Active surveillance (AS), the ongoing reassessment of low-risk cancer with delayed treatment if clinically indicated, is a management strategy intended to minimize unnecessary treatment of localized prostate cancer (PCa) without compromising mortality rates [1]. Given increasing enthusiasm for AS, our objective was to analyze temporal trends in US men with localized PCa meeting standard low-risk criteria.
All men diagnosed with nonmetastatic PCa and known information on prostate-specific antigen (PSA), clinical stage, and Gleason score were identified using data from Surveillance Epidemiology and End Results (SEER; 2004–2010, n = 310 875) and the National Cancer Data Base (NCDB; 2004–2011, n = 640 996) [2,3]. The proportion of patients with low-risk characteristics (PSA <10 ng/ml, stage cT1–cT2a disease, or Gleason ≤6) was measured, and multivariate logistic regression adjusting for age (continuous) and race (white vs black vs other) were used to compare these proportions by year of diagnosis. All statistical analyses were conducted using Stata v.12.0 (StataCorp, College Station, TX, USA), and p values <0.05 were considered significant.
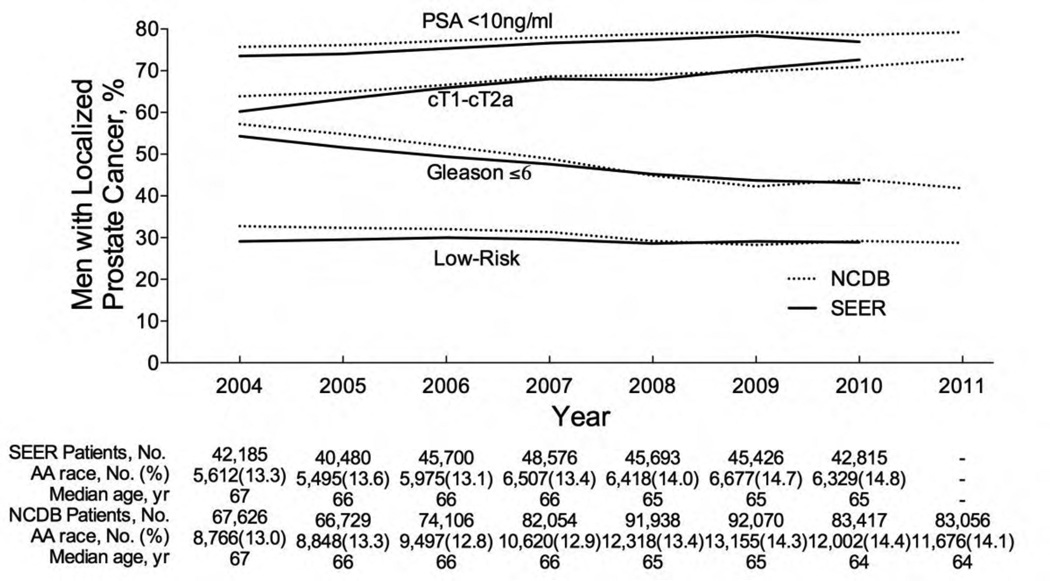
On multivariate analysis, SEER showed that the proportion of men with PSA <10 ng/ml increased from 73% to 77% (odds ratio [OR] 1.15; 95% confidence interval [CI], 1.11–1.18; p < 0.001), cT1-cT2a disease increased from 60% to 73% (OR: 1.71; 95% CI, 1.67–1.77; p < 0.001), and Gleason ≤6 decreased from 54% to 43% (OR: 0.62; 95% CI, 0.60–0.64; p < 0.001), and the proportion of men meeting all three low-risk criteria decreased from 29.0% to 28.9% (OR: 0.97; 95% CI, 0.94–0.99; p = 0.02) from 2004 to 2010 (Fig. 1). In the NCDB, the proportion of men with PSA <10 ng/ml increased from 76% to 79% (OR: 1.14; 95% CI, 1.11–1.17; p < 0.001), cT1-cT2a disease increased from 64% to 73% (OR: 1.47; 95% CI, 1.44–1.50; p < 0.001), and Gleason ≤6 decreased from 57% to 42% (OR: 0.51; 95% CI, 0.50–0.62; p < 0.001), and the proportion of men meeting all three low-risk criteria decreased from 32.7% to 28.8% (OR: 0.79; 95% CI, 0.77–0.80; p < 0.001) from 2004 to 2011.
Fig. 1.
Trends in prostate-specific antigen (PSA), clinical stage, and Gleason score for localized prostate cancer patients in the United States. Patient population is all males in the SEER (2004–2010) and NCDB (2004–2011) databases with localized prostate cancer and known data on PSA, clinical stage, and Gleason score diagnosed. Low risk was defined as PSA <10 ng/ml, stage cT1–cT2a, and Gleason ≤6.
NCDB = National Cancer Data Base; PSA = prostate-specific antigen; SEER = Surveillance Epidemiology and End Results.
This is the first large population-based study demonstrating an ongoing Gleason grade migration through 2011, as there was a substantial decrease in localized PCa patients with Gleason ≤6 and a slight to modest decrease in low-risk cancers. This cannot be easily explained by a change in screening or biopsy patterns because PSA and clinical stage at diagnosis decreased over our study period, suggesting that men should have been more likely to have low-grade cancers [4]. The implications of a Gleason grade migration are profound, particularly given its use as an eligibility criterion for AS. Grade migration may be attributable to a more restrictive definition of Gleason 6 proposed by the 2005 International Society of Urologic Pathology Consensus Conference [5]. The grading modification will likely improve the outcomes of AS by homogenizing Gleason 6 cancers, leading to a statistical artifact of spuriously improved outcomes due to stage migration, referred to as the Will Rogers phenomenon [5]. More important, with fewer Gleason 6 cancers diagnosed, fewer men will have the opportunity to utilize AS, limiting the potential for AS to minimize so-called overtreatment of PCa. To prevent further overtreatment due to grade migration, future investigations are needed to assess the safety of AS for selected intermediate-risk patients.
Take-home message.
In the United States, more men with prostate cancer are being diagnosed with prostate-specific antigen <10 ng/ml and stage cT1–cT2a; however, fewer are diagnosed with Gleason score 6 (likely due to 2005 changes in Gleason grading), which limits the number of men eligible for active surveillance.
Acknowledgments
Funding support
This research was sponsored by the Pritzker Summer Research Program and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health (grant number 2T35DK062719-26). The sponsors were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have nothing to disclose.
References
- 1.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 2.Harlan LC, Hankey BF. The Surveillance, Epidemiology, and End-Results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21:2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 3.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 4.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433–440. doi: 10.1016/j.juro.2009.10.046. [DOI] [PubMed] [Google Scholar]