Abstract
Objective
This study examined pre- and post-prandial glucagon-like peptide 1 (GLP-1) levels in women with bulimia nervosa (BN), purging disorder (PD), and non-eating disorder control women to better understand whether alterations in satiation-related hormones in BN may be linked to binge-eating episodes or other altered ingestive behaviors.
Method
Participants included women with BN (n = 19), PD (n = 14), or controls (n = 14). Participants provided subjective ratings for hunger and fullness and plasma samples before and after consumption of a standardized test meal.
Results
As expected, GLP-1 levels increased significantly following test meal consumption; however, participants with BN displayed significantly lower GLP-1 levels compared to PD and control participants both before and after consumption of the test meal. There were no significant differences between PD and control participants in GLP-1 levels, but individuals with PD displayed significantly higher levels of fullness throughout the test meal as compared to both control and BN participants.
Discussion
Our findings provide preliminary evidence that reduced GLP-1 levels in individuals with BN may be associated with binge-eating episodes. Additionally, increased fullness in individuals with PD does not appear to be accounted for by exaggerated post-prandial GLP-1 release.
Bulimia nervosa (BN) is characterized by recurrent objectively large binge-eating episodes, defined by a “loss of control” during consumption of excessive quantities of food. These objectively large feeding bouts are accompanied by compensatory behaviors (e.g., self-induced vomiting), which are used in an effort to prevent weight gain and/or influence body shape (1). Several studies have reported that individuals with BN experience altered perceptions of hunger, satiation, and fullness (2, 3, 4, 5, 6). These experiences suggest deficits in satiation which have been implicated in the propensity to consume large quantities of food, and investigators have sought possible physiological alterations that could account for satiation deficits. Examination of pre- and post-prandial levels of feeding-related hormones (e.g., cholecystokinin [CCK]) has revealed reductions in individuals with BN compared to controls, providing insight about the physiological correlates for the reported alterations in satiation in this group (7, 4, 5, 8). However, most studies have compared individuals with BN to healthy control participants. Thus, differences in satiation-related hormones may be linked to binge-eating episodes in BN or other altered ingestive behaviors in this group, such as dietary restriction or purging behavior.
Purging disorder (PD) is another specified feeding and eating disorder (OSFED) (1) that is characterized by purging behaviors (e.g., self-induced vomiting) after consumption of normal or small amounts of food among individuals who are not underweight (9). Thus, a key difference between PD and BN is the absence of objectively large binge-eating episodes in PD, making PD an excellent comparison group for understanding factors that are uniquely related to the presence of binge-eating episodes in BN. Keel and colleagues (5) found that individuals with BN reported both lower satiation and postprandial fullness in response to a standardized test meal compared to individuals with PD. Furthermore, individuals with BN demonstrated significantly lower post-prandial CCK response compared to individuals with PD. Of interest, participants with PD reported greater levels of post-prandial fullness as compared to controls. This elevation in post-prandial fullness could not be completely attributed to alterations in CCK levels, as those with PD did not differ from controls on postprandial CCK response. To follow up on these observations, the goal of this preliminary investigation was to examine pre- and post-prandial glucagon-like peptide-1 (GLP-1) levels in individuals with BN, PD, and non-eating disorder controls.
GLP-1 is a feeding-related hormone that is synthesized in intestinal enteroendocrine L cells and released in response and in proportion to nutrient stimulation of the gut (10, 11). Delivery of GLP-1 reduces food intake and increases satiation in a dose-dependent manner in humans (12). Furthermore, rodent studies have provided evidence that peripherally released GLP-1 plays a physiological role in satiation (13) and central GLP-1 receptors mediate nutrient-induced satiation (14, 15). Thus, alterations in GLP-1 release may be a physiological mechanism that underlies deficits in satiation in individuals with BN, elevated fullness in individuals with PD, or both.
Methods
Participants in the current study included women with BN (n=19), PD (n=14), and no history of any eating disorder (n=14) who participated in one of two larger studies examining the pathophysiology of BN and PD (5, Keel et al., in prep). GLP-1 has gained relatively recent attention in the ingestive behaviors literature, and, thus, assays of GLP-1 were not included among the original specific aims of either study. However, examination of peptides that influence ingestive behaviors was included among broad aims and was described in consent forms completed by all participants.
Participants
Pre- and post-prandial plasma GLP-1 levels were assessed in back-up samples for 47 female participants (RIA: 12 BN, 8 PD, 8 Controls; ELISA: 7 BN, 6 PD, 6 Controls). All BN participants met DSM-IV (16) diagnostic criteria that were modified in light of anticipated revisions to be made in DSM-5 criteria, including engaging in binge-eating and purging episodes (e.g., self-induced vomiting, laxative misuse) at least once per week, averaged over the past three months, and endorsing undue influence of shape and weight on their self-evaluation. Similarly, individuals in the PD group met research criteria (17) which included purging episodes (e.g., self-induced vomiting, laxative misuse) in the absence of objectively large binge-eating episodes at least once per week, averaged over the past three months, to control their weight or shape and undue influence of weight or shape on their self-evaluation. Individuals with PD were excluded if they endorsed a history of recurrent objectively large binge-eating episodes. Additionally, participants were excluded if they met DSM-IV (16) criteria for current mood disorder or substance dependence. Exclusion criteria for the control participants were current dieting to lose weight and/or a history of any eating or other major psychiatric disorder.
Participants were recruited from the surrounding communities via local advertisements. Telephone screens were conducted to determine initial eligibility, which was later confirmed via face-to-face structured clinical interview. All participants were between the ages of 18–45 years and fell within a body mass index (BMI) range of 18.5–26.5 kg/m2, as assessed by objective measurements during the study visits. Participants were free of medication, with the exception of oral contraceptives, and were instructed to abstain from consumption of alcohol for at least 72 hours prior to the visit in which blood samples were taken.
Procedures
During the first study visit, eating disorder diagnoses and control status were confirmed using the Eating Disorder Examination (EDE) (18) and Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (19). The study visit from which samples for GLP-1 were obtained occurred after an overnight fast (i.e., participants were instructed to consume nothing but water from 11:00 pm until arrival at the study center at 8:00 am). Following a brief medical screen, participants had an indwelling intravenous catheter inserted into their arm. Participants were then given a standardized test meal (Ensure Plus, 900 kcal in 600 g of liquid) and instructed to consume it over a 5-minute window. For one study from which samples were taken (5), blood was collected into chilled EDTA-treated tubes with 150 μl of aprotinin prior to, 15 and 30 minutes (min) after test meal consumption. In the other study (Keel et al., in prep), blood samples were collected prior to, 10, 20, 30, 50, 90, and 120 min after test meal consumption. Given the time over which GLP-1 is released following food ingestion, samples taken at baseline, 10, 15, or 20, and 30 min after the test meal were used in analyses for the current study. After collection, blood samples were immediately centrifuged at 4° C and plasma was moved to separate aliquot tubes and stored at −80° C until assayed (samples for radioimmunoassay stored for 8.2 (± 0.4) years; ELISA stored for 2.4 (± 1.3) years). Additionally, during each time point, participants completed visual analog scales (VAS) to obtain subjective measures of hunger and fullness. Responses were indicated by placing a vertical line on a 100 mm horizontal scale anchored from “not at all” to “extremely.”
GLP-1 analysis
Dependent on the study sample, plasma GLP-1 (total) concentrations were determined using either commercially available radioimmunoassay (RIA) or commercially available enzyme-linked immunosorbant assay (ELISA) kits (EMD/Millipore, St. Charles, Missouri). Total GLP-1 was assayed because preservation of back-up samples did not permit evaluation of the active form of GLP-1, which has been shown to degrade within 2 min of release. However, total GLP-1 levels reflect the accumulated release of the active form and thus provide a good initial proxy for a preliminary examination of GLP-1 levels in these groups. The detection limits were 3 pM and 1.5 pM for the RIA and ELISA assays, respectively. The maximum accepted coefficient of variation for each assay type was 15%, representing an acceptable range between duplicate samples using these assay methods.
Data analyses
Repeated measures collected before and after the standardized test meal were analyzed using multilevel model (MLM) analyses to examine change over time as a consequence of the test meal intake (level 1) and between-subjects predictors of change (level 2). This method was utilized above repeated measures ANOVA because it allowed assay and assay method (RIA vs. ELISA) to be included as covariates in analyses. Because assay method varied by the study from which samples were taken, controlling for assay method also controlled for study. In addition, MLM permits variation in timing of repeated observations (e.g., these models treat time as a continuous variable such that individuals may differ in whether their mid-point GLP-1 concentration was sampled 10, 15, or 20 min post-prandially). Within these models, time and time-squared were entered as continuous linear and quadratic predictors, respectively, of within-person change in GLP-1 levels, and group was entered as a between subjects predictor of GLP-1 levels. Finally, the interaction of Group × Time was included to test whether groups differed on change in GLP-1 levels in response to the standardized test meal. We also tested for the significance of potential covariates of GLP-1 levels, including age, BMI, hormonal contraceptive use in all participants, duration of illness and purging frequency in BN and PD participants, and binge-eating frequency in BN participants. Of these, only BMI was a significant covariate and was therefore included in models.
Results
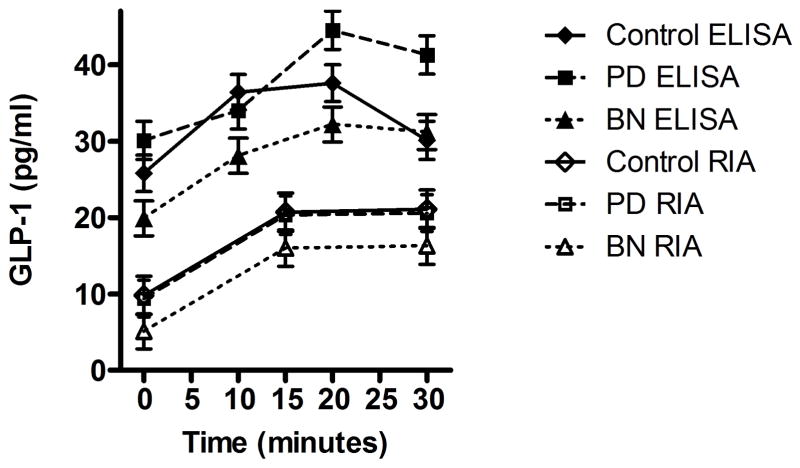
A model including the main effects of time, time-squared, group, and covariates of BMI, assay and assay method supported that each variable was a significant predictor of GLP-1 levels over the course of the test meal visit. Consistent with the effect of food intake on GLP-1 release, GLP-1 levels increased over time (Estimate (SE) = 1.08 (0.24), t(138)=4.45, p<0.001). Consistent with the short-term response of GLP-1, rate of growth decreased as a quadratic effect of time (Estimate (SE) = −0.02 (0.008), t(138)= −2.98, p=0.003). In addition to these main effects of time and time-squared, there was a main effect of group (F(2, 138)=8.23, p<0.001). Post-hoc comparisons supported significantly lower GLP-1 levels throughout the test meal in BN participants compared to both control (t(138)=−2.36, p=0.019) and PD participants (t(138)= −4.00, p<0.001). There was no significant difference between control and PD participants on GLP-1 levels (p=0.15). BMI was a significant predictor of GLP-1 levels (Estimate (SE) = 1.55 (0.39), t(138)=4.00, p<.001). Assay method (RIA vs. ELISA) was a significant predictor of GLP-1 levels, with values from ELISA significantly higher than values from the RIA (t(138)=8.60, p<0.001). Higher values from ELISA likely reflect both differences in sensitivities of methods and differences in duration of storage of back-up plasma samples from the studies from which samples were drawn (see Figure 1).
Figure 1. GLP-1 Concentrations over Time across Groups by Assay Method.
Plasma levels of GLP-1 in women with bulimia nervosa (BN), purging disorder (PD), and healthy control subjects (Control) in response to a test meal. Baseline pre-prandial measurement is designated as 0 on the x-axis. Values obtained via ELISA represented by filled markers and values obtained via RIA are represented by open markers. Plasma concentrations of GLP-1 pre- and post-prandially are lower in women with BN as compared to Controls (p<0.05) and PD participants (p<0.001). GLP-1, glucagon-like peptide 1. RIA: n = 12 BN, n = 8 PD, n = 8 Controls; ELISA: n = 7 BN, n = 6 PD, n =6 Controls.
Adding the interaction of Group × Time to the main effects model resulted in worse model fit compared to the main effect models (BIC=1068.262 vs. BIC=1059.334, respectively), and there was no significant interaction for group × time (F(2, 138)=0.47, p=0.63). Thus, group differences in GLP-1 levels did not change over time or in response to the test meal. This may reflect our measurement of total GLP-1 rather than active GLP-1 levels.
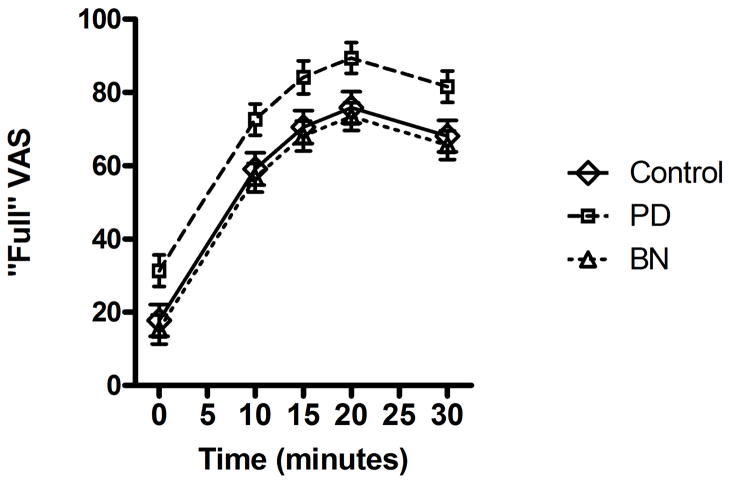
For analyses of subjective ratings of fullness, a model including the main effects of time, time-squared, and group supported that each variable was a significant predictor over the course of the test meal. Subjective feelings of fullness increased after the test meal (Estimate (SE) = 5.36 (0.57), t(140)=9.36, p<0.001). In addition, the quadratic effect of time indicated that feelings of fullness peaked and then subsided somewhat by 30 min after the meal (Estimate (SE) = −0.12 (0.02), t(140)= −6.60, p<0.001). In addition to these main effects of time and time-squared, there was a main effect of group (F(2, 140)=6.78, p=0.002). Post-hoc comparisons supported significantly higher fullness levels throughout the test meal in PD participants compared to both control (t(140)=2.77, p=0.006) and BN participants (t(140)= 3.53, p=0.001), who did not differ significantly from one another (p=0.59) (see Figure 2). Adding the interaction of Group × Time to the main effects model resulted in worse model fit compared to the main effect models (BIC=1301.894 vs. BIC=1295.428, respectively), and there was no significant interaction for group × time (F(2, 140)=1.73, p=0.18). Thus, changes in fullness following the test meal did not differ by group; however, this may reflect limited power to detect the interaction effect.
Figure 2. Subjective Ratings of Fullness over Time across Groups.
Visual analog scale (VAS) ratings of fullness collected from women with bulimia nervosa (BN, n = 19), purging disorder (PD, n = 14), and healthy control subjects (Control, n = 14) in response to a standardized test meal. Participants with PD report significantly elevated fullness as compared to control (p=0.006) and BN participants (p=0.001).
For analyses of subjective ratings of hunger, a model including the main effects of time, time-squared, and group supported significant effects for time and time-squared. Subjective feelings of hunger decreased after the test meal (Time: Estimate (SE) = −2.84 (0.60), t(141)=−4.74, p<0.001), and change over time was non-linear (Time Squared: Estimate (SE) = 0.06 (0.02), t(141)=3.26, p=0.001). There was no significant effect of group or group × time interaction in either the main effects model or a model including the two-way interaction (p-values > 0.10).
Discussion
This is the first study to examine GLP-1 levels in individuals with BN in direct comparison with individuals with PD as well as controls. Previous reports compared individuals with BN to controls, which does not permit evaluation of whether alterations in gut peptides are linked to objective binge-eating episodes or purging behavior. The current study found that individuals with BN displayed lower pre- and post-prandial GLP-1 levels compared to PD and control participants. In addition, we found that individuals with PD displayed similar GLP-1 levels as controls, but reported significantly higher fullness throughout the test meal study. These findings provide evidence that alterations in peripheral total GLP-1 in individuals with BN may be linked to objective binge-eating episodes rather than to purging behavior. Additionally, the lower concentrations of GLP-1 in participants with BN before and after the test meal also resemble patterns for subjective ratings of reduced satiation reported by the larger sample of BN participants from which these samples were taken (Keel et al., 2007). These findings extend our understanding of physiological mechanisms that may contribute to decreased satiation and increased vulnerability to binge-eating episodes in individuals with BN to another feeding-related hormone important in the regulation of food intake.
Our findings of reduced pre- and post-prandial total GLP-1 levels in individuals with BN compared to controls are consistent with those of Naessén et al., (8). Indeed, Naessén and colleagues (8) found both lower fasting and lower post-prandial serum concentrations of total GLP-1 in women with BN compared to control participants. However, our findings are inconsistent with findings from Brambilla et al. (20) who found no differences in GLP-1 levels between controls and participants with BN at any time point examined. This latter discrepancy may be due to differences in the composition of the test meal used between studies, particularly with regard to protein. Consumption of protein has a dose-dependent relationship with GLP-1 release and subjective appetite ratings in humans (21). Additionally, examination of meal composition on β-cell response found that a fat-rich test meal was less effective at stimulating GLP-1 release as compared to a carbohydrate-rich test meal (22). The test meal in the current study and the study by Naessén et al. (8) were rich in carbohydrate and protein (30% vs. 28% fat, 55% vs. 54% carbohydrate, 15% vs. 18% protein, respectively) and both studies found a significant post-prandial rise in GLP-1. In contrast, the test meal used by Brambilla et al. (20) was primarily comprised of fat (75% fat, 15% carbohydrate, 10% protein), which may not have been sufficient to elicit group differences in GLP-1 release. However, there is a clear need for future studies to systematically examine GLP-1 release following ingestion of different types and amounts of macronutrients, which will allow for clear interpretation of assay results in comparison to other studies.
One explanation for the reduction in GLP-1 levels in BN participants may be that these individuals have an enlarged gastric capacity (23) and delayed gastric emptying (4). Previous studies have shown individuals with BN require more nutrients delivered at a faster rate to achieve similar satiation levels as control subjects (24). This method of ingestion may compensate for reduced release of GLP-1 and CCK in individuals with BN. Such patterns may contribute to rapid consumption of large amounts of food during binge-eating episodes before feelings of satiation are stimulated. Consumption of a large amount of food may then increase gastric capacity over time, and enlarged gastric capacity may reduce gastric stretch-induced vagal stimulation in individuals with BN. This vagal stimulation plays an important role in signaling satiation to the central nervous system (25) and alterations in this signaling could contribute further to overconsumption of food, capturing women with BN in a vicious cycle.
In contrast to the GLP-1 levels observed in BN, GLP-1 levels in individuals with PD did not differ significantly from levels observed in controls, despite increased fullness reported by the PD participants. These findings suggest that GLP-1 levels do not contribute to excessive “fullness” reported by this group. Although total GLP-1 levels failed to explain the elevations in post-prandial fullness in participants with PD, alterations in other satiety-related hormones, such as peptide YY (PYY), should be examined for their possible relevance. In Sedlackova et al., (26) women with BN displayed increased post-prandial levels of PYY as compared to controls, which is opposite of what is observed with GLP-1. These differences suggest that GLP-1 and PYY may be differentially influenced by eating disorders such as BN and PD. Another possible explanation for elevated fullness to a fixed-volume meal in PD compared to the other participants is a reduction of gastric capacity in PD. A previous study by Geliebter et al. (27) revealed that dietary restriction significantly reduced gastric capacity, so dietary restriction in the absence of objectively large binge-eating episodes may decrease gastric capacity in PD. A reduction in gastric capacity would lead to greater gastric distention, which in turn stimulates vagal afferents (28) that activate GLP-1 neurons within the hindbrain (29). Stimulation of these hindbrain GLP-1 neurons leads to the release of GLP-1 via ascending projections to the forebrain (30) where activation of GLP-1 receptors can ultimately influence food intake (31, 32). As such, it would be important for future studies to examine gastric capacity in individuals with PD as well as PYY response to a test meal in disorders characterized by binge-eating, purging, or both.
This study had several strengths and limitations to acknowledge. Participants were well-matched on BMI, age, race and educational status, which is often not possible when studying consecutive referrals in a clinical sample. We used a commercially available standardized test meal that has been used in prior investigations (4), and this allows for precise replication of our study procedures in future studies. Finally, we used a sophisticated method of analysis that allowed for evaluation of changes over time in GLP-1 levels and subjective test meal responses, despite differences in assay methods and timing of samples and assessments between the parent studies from which back-up samples were drawn. In terms of limitations, we examined total rather than active GLP-1 due to limitations in preservation of samples originally collected as back-up samples for assay of other gut peptides. GLP-1 is rapidly degraded by the ubiquitous enzyme dipeptidyl peptidase IV, leaving the amount of biologically active GLP-1 greatly reduced within minutes of release. Active rather than total GLP-1 would more precisely reflect factors that influence stimulation of the vagus nerve and might better capture patterns related to subjective test meal response. Because we used back-up samples, these samples were stored at −80°C for more than one year, this duration of storage may have compromised the amount of detectable GLP-1 in our study. Additionally, because we utilized back-up samples, we had a relatively small sample. Thus, replication of this preliminary study is needed, using larger samples and collection methods to allow for assay of active GLP-1. Future studies also should examine GLP-1 in individuals who experience binge-eating episodes in the absence of purging (e.g., BN with non-purging compensatory behaviors), which may provide insight into the underlying pathological hormonal profile as it relates to binge-eating more broadly. Finally, in the absence of official diagnostic criteria for PD in the DSM-5, we employed a definition that has been recommended in the literature but that may differ from that used by other researchers. In addition, for the purposes of this study, we excluded participants with PD who had a history of BN as a means of ensuring that we were not studying one illness at different stages. However, in future nosological schemes, this requirement may not have clinical utility.
In summary, we documented lower total GLP-1 levels at baseline and following a standardized test meal in individuals with BN compared to both PD and control participants, who did not differ significantly from each other. Alterations in GLP-1 release in individuals with PD do not appear to explain the elevated fullness reported post-prandially in these individuals, suggesting that other meal-related hormones may contribute to these feelings. Findings of the present study extend the current literature of the pathophysiology of binge-eating episodes in BN, which may ultimately help inform potential targets for intervention.
Acknowledgments
This work was supported by R01 MH61836 (Keel) and T32 MH093311 (Keel and Eckel) from National Institute of Mental Health.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Halmi KA, Sunday S, Puglisi A, Marchi P. Hunger and satiety in anorexia and bulimia nervosa. Ann N Y Acad Sci. 1989;575:431–44. doi: 10.1111/j.1749-6632.1989.tb53264.x. discussion 44–5. [DOI] [PubMed] [Google Scholar]
- 3.Kissileff HR, Wentzlaff TH, Guss JL, Walsh BT, Devlin MJ, Thornton JC. A direct measure of satiety disturbance in patients with bulimia nervosa. Physiol Behav. 1996;60(4):1077–85. doi: 10.1016/0031-9384(96)00086-8. [DOI] [PubMed] [Google Scholar]
- 4.Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;65(1):114–20. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- 5.Keel PK, Wolfe BE, Liddle RA, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry. 2007;64(9):1058–66. doi: 10.1001/archpsyc.64.9.1058. [DOI] [PubMed] [Google Scholar]
- 6.Bello NT, Coughlin JW, Redgrave GW, Moran TH, Guarda AS. Oral sensory and cephalic hormonal responses to fat and non-fat liquids in bulimia nervosa. Physiol Behav. 2010;99(5):611–7. doi: 10.1016/j.physbeh.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geracioti TD, Liddle RA. Impaired cholecystokinin secretion in bulimia nervosa. N Engl J Med. 1988;319(11):683–8. doi: 10.1056/NEJM198809153191105. [DOI] [PubMed] [Google Scholar]
- 8.Naessén S, Carlström K, Holst JJ, Hellström PM, Hirschberg AL. Women with bulimia nervosa exhibit attenuated secretion of glucagon-like peptide 1, pancreatic polypeptide, and insulin in response to a meal. Am J Clin Nutr. 2011;94(4):967–72. doi: 10.3945/ajcn.111.014837. [DOI] [PubMed] [Google Scholar]
- 9.Keel PK, Haedt A, Edler C. Purging disorder: an ominous variant of bulimia nervosa? Int J Eat Disord. 2005;38(3):191–9. doi: 10.1002/eat.20179. [DOI] [PubMed] [Google Scholar]
- 10.Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88(6):2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 12.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150(4):1680–7. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–9. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab. 2013;304(12):E1314–20. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Publishing; 2000. text rev. [Google Scholar]
- 17.Keel PK, Striegel-Moore RH. The validity and clinical utility of purging disorder. Int J Eat Disord. 2009;42(8):706–19. doi: 10.1002/eat.20718. [DOI] [PubMed] [Google Scholar]
- 18.Fairburn CG. The eating disorder examination. In: Cooper Z, editor. Binge eating: Nature, assessment and treatment. 12. Guilford Press; New York: 1993. pp. 317–60. [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. In: Structured Clinical Interview for DSM-IV Axis I Disorders-Research Edition (SCID/I) Gibbon M, editor. New York: Biometrics Research; 1996. [Google Scholar]
- 20.Brambilla F, Monteleone P, Maj M. Glucagon-like peptide-1 secretion in bulimia nervosa. Psychiatry Res. 2009;169(1):82–5. doi: 10.1016/j.psychres.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97(5):980–9. doi: 10.3945/ajcn.112.047563. [DOI] [PubMed] [Google Scholar]
- 22.Rijkelijkhuizen JM, McQuarrie K, Girman CJ, Stein PP, Mari A, Holst JJ, et al. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism. 2010;59(4):502–11. doi: 10.1016/j.metabol.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56(4):656–61. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- 24.Hadigan CM, Walsh BT, Devlin MJ, LaChaussée JL, Kissileff HR. Behavioral assessment of satiety in bulimia nervosa. Appetite. 1992;18(3):233–41. doi: 10.1016/0195-6663(92)90200-p. [DOI] [PubMed] [Google Scholar]
- 25.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39(4):1824–31. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Sedlackova D, Kopeckova J, Papezova H, Hainer V, Kvasnickova H, Hill M, Nedvidkova J. Comparison of a high-carbohydrate and high-protein breakfast effect on plasma ghrelin, obestatin, NPY and PYY levels in women with anorexia and bulimia nervosa. Nutr Metab (Lond) 2012;9(1):52. doi: 10.1186/1743-7075-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geliebter A, Schachter S, Lohmann-Walter C, Feldman H, Hashim SA. Reduced stomach capacity in obese subjects after dieting. Am J Clin Nutr. 1996;63(2):170–3. doi: 10.1093/ajcn/63.2.170. [DOI] [PubMed] [Google Scholar]
- 28.Phillips RJ, Powley TL. Gastric volume detection after selective vagotomies in rats. Am J Physiol. 1998;274(6 Pt 2):R1626–38. doi: 10.1152/ajpregu.1998.274.6.R1626. [DOI] [PubMed] [Google Scholar]
- 29.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R470–8. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 30.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257–70. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 Pt 2):R848–56. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 32.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]