Proteins of the BCL-2 family control the permeabilisation of the mitochondrial outer membrane (MOMP), which is a key regulatory mechanism for the induction of apoptosis. The BCL-2 protein MCL-1, which prevents MOMP, was shown to be essential for the survival of T- and B-cells, as well as of neutrophils and hematopoietic stem cells1–4.
As MCL-1 represents the main barrier for the response to the BH3 mimetic ABT-7375, the modulation of MCL-1 activity and stability by interfering with its posttranslational modifications has gained particular attention.
We have previously demonstrated that the cytokine IL-3 maintains the survival of pro B-cell lines by stabilization of MCL-1. IL-3 induces the PI3K/AKT pathway, which negatively regulates Glycogen Synthase Kinase-3 (GSK-3) through inhibitory phosphorylation6. Conversely, upon loss of PI3K signalling, we found that GSK-3 phosphorylates MCL-1 on S159, earmarking MCL-1 for enhanced ubiquitylation and degradation. Thus, inhibition of GSK-3, or replacement of S159 by alanine, resulted in MCL-1 stabilization and prevention from apoptosis7. While similar findings were reported by a number of subsequent studies by others (reviewed in ref. 8), the in vivo role of this posttranslational modification of MCL-1 has not yet been addressed.
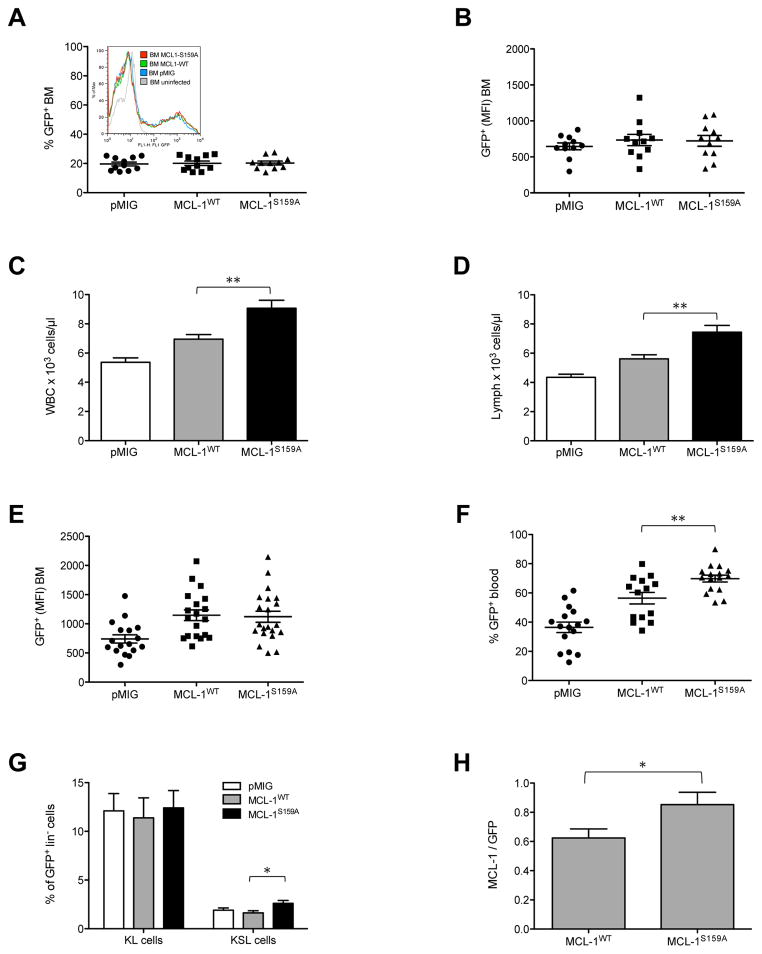
In this study, we investigated the relevance of MCL-1 S159 phosphorylation in vivo. We employed a murine bone marrow (BM) transplantation strategy, retrovirally introducing MCL-1, or the GSK-3 phosphorylation-deficient mutant MCL-1S159A in BM cells, followed by adoptive transfer of infected donor cells to lethally irradiated recipient mice. Equal expression levels of pMIG control, wild-type or mutant MCL-1 were crucial for our experiments, and were assured by testing virus supernatants for equal MOI and analysing infected donor bone marrow for equal levels of GFP and thus inferred MCL-1 or MCL-1S159A mRNA expression, by flow cytometry (Fig. 1A and B).
Figure 1. Effect of S159 phosphorylation-deficient MCL-1 on leukocyte numbers.
(A) Proportion of GFP+ BM cells infected with empty control vector (pMIG) or vectors encoding MCL-1WT-IRES-GFP (MCL-1WT) or MCL-1S159A-IRES-GFP (MCL-1S159A) as determined by flow cytometry. Inset shows a histogram of a representative individual experiment: pMIG (blue) 23% GFP+ infected BM (MFI=574); MCL-1WT (green) 25.5% GFP+ infected BM (MFI=704); MCL-1S159A (red) 25,7% GFP+ infected BM (MFI=704). (B) Mean fluorescence intensity of GFP+ BM cells indicating expression levels of MCL-1WT-IRES-GFP and MCL-1S159A-IRES-GFP as determined by flow cytometry. Points represent infected bone marrow used for transfer to the recipient mice as shown in Fig 1C–G. (C) Peripheral white blood cell count (WBC) was determined using the ADVIA hematology analyzer 4 weeks after engraftment. Empty vector control pMIG 5.37 x 103/μl ± 0.29 (n=29), MCL-1WT 6.95 x 103/μl ± 0.32 (n= 29) and MCL-1S159A 9.07 x 103/μl ± 0.54 (n= 30). Mice, which had received MCL-1S159A BM had significantly higher WBC count than MCL-1WT BM recipients. (D) Lymphocyte count: pMIG 4.35 x 103/μl ± 0.22 (n=29), MCL-1WT 5.62 x 103/μl ± 0.28 (n= 29) and MCL-1S159A 7.44 x 103/μl ± 0.47 (n= 30). MCL-1WT versus MCL-1S159A. (E) Mean fluorescence intensity of GFP+ cells indicating expression levels of MCL-1WT and MCL-1S159A as determined by flow cytometry. Points represent individual BM samples 6 weeks after engraftment. (F) Percentage of GFP+ peripheral blood cells, expressing empty vector control pMIG, MCL-1WT-IRES-GFP or MCL-1S159A-IRES-GFP as determined by flow cytometry. (G) Bars represent the percentage of sca1− c-kit+ precursor (KL) cells and ckit+ and sca1+ (KSL) hematopoietic stem cells, from lin− GFP+ BM cells. The proportion of sca1+ c-kit+ cells among lin− GFP+ BM expressing MCL-1S159A was significantly elevated, compared to MCL-1WT GFP+BM cells. *p<0.05. **p<0.1. (H) Expression of MCL-1 and GFP (as normalization) were analyzed by Western blotting, and quantified with a LUMI-Imager. Bars represent the ratio of MCL-1 to GFP of six independent Western blots for MCL-1WT and MCL-1S159A. Values are means ± SEM (n= 19) * p<0.05.
Four weeks after transplantation, the mice which had received bone marrow infected with MCL-1S159A BM (n=30) exhibited a significantly elevated WBC count (9.07 x 103/μl) as compared to mice expressing wild-type MCL-1 (6.95 x 103/μl, n=29, p=0.0014), while mice expressing control vector pMIG (n=29) had the lowest average WBC count (5.37 x 103/μl) (Fig. 1C). Likewise, lymphocyte (p=0,0015, Fig. 1D) and neutrophil granulocyte (Fig. S1A) numbers were highest in mice, which had received BM infected with MCL-1S159A, while the numbers of monocytes, platelets and red blood cells (RBC) were independent of expression of MCL-1wt or MCL-1S159A (Fig. S1B, C and D). This effect of MCL-1 phosphorylation on lymphocyte and neutrophil granulocytes numbers may reflect the general requirement of MCL-1 for the survival of these cell types1–3.
Six weeks after adoptive transfer, BM, thymocytes, splenocytes and lymph node (LN) lymphocytes were examined. Importantly, the mean fluorescence intensity (MFI) of GFP in BM cells expressing MCL-1-IRES-GFP or MCL-1S159A-IRES-GFP was equal, indicating that the mRNA levels for MCL-1wt and MCL-1S159A were similar (Fig. 1E), and thus the effects of MCL-1S159A expression on cell numbers were not attributable to different vector expression levels, but correlated with the mutation status of MCL-1. We did not observe an effect of MCL-1 or MCL-1S159A expression on proliferation in vivo or in cell lines, ruling out that this was the cause for the difference in cell numbers (Fig. S1E and data not shown).
The proportion of GFP+ cells was significantly higher in peripheral blood with GFP+ cells expressing MCL-1S159A (Fig. 1F), most likely because during maturation, MCL-1S159A cells outcompeted uninfected cells more efficiently than did cells expressing MCL-1wt.
While the proportions of KL cells (Lin−/Sca−/Kit+) were similar among GFP+ BM cells of each genotype, we found a significantly elevated KSL (Kit+Sca+Lin−, stem cell) population among MCL-1S159A cells, compared to pMIG control and MCL-1wt cells (Fig. 1G). In contrats, the distribution of the B-cell subpopulations in the BM was independent of the expression of MCL-1wt or MCL-1S159A (Fig. S2A).
In the spleen and lymph nodes, the distribution of T and B cells cells was not influenced by expression of MCL-1wt or MCL-1S159A (Fig. S2B and C), and the distribution of splenic B cells at maturation stages T1, T2 and FO remained unchanged (Fig. S2D). Likewise, the percentage of CD4+ and CD8+ cells among GFP+ thymocytes, splenocytes and lymph node lymphocytes was similar in mice with bone marrow expressing pMIG control, MCL-1wt or MCL-1S159A (Fig S2E, F and G). In sum, this demonstrates that expression of MCL-1 or MCL-1S159A does not affect the differentiation potential of one or the other leukocyte subset in vivo, but leads to a comparable expansion of all cell types expressing the transgene. We analysed the protein expression levels of MCL-1 in splenocytes derived from mice, which had received BM expressing MCL-1 or MCL-1S159A. Consistent with previous results, showing that MCL-1S159 phosphorylation decreases MCL-1 stability7, 8, splenocytes expressing the phosphorylation-deficient mutant exhibited significantly elevated protein levels (Fig. 1H).
Anti-apoptotic BCL-2 family members such as BCL-2 and, as shown more recently, MCL-1, strongly accelerate the development of c-Myc-induced lymphoma9, 10. Here, we set out to investigate to investigate the effect of S159 phosphorylation of MCL-1 on the acceleration of lymphoma development by cooperation of MCL-1 with oncogenic Myc. We infected pooled BM cells from young Eμ-Myc donor mice (age <5 weeks) with control vector pMIG, MCL-1wt, or MCL-1S159A, followed by flow cytometry analysis for equal expression of GFP and thus pMIG, and MCL-1wt-, or MCL-1S159A-encoding vectors, and transplantation into irradiated recipient mice.
We employed an elevated white blood cell count in peripheral blood as an early indicator for the onset of leukaemia, as palpable tumours occurred only at later stages (6–8 weeks after BM transfer).
Four weeks after BM transfer, pre-malignant mice, which had received Eμ-Myc MCL-1S159A bone marrow (as compared to Eμ-Myc MCL-1wt mice) exhibited a trend to elevated WBC counts (Fig. S3A), lymphocyte and neutrophil granulocyte counts (Fig. S3B and C). No difference was observed for numbers of red blood cells and platelets (Fig. S3D and E). As observed before with non-malignant bone marrow, Eμ-Myc cells expressing MCL-1S159A competed out against uninfected cells more efficiently than did Eμ-Myc cells expressing MCL-1wt (Fig. S3F).
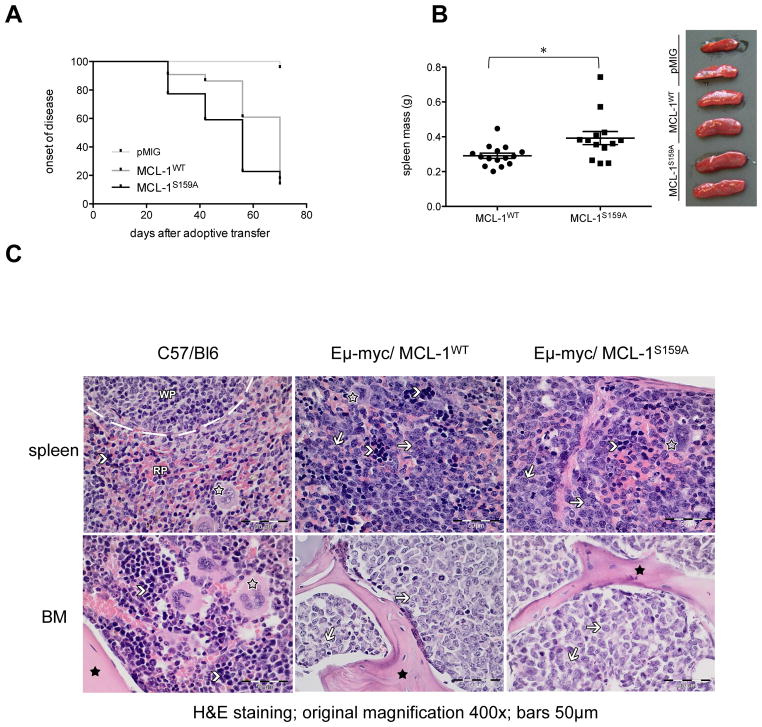
As shown by Kaplan-Meier-plot and statistical comparison by log-rank test, Eμ-Myc/MCL-1S159A-expressing mice exhibited a significantly earlier onset of leukemia than Eμ-Myc/MCL-1wt-expressing animals (p=0.0424), while only one control (vector-expressing) Eμ-Myc/pMIG mouse became leukemic in the respective time period (Fig. 2A). Consistently, Eμ-Myc/MCL-1S159A mice, in comparison to Eμ-Myc/MCL-1wt-expressing mice, exhibited elevated spleen weight when terminated after exhibiting palpable tumors (Fig. 2B).
Figure 2. S159 phosphorylation-deficient-MCL1 accelerates lymphoma.
(A) Kaplan–Meier curve of disease-free survival (defined as a WBC <22 x 103/μl, which is four times the normal WBC): MCL1WT (n= 22), median 70 days; MCL-1S159A (n=22), 56 days, the number for empty vector control pMIG was n=22. Mice which had received Eμ-myc/MCL-1S159A infected BM developed leukemia significantly earlier than Eμ-myc/MCL-1WT animals (p=0.0424) (B) Macroscopic appearance and spleen weight of diseased mice (*p<0.05) (C) Histology of formalin-fixed and paraffin-embedded murine spleen and bone marrow tissue slices (H&E staining, original magnification 400x). Black asterisks: bone, open asterisks: megakaryocytes, arrowheads: erythropoietic cells. C57/Bl6: Healthy control with normal tissue architecture of spleen and bone marrow (BM) including intact hematopoiesis (WP: white pulp, RP: red Pulp).
Eμ-myc/MCL-1WT and Eμ-myc/MCL-1S159A: Lymphoblasts (arrows) infiltrate and destroy the architecture of the spleen and the bone marrow where hematopoiesis is completely replaced by the lymphoid blasts.
All of the MCL-1/Eμ-Myc and Eμ-Myc/MCL-1S159A-expressing mice developed malignant lymphoma with massive B-cell infiltration into spleen and BM (Fig. 2C). Interestingly, in contrast to recent findings of B220+IgM+ lymphoma with vav-MCL-1/Eμ-Myc double transgenic animals10, these tumours were, with the exception of a single MCL-1/Eμ-Myc animal, all B220+IgM− pre B cell lymphoma.
High PI3K signalling has been observed in many tumours, including lymphoblastic leukemia11,12 and has recently been shown to cooperate with c-MYC in Burkitt lymphomagenesis13. Likewise, the frequent amplification of MCL-1 in a variety of tumors14 and the protection from Myc-induced leukaemia by the absence of a single MCL-1 allele15 underscores the dosage-dependent role of MCL-1 protein levels for tumour cell survival.
Here we show in an animal model, that the absence of MCL-1 S159 phosphorylation, usually achieved by cytokine-dependent, AKT-mediated GSK-3 inactivation, represents a mechanism by which constitutive PI3K signalling contributes to lymphadenopathy fostering malignancy, and possibly therapy resistance. Our results provide a rationale for the combination of PI3K inhibitors with BCL-2 antagonists that lack the capacity to directly target MCL-1 such as ABT737, or its more bioavailable derivative, ABT263.
Methods
Mice
Mice used in these experiments were on the C57BL/NCrl background. Eμ-myc transgenic mice have been described. All animal experiments were performed according to German law for animal protection (Tierschutzgesetz) as published on May 25, 1998. Genotyping was performed by PCR analysis of tail DNA using the following primers: mycS, 5′-CGGACACACAACGTCTTGGA-3′, and mycAS, 5′-CTCTCACGAGAGATTCCA GC-3′.
Constructs, transfection and retroviral infection
Human MCL-1 or the phosphorylation-deficient mutant, MCL-1S159A, were subcloned into the pMIG vector. BM cells were infected with retrovirus supernatants, followed by adoptive transfer to irradiated recipient mice.
Human MCL-1 was amplified by PCR and cloned into the pMIG retroviral vector using the following primers: hMCL-1S, 5′-CGCGGATCCACCATGTTTGGCCTCAAAAGAAACGC-3′, and hMCL-1AS, 5′-CCGGAATTCCGGCTATCTTATTAGATATGCCAAACCA-3. The MCL-1 mutant S159A was generated using the Quikchange Kit (Agilent) following primers: hMCl-1S, 5′-ACGGACGGGGCACTACCCTCGA-3′, and hMCL-1AS, 5′-TCGAGGGTAGTGCCCCGTCCGT-3. 293T cells were infected with Hit60, VsVg and pMIG constructs using superfect (Qiagen) according to manufacturers protocol. After 16h, sodium butyrate was added (5mM) for 8h. Retrovirus-containing supernatant was harvested after 12h and 24h, respectively. C57BL/NCrl or Eμ-myc mouse BM was mobilized with 5-Fluoruracil (150mg/kg), isolated 3 days after injection and taken in culture with IMDM containing IL-3 (10ng/ml), IL-6 (10ng/ml) and mSCF (50ng/ml). After 12h in culture, the BM cell culture was infected with the respective virus supernatants, which was repeated after 10h. The infection level (GFP) of BM cells was monitored after 10h by FACS and 5x105 infected BM cells were engrafted in 6–8 weeks old irradiated (9.5Gy) female C57BL/NCrl mice.
ADVIA hematology analyzer and flow cytometric analysis
Peripheral blood was analysed using the ADVIA hematology analyser. Flow cytometry analysis was done on FACSCalibur (Becton Dickinson) and Gallios™ (Beckman Coulter). Hematopoietic organs were isolated and single-cell suspensions of bone marrow, lymph nodes, Spleen and Thymus prepared. Cells were counted with the Z1 Coulter Counter and 1x106 cells stained for certain cell populations using specific monoclonal antibodies: 500A2, anti-CD3e; H129.19, anti-CD4; 53-6.7, anti-CD8a; RA3-6B2, anti-CD45R-B220 (Becton Dickinson); M1/70, anti-CD11b; 1B11, anti-CD43; RMM-1, anti-IgM; 11-26c.2a, anti-IgD; D7, anti-Sca-1; RB6-8C5, anti-Gr-1 (BioLegend); 3C1, anti-c-Kit (Milteny Biotec); hematopoietic lineage biotin panel (CD3e, CD11b, CD45R-B220, Ter-119, and Ly6G-Gr-1) (eBioscience).
Western blots
Western blots were analysed by Lumi-Imager (Roche).
Histology
Tissues were fixed in 4% buffered formalin, decalcified in a mixture of 10 % ethylene-diamine-tetraacetic acid disodium salt (EDTA, Serva, Cat. No. 11280.02) and 3.3 % tris-(hydroxymethyl) aminomethane (THAM, AppliChem, Cat. No. A1086,1000) in dd H2O at pH: 7.0 – 7.2 and paraffin embedded. Histological examination of all the specimens was performed using hematoxylin-eosin (H&E) staining.
Proliferation using the RTCA analyzing system
HeLa cells were plated (5000/well) onto 96-well E-plates and recorded every 15 min for 49 h over time.
Statistical Analysis
Statistical analysis was carried out with Graph Pad Prism (Version 5.0c) and statistical significance was calculated with the unpaired student’s t-test.
For further details, see supplemental methods.
Supplementary Material
Figure S1. Effect of S159 phosphorylation-deficient MCL-1 on neutrophil, monocyte, eosinophil and red blood cell numbers, and on cell proliferation (A) Neutrophil granulocyte numbers in the peripheral blood were determined using the ADVIA hematology analyzer 4 weeks after engraftment. Empty vector control pMIG 0.44 x 103/μl ± 0.04 (n= 29), MCL-1WT 0.72 x 103/μl ± 0.11 (n= 29) and MCL-1S159A 0.88 x 103/μl ± 0.14 (n= 30). (B) Monocyte count: pMIG 0.36 x 103/μl ± 0.12 (n= 29), MCL-1WT 0.35 x 103/μl ± 0.11 (n= 29) and MCL-1S159A 0.41 x 103/μl ± 0.1 (n= 30). (C) Platelets count: pMIG 581.8 x 103/μl ± 71.24 (n= 29), MCL-1WT 584.8 x 103/μl ± 57.77 (n= 29) and MCL-1S159A 636.3 x 103/μl ± 66.65 (n= 30). (D) Red blood cell count (RBC): pMIG 9.09 x 103/μl ± 0.1 (n= 29), MCL-1WT 9.55 x 103/μl ± 0.15 (n= 29) and MCL-1S159A 9.69 x 103/μl ± 0.13 (n= 30). (E) HeLa cell were stably infected with empty vector (blue), vectors encoding MCL-1WT-IRES-GFP (green) or Mcl-1S159A-IRES GFP (red) and sorted for GFP, indicating equal expression levels of MCL-1. Proliferation was monitored using the RTCA DP Analyzer. From the data of the cell growth curves, doubling times were calculated.
Figure S2: Cellular composition of pMIG, MCL-1WT and MCL-1S159A in BM, spleen, lymph nodes and thymus. Flow cytometric analysis of of primary and secondary hematopoietic organs. Total cells from mice of all three genotypes were isolated 6–8 weeks after engraftment. (A) Proportion of pro B (B220+IgM−CD43+), pre B (B220+IgM−CD43−), immature (B220+IgM+CD43−) and mature (B220hiCD43−IgM+) cells among GFP+B220+ BM cells (B) Proportion of splenic B (B220+) and T cells (CD3+) among GFP+ splenocytes (C) Proportion of LN B (B220+) and T cells (CD3+) among GFP+ LN lymphocytes (D) assessment of B cell maturation stages, bars represent percentage of transitional type 1 (T1) B cells (IgM+ IgD−), transitional type 2 (T2) B cells (IgM+ IgD+) and follicular (FO) B cells (IgMlow IgD+) among GFP+B220+ splenocytes (E) CD4+ and CD8+ T cell subpopulations (DN (CD4−CD8−), DP (CD4+CD8+), CD4+CD8− and CD4−CD8+ SP maturation stages) among thymocytes (F) CD4+ and CD8+ T cell subpopulations among CD3+ splenocytes and (G) CD4+ and CD8+ T cell subpopulations among CD3+ LN lymphocytes. Mouse numbers (Fi. S2A–C, E) pMIG (n= 11), MCL-1WT (n= 9) and MCL-1S159A (n= 8); (Fig. S2D, F–I) pMIG (n= 26), MCL-1WT (n= 28) and MCL-1S159A (n= 28).
Figure S3: Cooperation of S159 phosphorylation-deficient MCL-1 with Eμ-myc to induce lymphoma. (A) BM cells from young Eμ-myc transgenic mice were infected with pMIG, MCL-1WT or MCL-1S159A and analyzed by flow cytometry before engrafting into recipient mice. Peripheral white blood cell count was determined using the ADVIA hematology analyzer 4 weeks after engraftment. pMIG 7.37 x 103/μl ± 0.78 (n= 22), MCL-1WT 11.91 x 103/μl ± 1.44 (n= 22) and MCL-1S159A 15.23 x 103/μl ± 2.04 (n= 22). (B) Lymphocyte count: pMIG 4.59 x 103/μl ± 0.43 (n= 22), MCL-1WT 9.61 x 103/μl ± 1.39 (n= 22) and MCL-1S159A 13.08 x 103/μl ± 1.52 (n= 22). (C) Neutrophil granulocyte count: pMIG 1.22 x 103/μl ± 0.22 (n= 22), MCL-1WT 1.93 x 103/μl ± 0.51 (n= 22) and MCL-1S159A 2.33 x 103/μl ± 0.42 (n= 22). (D) RBC: pMIG 10.83 x 103/μl ± 0.23 (n= 22), MCL-1WT 10.95 x 103/μl ± 0.14 (n= 22) and MCL-1S159A 11.24 x 103/μl ± 0.23 (n= 22). (E) Platelets count: pMIG 772.4 x 103/μl ± 35.29 (n= 22), MCL-1WT 706.2 x 103/μl ± 46.52 (n= 22) and MCL-1S159A 751.8 x 103/μl ± 30.98 (n= 22). (F) Percentage of GFP+ peripheral blood cells, expressing MCL-1WT-IRES-GFP or MCL-1S159A-IRES-GFP as determined by flow cytometry.
Acknowledgments
The authors would like to thank Martin Brandenburg, Katharina Thumm, Heiko Bauer, Klaus Geiger and Jan Bodinek-Wersing.
This study was supported by grants Ma 1967/1 and Ma 1967/2 from the Deutsche Froschungsgemeinschaft to UM and a fellowship from the Dr. Heinrich Kircher-Stiftung to SEL.
Footnotes
We declare that there is no competing financial interest in relation to the work described.
References
- 1.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109(4):1620–6. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 3.Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113(12):2805–15. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307(5712):1101–4. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 5.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 7.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Perciavalle RM, Opferman JT. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013;23(1):22–9. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunelle JK, Ryan J, Yecies D, Opferman JT, Letai A. MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts. J Cell Biol. 2009;187(3):429–42. doi: 10.1083/jcb.200904049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116(17):3197–207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647–50. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sander S, Calado DP, Srinivasan L, Kochert K, Zhang B, Rosolowski M, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22(2):167–79. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiang Z, Luo H, Payton JE, Cain J, Ley TJ, Opferman JT, et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest. 2010;120(6):2109–18. doi: 10.1172/JCI39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of S159 phosphorylation-deficient MCL-1 on neutrophil, monocyte, eosinophil and red blood cell numbers, and on cell proliferation (A) Neutrophil granulocyte numbers in the peripheral blood were determined using the ADVIA hematology analyzer 4 weeks after engraftment. Empty vector control pMIG 0.44 x 103/μl ± 0.04 (n= 29), MCL-1WT 0.72 x 103/μl ± 0.11 (n= 29) and MCL-1S159A 0.88 x 103/μl ± 0.14 (n= 30). (B) Monocyte count: pMIG 0.36 x 103/μl ± 0.12 (n= 29), MCL-1WT 0.35 x 103/μl ± 0.11 (n= 29) and MCL-1S159A 0.41 x 103/μl ± 0.1 (n= 30). (C) Platelets count: pMIG 581.8 x 103/μl ± 71.24 (n= 29), MCL-1WT 584.8 x 103/μl ± 57.77 (n= 29) and MCL-1S159A 636.3 x 103/μl ± 66.65 (n= 30). (D) Red blood cell count (RBC): pMIG 9.09 x 103/μl ± 0.1 (n= 29), MCL-1WT 9.55 x 103/μl ± 0.15 (n= 29) and MCL-1S159A 9.69 x 103/μl ± 0.13 (n= 30). (E) HeLa cell were stably infected with empty vector (blue), vectors encoding MCL-1WT-IRES-GFP (green) or Mcl-1S159A-IRES GFP (red) and sorted for GFP, indicating equal expression levels of MCL-1. Proliferation was monitored using the RTCA DP Analyzer. From the data of the cell growth curves, doubling times were calculated.
Figure S2: Cellular composition of pMIG, MCL-1WT and MCL-1S159A in BM, spleen, lymph nodes and thymus. Flow cytometric analysis of of primary and secondary hematopoietic organs. Total cells from mice of all three genotypes were isolated 6–8 weeks after engraftment. (A) Proportion of pro B (B220+IgM−CD43+), pre B (B220+IgM−CD43−), immature (B220+IgM+CD43−) and mature (B220hiCD43−IgM+) cells among GFP+B220+ BM cells (B) Proportion of splenic B (B220+) and T cells (CD3+) among GFP+ splenocytes (C) Proportion of LN B (B220+) and T cells (CD3+) among GFP+ LN lymphocytes (D) assessment of B cell maturation stages, bars represent percentage of transitional type 1 (T1) B cells (IgM+ IgD−), transitional type 2 (T2) B cells (IgM+ IgD+) and follicular (FO) B cells (IgMlow IgD+) among GFP+B220+ splenocytes (E) CD4+ and CD8+ T cell subpopulations (DN (CD4−CD8−), DP (CD4+CD8+), CD4+CD8− and CD4−CD8+ SP maturation stages) among thymocytes (F) CD4+ and CD8+ T cell subpopulations among CD3+ splenocytes and (G) CD4+ and CD8+ T cell subpopulations among CD3+ LN lymphocytes. Mouse numbers (Fi. S2A–C, E) pMIG (n= 11), MCL-1WT (n= 9) and MCL-1S159A (n= 8); (Fig. S2D, F–I) pMIG (n= 26), MCL-1WT (n= 28) and MCL-1S159A (n= 28).
Figure S3: Cooperation of S159 phosphorylation-deficient MCL-1 with Eμ-myc to induce lymphoma. (A) BM cells from young Eμ-myc transgenic mice were infected with pMIG, MCL-1WT or MCL-1S159A and analyzed by flow cytometry before engrafting into recipient mice. Peripheral white blood cell count was determined using the ADVIA hematology analyzer 4 weeks after engraftment. pMIG 7.37 x 103/μl ± 0.78 (n= 22), MCL-1WT 11.91 x 103/μl ± 1.44 (n= 22) and MCL-1S159A 15.23 x 103/μl ± 2.04 (n= 22). (B) Lymphocyte count: pMIG 4.59 x 103/μl ± 0.43 (n= 22), MCL-1WT 9.61 x 103/μl ± 1.39 (n= 22) and MCL-1S159A 13.08 x 103/μl ± 1.52 (n= 22). (C) Neutrophil granulocyte count: pMIG 1.22 x 103/μl ± 0.22 (n= 22), MCL-1WT 1.93 x 103/μl ± 0.51 (n= 22) and MCL-1S159A 2.33 x 103/μl ± 0.42 (n= 22). (D) RBC: pMIG 10.83 x 103/μl ± 0.23 (n= 22), MCL-1WT 10.95 x 103/μl ± 0.14 (n= 22) and MCL-1S159A 11.24 x 103/μl ± 0.23 (n= 22). (E) Platelets count: pMIG 772.4 x 103/μl ± 35.29 (n= 22), MCL-1WT 706.2 x 103/μl ± 46.52 (n= 22) and MCL-1S159A 751.8 x 103/μl ± 30.98 (n= 22). (F) Percentage of GFP+ peripheral blood cells, expressing MCL-1WT-IRES-GFP or MCL-1S159A-IRES-GFP as determined by flow cytometry.