014;23:8–17. doi: 10.1097/CEJ.0b013e3283639fb8. [DOI] [PubMed] [Google Scholar]
- 40.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. Journal of the American Dietetic Association. 2010;110:911–16. e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15888–93. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey JB, Cipollone F, Abdullah SM, McGuire DK. Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): cardiovascular implications. Diabetes & vascular disease research: official journal of the International Society of Diabetes and Vascular Disease. 2009;6:7–14. doi: 10.3132/dvdr.2009.002. [DOI] [PubMed] [Google Scholar]
- 43.Norata GD, Garlaschelli K, Grigore L, Tibolla G, Raselli S, Redaelli L, et al. Circulating soluble receptor for advanced glycation end products is inversely associated with body mass index and waist/hip ratio in the general population. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2009;19:129–34. doi: 10.1016/j.numecd.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Choi KM, Han KA, Ahn HJ, Hwang SY, Hong HC, Choi HY, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. The Journal of clinical endocrinology and metabolism. 2012;97:3751–8. doi: 10.1210/jc.2012-1951. [DOI] [PubMed] [Google Scholar]
- 45.Sung JY, Chung W, Kim AJ, Kim HS, Ro H, Chang JH, et al. Calcitriol treatment increases serum levels of the soluble receptor of advanced glycation end products in hemodialysis patients with secondary hyperparathyroidism. The Tohoku journal of experimental medicine. 2013;230:59–66. doi: 10.1620/tjem.230.59. [DOI] [PubMed] [Google Scholar]
- 46.Jiao L, Taylor PR, Weinstein SJ, Graubard BI, Virtamo J, Albanes D, et al. Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1430–8. doi: 10.1158/1055-9965.EPI-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiao L, Chen L, Alsarraj A, Ramsey D, Duan Z, El-Serag HB. Plasma soluble receptor for advanced glycation end-products and risk of colorectal adenoma. International journal of molecular epidemiology and genetics. 2012;3:294–304. [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC cancer. 2010;10:203. doi: 10.1186/1471-2407-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer genomics & proteomics. 2013;10:55–67. [PubMed] [Google Scholar]
- 50.Saif MW. Is there a benefit from addiction to anti-VEGF therapy in patients with colorectal cancer? Anticancer research. 2013;33:2377–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.