Abstract
The study of human-specific infectious agents has been hindered by the lack of optimal small animal models. More recently development of novel strains of immunodeficient mice has begun to provide the opportunity to utilize small animal models for the study of many human-specific infectious agents. The introduction of a targeted mutation in the IL2 receptor common gamma chain gene (IL2rgnull) in mice already deficient in T and B cells led to a breakthrough in the ability to engraft hematopoietic stem cells, as well as functional human lymphoid cells and tissues, effectively creating human immune systems in immunodeficient mice. These humanized mice are becoming increasingly important as pre-clinical models for the study of human immunodeficiency virus-1 (HIV-1) and other human-specific infectious agents. However, there remain a number of opportunities to further improve humanized mouse models for the study of human-specific infectious agents. This is being done by the implementation of innovative technologies, which collectively will accelerate the development of new models of genetically modified mice, including; i) modifications of the host to reduce innate immunity, which impedes human cell engraftment; ii) genetic modification to provide human-specific growth factors and cytokines required for optimal human cell growth and function; iii) and new cell and tissue engraftment protocols. The development of “next generation” humanized mouse models continues to provide exciting opportunities for the establishment of robust small animal models to study the pathogenesis of human-specific infectious agents, as well as for testing the efficacy of therapeutic agents and experimental vaccines.
Abbreviations: APC, antigen-presenting cell; BLT, bone marrow/liver/thymus; BRG, C.129(cg)-Rag2tm1Fwa9Il2rgtm1Cgn; CMV, cytomegalovirus; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats CRISPR/Cas9; EBV, Epstein–Barr Virus; ESC, embryonic stem cell; FDC, follicular dendritic cell; G-CSF, granulocyte-colony stimulating factor; GM-CSF1, granulocyte/macrophage-colony stimulating factor; GVHD, graft-versus-host disease; HIV-1, human immunodeficiency virus-1; HSC, hematopoietic stem cell; IL2rgnull, IL2 receptor common gamma chain gene; JCV, JC virus; LT, lymphotoxin; LTi, lymphoid tissue inducer; MERS-CoV, Middle East respiratory syndrome coronavirus; NCF1, neutrophil cytosolic factor 1; NHEJ, non-homologous end joining; NK, natural killer; NOG, NOD.Cg-PrkdcscidIl2rgtm1Sug; NSG, NOD.Cg-PrkdcscidIl2rgtm1Wjll; NSG-(KbDb)null, NOD.Cg-PrkdcscidIl2rgtm1Wjl H2-K1tm1Bpe H2-D1tm1Bpe/Sz; PAMP, pathogen-associated molecular patterns; PBL, peripheral blood lymphocytes; PML, progressive multifocal leukoencephalopathy; PRR, pattern recognition receptors; SIRPa, signal regulatory protein alpha; SRC, scid repopulating cell; TALEN, transcription activator-like effector nuclease; TLR, Toll-like receptors; TNF, tumor necrosis factor; ZFN, zinc finger nucleases
Keywords: NSG, Humanized mice, Immunodeficient mouse, Immune response, Infectious agents, Animal model
Highlights
-
•
Humanized mice support pre-clinical analyses of human-specific infectious agents.
-
•
Novel technologies are generating new humanized mouse models.
-
•
Innovations to improve human immune responses in humanized mice are becoming available.
1. Introduction
There are a number of human-specific infectious agents for which small animal models are critically needed to permit efficient and cost-effective evaluation of disease pathogenesis, therapeutic responses in vivo, and for the development of new vaccines, all without putting individuals at risk. Since many of these agents only infect human cells and tissues (Baumler and Fang, 2013, Wolfe et al., 2007), traditional small animal models such as mice and rats cannot be used as hosts for infection. In addition to the human-specific nature of many infectious agents, there are also cell and tissue-specific requirements for infection (Baumler and Fang, 2013, Wolfe et al., 2007). For example, Neiserria gonorrhoeae infects only human epithelial cells due to their requirement for binding to human CEACAM1 glycoprotein to enter the cell, a protein that differs between humans and other species (Voges et al., 2012). Thus, development of new small animal models for the study of these human-specific and cell and tissue-specific agents requires engraftment into animals of multiple types of human cells and tissues, including those from human hematopoietic and immune systems. The development of “next generation” humanized mice will accelerate investigation of currently known human-specific infectious agents including, for example, human immunodeficiency virus type 1 (HIV-1) and will support rapid identification and study of new emerging human-specific infectious agents for example the Middle East respiratory syndrome coronavirus (MERS-CoV, http://www.who.int/csr/don/2013_05_22_ncov/en/index.html).
2. Human immune system engrafted humanized mice
For the engraftment of functional human immune system in immunodeficient mice, three major model systems, described below, are commonly used. The protocols for establishing each of these models have been reviewed recently (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). Each of the model system has its strengths and limitations for the study of human immunobiology. It is these limitations that provide fresh opportunities for improvements in the models for the study of human infectious diseases and for the evaluation of vaccines.
2.1. Hu-PBL-SCID
The simplest approach to engraft a human immune system is by injection of human peripheral blood lymphocytes (PBLs) into adult immunodeficient mice, and is termed the Hu-PBL-SCID model (Mosier et al., 1988). In this system, PBLs are injected intraperitoneally or intravenously into non-irradiated or conditioned, usually sublethally-irradiation conditioning, recipients. The primary population of engrafting cells is the T cell (Mosier et al., 1988, King et al., 2009, Ito et al., 2002). All introduced T cells rapidly acquire an activated phenotype after one week, and few B cells, myeloid cells or other immune cells can be detected (Ito et al., 2002, King et al., 2009). This model is used to study effector T cell activity, and resulting Hu-PBL-SCID mice have been shown to be capable of mediating human skin and islet allograft rejection (King et al., 2008, Racki et al., 2010). However, the model is limited with the window for experimental observation being relatively short as all engrafted mice will develop a lethal xenogeneic graft-versus-host disease (GVHD) within a few weeks (Ito et al., 2002, King et al., 2009).
2.2. Hu-SRC-SCID
A second model, known as Hu-SRC-SCID, is established by the injection of human CD34+ hematopoietic stem cells (HSCs), defined functionally as scid-repopulating cells (SRCs), into newborn or adult immunodeficient recipients (Lapidot et al., 1992). Human HSCs are usually obtained from the bone marrow, umbilical cord blood, granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood or fetal liver, with fetal liver and cord blood being the most commonly used as sources as they are more efficient in repopulating immunodeficient mice than adult HSCs (Matsumura et al., 2003, Lepus et al., 2009). In the Hu-SRC-SCID model complete human hematopoietic and immune systems develop, however human T cells undergo thymic education through positive and negative selection on mouse thymus and are mouse MHC (H2)-restricted, precluding appropriate HLA-restricted interaction of human antigen-presenting cells (APCs) and human T cells in peripheral tissues (Watanabe et al., 2009). The Hu-SRC-SCID model has been used extensively for the study of human hematopoiesis, cell-mediated immunity, as well as infectious diseases such as HIV and Epstein–Barr Virus (EBV) (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012).
2.3. BLT
A third model system which overcomes some of the challenges seen in those above is established by subrenal capsule transplantation of fragments of human fetal liver and thymus into adult immunodeficient mice. This is accompanied by intravenous injection of autologous CD34+ HSC from the same fetal liver (McCune et al., 1988), and is termed the bone marrow/liver/thymus (BLT) model (Lan et al., 2006, Melkus et al., 2006). Engraftment of mice using the BLT model allows for the development of a complete human hematopoietic and immune system develops, and the human T cells are educated on a human thymus and are HLA-restricted (Rongvaux et al., 2013, Shultz et al., 2012). Of the three models, the BLT model system provides the most robust human immune system engraftment, and has become the model system of choice for studies of infectious agents targeting the human hematopoietic or immune systems. BLT mice also develop human mucosal immune systems, permitting the study of mucosal immunity following, for example, HIV infection via oral, vaginal or rectal routes (Chateau et al., 2013a, Chateau et al., 2013b, Denton and Garcia, 2012). A caveat of the BLT model is the eventual development of a wasting syndrome resembling a GVHD and has been reported by many (Greenblatt et al., 2012, Lockridge et al., 2013, Covassin et al., 2013, Ali et al., 2012) but not by all (Onoe et al., 2011) laboratories. In one report, HSCs allogeneic to the thymus were injected along with anti-CD2 to remove any pre-existing mature T cells, and no GVHD was observed (Kalscheuer et al., 2012). The variability in GVHD development among different research groups may be due to varying levels of mature T cells in the inoculum or colony variables, for example variable microbiome flora, antibiotic administration, or exposure to other variables such as bedding. The mechanism underlying the development of this wasting syndrome remains an open question, but based on observations in the Hu-PBL-SCID model of GVHD (King et al., 2009), it may be due to loss of tolerance of the human immune system to murine MHC antigens.
3. Immunodeficient IL2rgnull mouse strains
There have been three major advances in the development of small animal models that can be engrafted with functional human cells, tissues, and immune systems. First, the discovery of the immunodeficient C.B-17-Prkdcscid (CB17-scid) mouse in 1983 (Bosma et al., 1983) which provided the foundation for subsequent descriptions of human hematopoietic and immune cell engraftment in 1988 (Mosier et al., 1988, McCune et al., 1988). These reports soon led to the establishment of the first small animal models for the study of HIV infection (Namikawa et al., 1988, Mosier et al., 1991). However, only low levels of human hematopoietic and immune cell engraftment could be established in CB17-scid mice, severely limiting its utility. Subsequent development of NOD-scid mice in the mid-1990's (Shultz et al., 1995, Koyanagi et al., 1997) permitted much higher levels of human hematopoietic and immune cell engraftment, however complete restoration of the human immune system and development of human T cells from HSC were not achieved (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). A breakthrough in the field came with the development of immunodeficient scid, Rag1null, or Rag2null mice in the early 2000s bearing targeted mutations in the IL2 receptor common gamma chain (IL2rgnull) (Ito et al., 2002, Shultz et al., 2005, Traggiai et al., 2004). The IL2r common gamma chain is required for high-affinity signaling for the IL2, IL4, IL7, IL9, IL15, and IL21 cytokine receptors (Rochman et al., 2009). Blocking high-affinity signaling through these receptors severely dampens innate immunity, promoting heightened engraftment of functional human cells. IL2rgnull immunodeficient mice show multiple deficiencies in innate immunity, and completely lack natural killer (NK) cells that require IL15 for their development and function (Ito et al., 2002, Shultz et al., 2005, Traggiai et al., 2004). NK cells are one of the primary host innate immune factors that hinder human cell engraftment (Shultz et al., 2003). Importantly, NOD-scid IL2rgnull mice do not develop mouse thymic lymphomas (Shultz et al., 2005, Kato et al., 2009), which are dependent on IL2, and achieve a normal life span approaching 2 years. This is in contrast to NOD-scid mice that have a relatively short lifespan due to the development of thymic lymphomas and begin to die starting at 5 months of age (Shultz et al., 1995). Although immunodeficient mice bearing the IL2rgnull gene permitted for the first time the establishment of “humanized mice” bearing a complete human immune system following the engraftment of human HSCs, it was found that NK cell deficiency is not the sole factor regulating engraftment. Depletion of NK cells in NOD-scid or C57BL/6-scid mice following treatment with anti-CD122 monoclonal antibody surprisingly did not lead to the high levels of human engraftment levels and immune function that are attained in immunodeficient IL2rgnull mice (Christianson et al., 1996, Shultz et al., 2003). Although some reports suggest that in the BLT model, similar engraftment levels are observed in NOD-scid and NSG mice (Brainard et al., 2009, Denton et al., 2012), other reports continue to demonstrate superior engraftment in NSG mice as compared to NOD-scid mice in this model (Stoddart et al., 2011). Again the reason for these observational differences is not understood.
There are now three major strains of immunodeficient IL2rgnull mice commonly used by investigators. NOD.Cg-PrkdcscidIl2rgtm1Wjll (abbreviated as NOD-scid Il2rγnull or NSG) (Shultz et al., 2005, Ishikawa et al., 2005), NOD.Cg-PrkdcscidIl2rgtm1Sug (NOG) (Ito et al., 2001, Yahata et al., 2003), and C.129(Cg)-Rag2 tm1Fwa9 Il2rgtm1Cgn (abbreviated as BALB/c-Rag2nullIl2rγnull or BRG) mice (Traggiai et al., 2004). The origins, similarities, and differences of these three strains of immunodeficient IL2rgnull mice have been extensively reviewed, and each strain has advantages and disadvantages, depending upon the engraftment model system used to establish humanized mice (Shultz et al., 2007, Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012).
4. Opportunities for improvement of humanized mouse models
The development of immunodeficient IL2rgnull mice has been important for studies of regenerative medicine, immunity, hematopoiesis, cancer and infectious diseases (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). However, there are many opportunities to further improve these models for the study of infectious agents. For example, for the studies of human immunity, opportunities include enhancement of human primary and recall humoral immune responses, promotion of more efficient class switching and immunoglobulin G antibody production, and the generation of memory T cells. These opportunities also include improvement in the formation of lymphoid structures and development of germinal centers, which are currently poorly developed in immunodeficient IL2rgnull mice. The lack of the IL2rg expression leads to a decrease in mouse lymphoid tissue inducer (LTi) cells as well as poorly developed lymph nodes. Discovery of approaches to increase the numbers and function of LTi cells should lead to enhanced lymph node structure and improve immune responses. Additional future directions include provision of human-specific growth factors and cytokines that are required for optimal human cell engraftment and function within the model animal (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). New techniques for engraftment of human cells and tissues such as human hepatocytes, are being developed and once established, will optimize the study of infectious agents such as Plasmodium falciparum that infect hepatocytes as part of their life cycle (Vaughan et al., 2008). Finally, recently developed novel technologies permit rapid genetic modification of mice, accelerating the generation of new models of immunodeficient IL2rgnull mice needed to capitalize on the discovery of new approaches to enhance the engraftment and function of human tissues (see below).
Overall, improvements in humanized mouse models encompass further reductions in murine host innate immunity while simultaneously enhancing human innate and adaptive immunity and reducing the development of xenogeneic GVHD.
4.1. Reduction of murine innate immunity and GVHD
Although the introduction of the IL2rgnull gene into scid, Rag1null or Rag2null background severely cripples host innate immunity thereby enhancing human cell engraftment and function, a number of remaining host innate immune factors still need to be manipulated in order to achieve optimal human cell engraftment. Furthermore, in all models of human immune system engraftment, particularly in the Hu-PBL-SCID model, development of a lethal xenogeneic GVHD limits long-term studies and this needs to be addressed (Ito et al., 2002, King et al., 2009).
One of the major factors that regulate human cell engraftment in immunodeficient IL2rgnull strains is the signal regulatory protein alpha (SIRPA)-CD47 receptor–ligand interaction. SIRPA is highly expressed on macrophages, dendritic cells and neutrophils, cells of the innate immune system that have phagocytic activity and function to clear infectious agents, dead and dying cells, and foreign cells including xenogeneic human cells transplanted into murine recipients (Tsai and Discher, 2008, Matozaki et al., 2009, Barclay and Brown, 2006). The ligand for SIRPA is CD47, which is ubiquitously expressed on almost all cells of the body including hematopoietic and lymphoid cells (Yamauchi et al., 2013). Appropriate signaling through the SIRPa-CD47 complex provides a negative regulatory signal to phagocytic cells, in essence a “do not eat me” signal. This signaling complex is critical for regulating the engraftment of human cells in immunodeficient IL2rgnull mice (Yamauchi et al., 2013, Takenaka et al., 2007). NSG mice have a NOD strain-derived polymorphism of SIRPa that closely resembles that of human SIRPa, permitting appropriate recognition of human CD47 on hematopoietic cells and enhancing engraftment efficiency (Takenaka et al., 2007). In contrast, BALB/c and C57BL/6 mice have SIRPa polymorphisms that are not closely related to human SIRPa, impeding appropriate signaling through the SIRPa receptor and limiting human hematopoietic cell engraftment due to the ineffective negative regulatory signaling in host innate immune cells (Yamauchi et al., 2013).
Transgenic expression of human SIRPA in BRG (Strowig et al., 2011) or NOD Sirpa in B6.Cg-Rag2tm1FwaIL2rgnull (Yamauchi et al., 2013) mice increases human hematopoietic cell engraftment (Yamauchi et al., 2013). Moreover, C57BL/6 and BALB/c mice have intact hemolytic complement permitting study of the involvement of the complement cascade in immune responses as complement activity is readily detectable in vivo (Yamauchi et al., 2013). In contrast, immunodeficient IL2rgnull strains based on the NOD background lack hemolytic complement due to a 2-bp deletion in the coding region of the hemolytic complement (Hc) gene that encodes the C5 complement component (Shultz et al., 1995). This mutation prevents the formation of the C5b-9 membrane attack complex. To address this, we have recently backcrossed the intact Hc gene from the CBA/J strain to generate NSG mice that have an intact complement system (LDS, unpublished data). Moreover, it was recently reported that B6.Cg-Rag2null IL2rgnull CD47null mice also have elevated human hematopoietic cell engraftment levels in the BLT model system (Lavender et al., 2013). B6.Cg-Rag2null IL2rgnull CD47null mice in addition to having an intact complement system also appeared to develop more appropriate lymphoid-like structures in the BLT model, which is in contrast to the observations in NSG and NOG mice (Greenblatt et al., 2012, Lockridge et al., 2013, Covassin et al., 2013, Ali et al., 2012). B6.Cg-Rag2null IL2rgnull CD47null mice show no signs of a wasting GVHD-like syndrome at 29 weeks after engraftment (Lavender et al., 2013). Our laboratories are currently developing NSG-Tg(SIRPA) CD47Tm1Fpl mice as well as NSG-CD47Tm1FplTg(huCD47) mice to determine whether these genetic modifications of the SIRPa–CD47 pathway enhance the development of lymphoid structures and reduce the development of xenogeneic GVHD in the BLT model.
Extending these observations and supporting the importance of the SIRPa–CD47 pathway to cell types other than hematopoietic cells, it was also shown that intrasplenic injection of human hepatocytes transduced to express murine CD47 increases human hepatocyte engraftment in BALB/c-Tg(Alb1-Plau)144Bri mice (Waern et al., 2012).
An alternative approach to potentially reduce the development of xeno-GVHD is to eliminate the murine targets of the human xeno-GVHD response. Using in vitro xeno-mixed lymphocyte reactions, we have determined that a major component of this xenogeneic response is directed at murine MHC class I and class II molecules (King et al., 2008). Furthermore, xeno-GVHD is delayed in the Hu-PBL-SCID model when NSG class I or class II deficient recipients are used as recipients (King et al., 2008). To determine whether human CD4 T cell xeno-reactivity is predominately directed at murine MHC class II, we injected purified human CD4 T cells into NSG MHC class II knockout mice. In this model system, the development of xeno-GVHD is significantly delayed, suggesting that the xeno-GVHD response of human CD4 T cells is predominately directed at the murine MHC class II loci (Covassin et al., 2011). Similar results are obtained when enriched human CD8 T cells are injected into NSG class I deficient recipients (MAB, unpublished data). However, when NSG MHC class I or NSG MHC class II deficient mice are used in the BLT model, little to no delay of the development of the wasting syndrome was observed (Covassin et al., 2013). To investigate further the role of murine MHC class I and class II in the development of xeno-GVHD in the Hu-PBL-SCID model and the wasting syndrome in the NSG-BLT model, we are generating NSG mice that are deficient in both MHC class I and class II using two approaches. First, we are crossing NSG beta-2 microglobulin knockout mice with NSG mice deficient in the MHC class II I-A locus. However, in our hands, NSG mice deficient in beta-2 microglobulin exhibit poor breeding characteristics. To address this issue, we are also generating NSG class I/II knockout mice using a second approach. NOD.Cg-PrkdcscidIl2rgtm1Wjl H2-K1tm1Bpe H2-D1tm1Bpe/Sz, abbreviated as (NSG-(KbDb)null), mice do not express murine MHC class I molecules and are excellent breeders. We are now using TALEN technology to rapidly target and disrupt the murine I-Ab MHC class II allele in NSG-(KbDb)null mice (Gaj et al., 2013).
Additional approaches to prevent or reduce the elimination of human cells by host macrophages include restricting their phagocytic ability or eliminating them from the host. The use of liposome-encapsulated CL2MDP (clodronate liposomes) to kill macrophages in vivo has been used to increase engraftment and survival of human RBCs, which macrophages normally remove rapidly from the circulation (Hu et al., 2011). However, this treatment is toxic when given long term, and is also toxic to any engrafted human macrophages. To address this, we are currently developing a number of NSG models that permit reduction of host macrophages. For example, we are analyzing NSG mice that express the diphtheria toxin receptor under control of the CD11b or CD11c promoters to specifically delete murine myeloid and dendritic cell populations. This would permit interactions of infectious agents with human macrophage populations without the confounding effects of activity due to murine host macrophages.
Another host factor that complicates the study of innate immune responses to infectious agents is the murine expression of Toll-like receptors (TLRs) on host cells. Almost all infectious agents have pathogen-associated molecular patterns (PAMPs) that react with pattern recognition receptors (PRRs) on host innate immune cells, including PRRs of the TLR family (Kawai and Akira, 2010, Hoebe et al., 2006). Furthermore, TLR receptors can differ between mice and humans as mice express TLR11 which is not present in humans (Savva and Roger, 2013). Thus, the study of innate immune responses of the human myeloid cells to infectious agents is confounded by the corresponding innate immune response of the host myeloid cells. To address this, we are generating NSG mice deficient in TLR4, MYD88 cytosolic adapter protein, and type 1 interferon (IFN) receptors. We have also developed NSG mice deficient in the neutrophil cytosolic factor 1 (Ncf1) by crossing NSG mice with NOD-Cg.Ncf1m1J mice (Thayer et al., 2011). This gene controls superoxide production by macrophages and neutrophils and is responsible for much of their cytotoxic activity (Nauseef, 2008).
4.2. Enhancement of human innate and adaptive immunity
There are a number of species-specific factors that are important in cell development and function that differ between mouse and human and many of these have been described in recent reviews (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). A number of technical approaches can be used to either supply, “knockout” or “knockin” these factors and target mouse host gene encoding factors that interfere with human cell engraftment or function (Fig. 1 ).
Fig. 1.
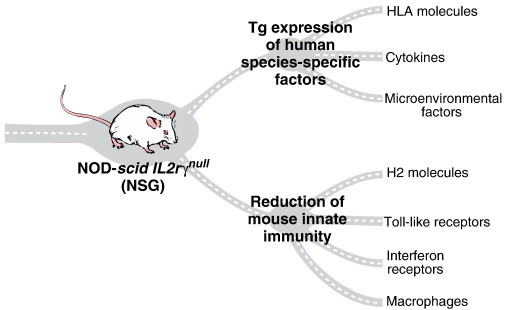
NSG mice provide a powerful platform for engraftment of human cells and tissues. Limitations in the development and function of certain lineages of human cells can be overcome by transgenic expression of human HLA molecules, cytokines, and other species-specific factors and by targeting mouse genes to eliminate host MHC antigens and other genes to further reduce innate immunity.
One of the opportunities in the generation of immune system engrafted humanized mice is to enhance the development and function of human innate immune cells. For example, few human myeloid cells are found in the circulation of human HSC engrafted NSG mice (Tanaka et al., 2012), and the myeloid cells that are present phenotypically immature and exhibit functional impairments (Gille et al., 2012). To begin to address this, investigators have administrated recombinant human G-CSF to increase human myeloid cells in the circulation, including granulocytes and macrophages that are rarely observed in the blood of human HSC-engrafted mice (Tanaka et al., 2012, Rathinam et al., 2011). Transgenic mice expressing human IL3 and granulocyte/monocyte-colony stimulating factor (GM-CSF) also show enhanced human myeloid cell levels in the circulation (Willinger et al., 2011). Another approach is to introduce into mice by hydrodynamic shock expression plasmids containing IL4 and GM-CSF leading to increased numbers of human dendritic cells (Chen et al., 2012). Next generation models of humanized mice will provide factors that are important for human myeloid, granulocyte, and dendritic cell development and will improve further human innate immunity. To enhance human adaptive immunity, a number of approaches can be used. The first challenge is that many of the cytokines needed for the development and function of human immune cells are species-specific, and human cytokines need to be provided for optimal immune system development (for review, see (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012)). As described in these recent reviews, there has been great progress in providing these factors to immunodeficient IL2rgnull NSG, NOG, and BRG mice. In particular, interest has centered on providing factors that enhance human hematopoiesis such as human stem cell factor (Brehm et al., 2012, Takagi et al., 2012) or thrombopoietin (Rongvaux et al., 2011) both of which are important in the development and function of human hematopoietic immune systems.
As noted above, one opportunity for improvement in human immune cell engrafted immunodeficient IL2rgnull mice is to enhance the ability of the engrafted immune system to mount primary and secondary immune responses. Following immunization, only sporadic reports suggest that antibody class switching during an immune response from IgM production to IgG production with affinity maturation occurs. Further, this has been primarily reported in mice transgenically expressing the human HLA class II molecule DR4 (Danner et al., 2011, Suzuki et al., 2012). Most human B cells in HSC-engrafted humanized mice are of the “immature” or “transitional” phenotype in that they express high levels of CD5, which is in contrast to that normally observed in humans (Biswas et al., 2011, Matsumura et al., 2003, Watanabe et al., 2009). Approaches to enhance B cell differentiation and function include provision of such factors as recombinant BLyS (BAFF) (Schmidt et al., 2008), however NSG transgenically expressing human BLyS do not show enhanced human B cell function (LDS and MAB, unpublished observations). This difference in observations may be due to the high level of mouse BLyS in immunodeficient mice which can bind to the human BLyS receptor but cannot signal following binding (Schmidt et al., 2008). In essence this becomes a “decoy” molecule blocking the human BLyS from signaling human B cells. The inability of transgenically expressed human BLyS to support human B cell development and survival is being investigated by further genetic crosses with NSG BLyS knockout mice.
Additional opportunities focus on improvements in the organization of lymphoid structures and germinal centers in humanized immunodeficient IL2rgnull mice, which lack well-organized lymphoid tissues (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012). Lymphoid tissue inducer (LTi) cells are members of an emerging family of innate lymphoid cells (ILCs) that are crucial for lymph node development. LTi cells are hematopoietically derived CD4+CD3− IL7-receptor-α+ cells (Eberl et al., 2004). They are absent in immunodeficient IL2rgnull mice due to the lack of signaling through the IL7 receptor. Their role in activating mesenchymal cells in the lymph nodes and Peyer's patch appears to be mediated through their expression of membrane-bound LTα1β2 (Yoshida et al., 1999, Mebius et al., 1997), which binds to VCAM1, ICAM1, and LTβR expressed by mesenchymal cells (Honda et al., 2001). Approaches to activate the mesenchymal cells through these receptors in the absence of LTi cells may be an alternative approach for inducing the development of lymph node anlagen in immunodeficient IL2rgnull mice.
However, simple induction of lymph node anlagen may not be sufficient for the development of desired structural components of lymph nodes and splenic germinal centers in humanized mice. A second population important in the formation, structure, and maintenance of lymph nodes and spleen germinal centers is the follicular dendritic cells (FDCs) (Aguzzi et al., 2013). These cells are stromal in origin (Krautler et al., 2012), so in immunodeficient IL2rgnull mice that have been engrafted with HSC, the FDCs are of mouse origin. These cells are required for bridging innate and adaptive B cell immune responses by stimulation via TLRs (Aguzzi et al., 2013). FDCs require B cell provision of tumor necrosis factor (TNF) and lymphotoxin (LT) for their development and maintenance (Mackay et al., 1997, Allen and Cyster, 2008, Ware, 2005), and it is currently not known whether the predominantly human CD5+ B cell population generated in humanized mice following HSC engraftment can provide this help. FDCs also have an important role in the recruitment of follicular helper T cells. Alternative approaches to enhance FDC development and maintenance in humanized mice are an opportunity to optimize adaptive immune responses by the engrafted human immune system.
MHC molecules are required for normal T cell development and function, and also for the appropriate interaction with antigen-presenting cells involved in the generation of an adaptive immune response. In humans, the MHC complex is termed HLA, and a number of immunodeficient IL2rgnull HLA transgenic mice have been generated. Almost all HLA transgenic mice have been generated exclusively on the NSG strain with just a few on the NOG strain. For HLA class I, the majority of experimental effort has focused on the HLA-A2 molecule as an ~ 30–40% of Caucasians in North America express this HLA class I molecule (for reviews, see (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012)). Published work using NSG-HLA-A2 transgenic mice in the Hu-SRC-SCID model has shown that human T cells can develop HLA-A2 restricted responses to EBV infection (Shultz et al., 2010, Strowig et al., 2009). A number of HLA-class II transgenic humanized mice have also been generated and NOD-Rag1tmMomIL2rgtm1Wjl HLA-DR4 transgenic mice engrafted with HLA-DR4 HSC appear to exhibit increased antibody responses (Danner et al., 2011, Suzuki et al., 2012). Crossing NSG mice expressing human HLA class I and class II with NSG murine MHC class I/II knockout mice that we have generated will permit the development of human T cells in NSG recipients restricted exclusively to the appropriate HLA.
5. Development of new human cell and tissue engraftment models for the study of human-specific infectious agents
Infectious agents of interest include bacterial, viral, and protozoan agents, and models to study each of these agents are highly dependent on the human cells and tissues required for cell entry, replication, and pathogenesis of each of the specific infectious agents (Wolfe et al., 2007, Baumler and Fang, 2013). For example, humans are the only known reservoir for Salmonella enterica serovar Typhi (Salmonella typhi) (Selander et al., 1990). Recently two reports used immunodeficient mice engrafted with HSCs to develop models of S. typhi (Libby et al., 2010, Song et al., 2010). In one model based on NSG mice (Libby et al., 2010), infection with S. typhi leads to progressive lethal infection (Libby et al., 2010). In contrast, infection of HSC engrafted BRG mice although leading to high organism levels does not appear as lethal as that observed in HSC-engrafted NSG mice (Song et al., 2010). This illustrates two important points. First, humanized mice can be used to study the pathogenesis of human-specific infectious agents such as S. typhi, and second, subtle but important genetic differences exist between model systems, and depending on the question being addressed, one system may be better suited to the experimental use than the other.
There are a number of human-specific infectious agents that can now be studied in small animal models due to the availability of humanized mice. These include hepatitis viruses, HIV, cytomegalovirus (CMV), Epstein–Barr virus (EBV), dengue virus, Neiserria gonorrhoeae and Neisseria meningitides, measles virus, and P. falciparum (for reviews, see (Shultz et al., 2012, Rongvaux et al., 2013, Ito et al., 2012, Brehm et al., 2013a, Brehm et al., in press 2013b, Tager et al., 2013, Denton and Garcia, 2012, Ikeno et al., 2013)). However, the humanized mouse model used to study each infectious agent is likely to depend on tissue tropism. For this, approaches to engraft various human tissues into humanized mice are critical (Fig. 2 ). For example, humanized mouse models engrafted with human immune systems have been developed as HIV infected cells of the lymphoid system such as CD4 + T cells and macrophages (Brehm et al., 2013a, Brehm et al., in press 2013b). The hepatitis viruses require human hepatocytes as their target cell, and approaches to engraft human hepatocytes into immunodeficient mice genetically modified to enhance xenogeneic human hepatocyte engraftment have been developed (Washburn et al., 2011, Dandri et al., 2001, Kosaka et al., 2013, Azuma et al., 2007, Brezillon et al., 2011, Lutgehetmann et al., 2011, Mercer et al., 2001, Brown et al., 2012, Morosan et al., 2006, Bissig et al., 2010, Vaughan et al., 2012). More recently, it has been reported that humanized mice can be engrafted with human hepatocytes and human HSC from the same donor, permitting a model system in which hepatitis virus infection can be studied in the liver in the presence of an autologous human immune system (Bility et al., 2012).
Fig. 2.
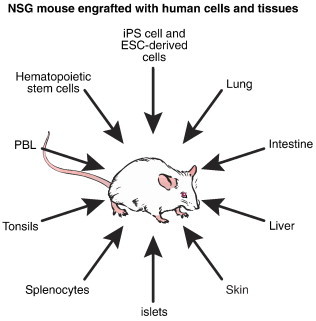
NSG mice and recently developed NSG-based models can support engraftment with multiple types of human cells and tissues. These engrafted human cells support infection with many different human pathogens.
For infectious organisms such as P. falciparum, both the human liver and the circulating human RBCs are required for a complete life cycle to occur (Tuteja, 2007). Human RBCs are present at only very low levels in the circulation of HSC-engrafted NSG mice, in large part due to their removal by host macrophages (Hu et al., 2011). As mentioned above, genetic modifications of the host are being developed to diminish mouse macrophage activity in HSC-engrafted humanized mice. In the short term however, the daily injection of human RBCs into NSG mice can lead to high circulating levels of human RBCs that support the erythrocyte portion of the P. falciparum life cycle (Jimenez-Diaz et al., 2009). The engraftment of human RBCs can also be enhanced by the injection of clodronate-loaded liposomes to kill host macrophages (Arnold et al., 2011). The use of daily injections of RBCs into human hepatocyte-engrafted mice would permit both the erythrocyte and hepatocyte-dependent parts of the P. falciparum life cycle in humanized mice to occur and be open to investigation.
Not all infectious agents target hematopoietic origin cells such as macrophages, lymphocytes or RBCs. For example, many infectious agents target stromal cells. For example N. meningitidis targets human nasopharyngeal epithelial cells (Stephens and Farley, 1991). An adaptation of humanized mice to study N. meningitidis uses engrafted human skin onto immunodeficient mice. This can be used as a model to study the pathogenesis of local vascular damage and the development of purpura (Melican et al., 2013). Another approach may be to engraft human nasal polyps (Bernstein et al., 2012), which would provide the preferred natural cell niche for the organism. Similar approaches may also be used for the study of the human-specific agent N. gonorrhoeae, which has a target niche preference for urogenital tracts (Hill et al., 2010). In addition, human cytomegalovirus infects human endothelial cells, and a humanized mouse model of CMV infection has been established by transplanting human CMV-infected internal mammary arteries into C57BL/6-Rag2null IL2rgnull mice that were then engrafted with HLA-matched PBLs (Abele-Ohl et al., 2012). The human PBLs infiltrated the grafts and created vascular lesions, similar to the pathogenesis observed in transplant arteriosclerosis.
Humanized mouse models are also needed for studies of viruses such as JC virus (JCV), a polyomavirus that causes progressive multifocal leukoencephalopathy (PML) (Tan and Koralnik, 2010). JCV is a highly species-specific virus and active replication is only permissive in the human host. The initial site of infection may be the tonsils, and tonsil material from humans can be engrafted into humanized mice to serve as a site of infection for this virus (Duchosal et al., 2000, Yamanaka et al., 2001, Vallet et al., 2005). The gastrointestinal tract can also serve as a site of infection, and human intestine transplanted humanized mice could potentially be developed (Buisine et al., 2003). Of concern is that the FDA has issued a warning for PML by JCV infection that may be associated with four commonly used drugs: Rituxan, natalizumab (Tysabri, which is a last resort medicine for severe cases of multiple sclerosis), efalizumab (Raptiva), and brentuximab vedotin. Understanding the activation and pathogenesis of JCV is a high priority to permit the use of these drugs for therapy. Towards this goal, the development of the BLT humanized mouse model has permitted the evaluation of a JC virus-specific human immune response that may be important for understanding how the human immune system responds to this virus (Tan et al., 2013).
An emerging infectious agent threat is the Middle East respiratory syndrome coronavirus (MERS-CoV, http://www.who.int/csr/don/2013_05_22_ncov/en/index.html). This virus has a tropism for nonciliated bronchial epithelial cells, and engraftment of human epithelial tissues such as human skin into immunodeficient mice (Racki et al., 2010, Kirkiles-Smith et al., 2009) may possibly establish this as a humanized mouse model for this emerging disease. Engraftment of tissues such as human pulmonary tissue (Peault et al., 1994) would provide a humanized mouse model not only for MERS-CoV but also for other coronaviruses and for other infectious agents that target lung epithelium. For infectious agents such as Salmonella that can cross the intestinal epithelium and are internalized by macrophages, neutrophils and dendritic cells, engraftment of human small intestine along with human hematopoietic stem cells into immunodeficient mice (Buisine et al., 2003) may represent a novel approach for studying this infectious agent in humanized mice.
An interesting approach to more closely recapitulate the pathogenesis of virus infection is to modify the way the infectious agent is delivered. In studies of dengue virus, NSG mice were engrafted with cord blood-derived CD34+ HSC, and dengue infected Aedes aegypti mosquitoes were allowed to bite the HSC-engrafted mice. The infected mice exhibited higher and more sustained viremia, erythema, and thrombocytopenia than did mice infected by direct intradermal injection (Cox et al., 2012). This use of this model will permit the factors that increase virulence following a mosquito bite, including potential factors in the saliva of the infected mosquito to be investigated.
Mycobacterium tuberculosis (TB) infects 8.6 million people annually and kills 1.3 million people (Tuberculosis Fact sheet, 2012, World Health Organization, Geneva, Switzerland; http://www.who.int/tb/publications/global_report/en/). Increased understanding of the human immune response to TB infection is critical to the development of effective vaccines. Adaptive human immune responses to TB have recently been demonstrated in NSG-HLA-A2 mice following intravenous infection with BCG using the BLT engraftment model (Lee et al., 2013). Other studies using BLT engrafted NSG mice infected intranasally with a strain of TB that expresses red tomato fluorophore have demonstrated the development of organized granulomatous lesions and other pathologic changes reminiscent of human TB infection (Calderon et al., 2013).
Humanized mice are also being used for the study of infectious agents classified as BSL4. Ebola virus can cause severe hemorrhagic fever in humans with a lethality rate of up to 90% (World Health Organization Fact Sheet No. 103, 2012, http://www.who.int/mediacentre/factsheets/fs103/en/). Human lymphocytes undergo apoptosis after Ebola virus infection. Using the NSG Hu-PBL model it was found that human lymphocytes underwent apoptosis after infection with mouse adapted Ebola virus but not after infection with wild-type Ebola virus (Bradfute et al., 2012), suggesting that the pathogenesis of Ebola infection can be studied in humanized mice.
Clearly, as cell and tissue tropism is identified for existing and newly emerging human-specific infectious agents, new humanized mouse models engrafted with appropriate target tissues are needed to be developed. The goal, however, will be to engraft the target cell or tissue in the presence of an autologous human immune system (Fig. 2). With the development of technologies for the generation of human induced pluripotent stem (iPS) cells (Romano et al., 2014, Bayart and Cohen-Haguenauer, 2013), this may become a future reality. For example, human hepatocytes have been generated from human iPS cells (Yoshida et al., 2011, Medine et al., 2013, Yanagida et al., 2013). However, current efforts to generate functional human HSCs from iPS cells that can generate complete human hematopoietic and immune systems in immunodeficient mice have, to date, not been successful (Klump et al., 2013). However, progress is continuing on identifying the final steps of in vitro differentiation that will be required for successful generation of true HSCs from human iPS cells. Once established this will be a major step forward in humanization approaches.
6. New technology available for manipulation of the mouse genome
Approximately a decade ago, a genetic engineering revolution began based on designer DNA nucleases capable of high efficiency precision modification of the genome (Lloyd et al., 2005, Porteus and Carroll, 2005). Over the last few years the development of these targeting nucleases has accelerated becoming the dominant biotechnology advance of the early 21st century. Using Zinc Finger Nucleases (ZFN) it became possible to target and modify the genome directly in mammalian oocytes (Carbery et al., 2010, Cui et al., 2011). The concept and capabilities to genetically target and modify almost any region in the genome rapidly engendered the development of transcription activator-like effector nuclease (TALEN) technology (Moscou and Bogdanove, 2009, Boch et al., 2009, Hockemeyer et al., 2011), and in 2012–13 the development of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats CRISPR/Cas9) technology (Jinek et al., 2012, Wang et al., 2013). Such tools now allow the exquisite efficient manipulation of the genome (Gaj et al., 2013). Here we briefly outline the basic commonalities and unique aspects of these approaches and their capabilities to create and improve humanized mouse models (Table 1 ).
Table 1.
Resource comparison, plus pros and cons for approaches to create genetic modified animals.
| Approach | Resources/time to genetically modified founder | Approx. time | Major pros | Major cons | Key ref's |
|---|---|---|---|---|---|
| ESC | $$$$$$ | ≥ 12 months | Can fully characterize multiple ESC lines before making mice. Can select for very rare events, including large (> 10 kb) modifications. |
Needs ESC tissue culture facility. Very limited genetic backgrounds available as ESC. Low efficiency of correct event Germline transmission required |
Doetschman et al. (1987), Capecchi (1989) |
| ZFN | $$$$ | ≤ 6 months | Works directly in oocytes. Highly characterized. Readily available Thought to be quite specific. |
Not easy to design or make Large modifications at low efficiency Some limitations on targeting sequence |
Carbery et al. (2010), Gaj et al. (2013), Orlando et al. (2010), Carbery et al. (2010) |
| TALEN | $$$ | ≤ 5 months | Works directly in oocytes. Moderately simple to design and make, or are readily available. Broad targeting capability. Thought to be quite specific. |
Requires an experienced molecular group to build and QC. Large modifications at low efficiency |
Moscou and Bogdanove (2009), Boch et al. (2009), Gaj et al. (2013), Hockemeyer et al. (2011) |
| CRISPR | $ | ≤ 4 months | Works in oocytes. Very simple to design and make, or readily available. Moderately broad targeting capability. |
The short targeting sequence may lead to off-target effects. | Wang et al. (2013), Gaj et al. (2013), Sander et al. (2013), Yang et al. (2013), Cho et al. (2014) |
Note: for producing deletion mutations, nucleases are microinjected into the oocyte cytoplasm. For knockins, the nuclease plus homologues DNA is microinjected into the pronucleus, although some groups have indicated that this is not an absolute requirement (Yang et al., 2013).
The common key advance in these approaches is their efficiency in precisely targeting almost any genomic region based on its DNA sequence (Fig. 3 ). This efficiency has risen to such levels that the use of embryonic stem cell (ESC) as intermediates to create precise genetic modifications in mice is, in general, no longer necessary, with modifications now being done directly in fertilized oocytes (Carbery et al., 2010). As indicated in Fig. 3, these modifications can include deletion of the targeted region, or at a lower frequency, the targeted integration of novel sequences into the region; e.g. one base, Frt/Cre sites or whole genes (Gaj et al., 2013). Crucially, by avoiding the use of ESC, the process can now be executed in a mouse strain/background that precisely fits the experimental design needs. Further, this now allows the rapid direct sequential modification of previously modified and characterized mouse strains. Thus by building on the past animal model data, these approaches facilitate a more rapid development and adoption by the community of the resulting genetically modified mouse lines. For example, we have used these approaches on NOD, NRG and NSG strains directly in oocytes, greatly speeding advances in their use for human xenotransplantation (unpublished observations). The general approach is outlined in Fig. 4 , and involves introducing into fertilized oocytes a protein, or more commonly an mRNA encoding for ZFN or TALEN which targets the genomic region of interest, or for CRISPR, mRNA encoding Cas9 plus a signal guide RNA (sgRNA) designed to complex with Cas9 protein, guiding and specifying its target (Carbery et al., 2010, Hockemeyer et al., 2011, Jinek et al., 2012).
Fig. 3.
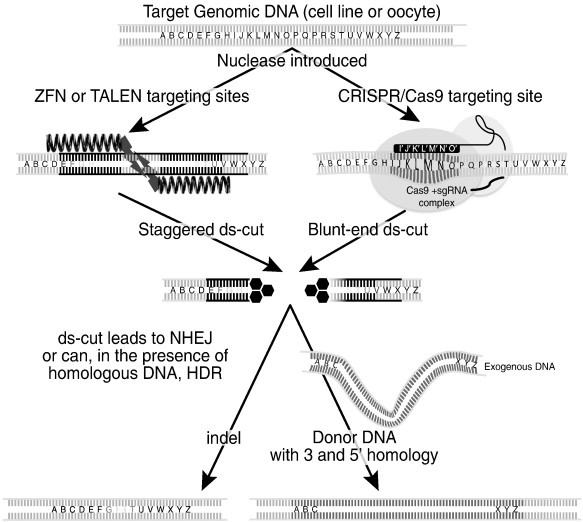
Simple schematic representation of ZFN, TALEN and CRISPR modes of action.
Starting with genomic DNA target sequence, nucleases are introduced into cells or oocytes. These can be, on the left ZFN or TALEN composed of a pair of proteins each designed to bind a defined 16–20 bases, each unit also carries an obligate homo or heterodimer of the FokI nuclease, which upon dimerization leads to a double stranded cut (ds-cut) in the intervening DNA spacer sequence. Or, on the right the protein Cas9 which causes a double stranded break at the target site when complexed with a signal guide RNA (sgRNA) defining a 17–20bp target sequence (plus a trinucleotide 5’NGG, protospacer adjacent motif (PAM) which is recognized by Cas9).Upon a double stranded DNA break occurring, a repair process is initiated leading to, in the absence of homologous DNA, nonhomologues end-joining (NHEJ) repair and the deletion of one, to many hundreds of bases, i.e. targeted deletion mutation; or if the presence of DNA with homology to the target cut site, homologous recombination or homologous directed repair (HDR) can occur providing precise DNA/gene sequence integration (Gaj et al., 2013; Orlando et al., 2010). Both NHEJ and HDR have been highly successful mouse in directing oocytes of multiple backgrounds, although at the time of writing the frequency of homologous recombination is general less than of NHEJ (Gaj et al., 2013; Low et al., 2014; Yang et al., 2013).
Fig. 4.
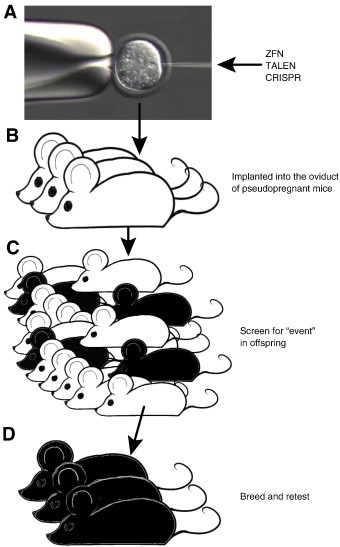
Sequence of events for gene modification using targeted nucleases.
A) ZFN and TALEN are introduced into fertilized oocytes generally as mRNAs encoding the binding site and nucleases (ZFN and TALEN) or for CRISPR, the Cas9 nuclease and its signal-guide RNA (sgRNA). B) Microinjected oocytes are introduced into pseudopregnant host females and carried to term. C) The resulting offspring is screened for the desired genetic modification event, generally by PCR and sequencing. D) Mice carrying desired modification events are bred to ensure germline transmission and eliminate any possible mosaicism.
The key challenge in using these approaches is achieving reproducibly high efficiencies of the desired genetic modification. This is especially relevant to modification requiring knockins where efficiency of the correct integration event is lower than that of non-homologous end joining (NHEJ) events. When using fertilized oocytes as the starting material, if efficiency of the correct targeted modification falls below ~ 3% the cost to make modified animals can become prohibitive due to the numbers of oocyte donors required. ZFN, TALEN and CRISPR are available from commercial vendors in various formats and restrictions. However TALEN and especially CRISPR can be constructed in a standard molecular lab. Assistance for TALEN and CRISPR construct design can be found on the web where a number of computer programs/sites exist; e.g. http://zifit.partners.org/ZiFiT/ or http://crispr.mit.edu. Further, many of the genetic elements required to build TALEN and CRISPR nucleases are available via https://www.addgene.org.
Although these systems are moderately simple for a molecular lab plus microinjection group to establish, as with all new technologies they also engender a host of new challenges (Table 1). These can range from difficulty in making high quality mRNA, the ability to microinject, especially if pronuclear injection is required, to screen for the correct event in founder animals. For example, a common and unexpected occurrence we and others have reported is that often founder animals are somatic and germ line (gamete) mosaics. This is thought to be due to the nuclease activity occurring post one cell (oocyte) stage; for example at 2, 4 or even 8 cell stage, with blastomeres having independent genetic modification events. The resulting animal of this event is often a mosaic of possibly quite different and independent genetic modification events. This can lead to the identification of founders with a correct event (in their tail derived DNA), but upon breeding they produce offspring with perhaps a whole series of different genetic modifications; i.e. tail derived DNA is not always the same as the gametes (Sung et al., 2013). However, as these approaches mature and the actual mechanisms involved in nuclease meditated homologous recombination are more fully understood, it is expected that modification events will rise to 100% in all somatic and germ cells.
An issue often raised in the use of nuclease modification is the possibility of off-target damage to the genome caused by lack of specificity (Wang et al., 2013, Sander et al., 2013, Yang et al., 2013). This is a valid concern, especially in the case of CRISPR where the recognition site is ~ 20 nucleotides which has a degree of non-specific wobble at the 5′ end can be detected (Fu et al., 2013, Hsu et al., 2013, Pattanayak et al., 2013). However, this challenge has to be viewed in the context of previous genetic engineering approaches based on ESC or random transgenesis both of which were not immune to collateral genome damage. The adage “buyer beware” is relevant here and it is strongly encouraged that good breeding records be maintained so that if untoward effects are seen that they can be examined and the potential off-target effect can be eliminated (or more rarely, confirmed).
Although we have focused on the genetic modification and humanization of mice, these approaches can also be used with any DNA organism where the embryonic stage or germ cells can be accessed, ranging from maize (Shukla et al., 2009) to parasitic protozoans (malaria) (Smidler et al., 2013), to rat (Mashimo et al., 2010) and human (Urnov et al., 2005). This opens a whole vista of possibilities in the development of humanized animal models.
7. Conclusions
Humanized mice are rapidly becoming important tools for the study of human-specific infectious agents. Models for many of the human agents are now available for the study of the pathogenesis of the infectious agent, for the evaluation of drug efficacy, and for the development and testing of vaccines. However, there remain opportunities to optimize further the immunodeficient host, increase the robustness of engrafted human immune systems, further identify novel models for engraftment of non-hematopoietic human cells and tissues as targets for the infectious agents, and for the implementation of novel technologies for rapidly generating new genetically modified hosts. Targeted nuclease based genetic engineering will greatly speed their development allowing rapid sequential modification of preexisting established strains. The use of humanized mice as small animal models for the study of human-specific agents will likely provide novel insights into the biology of the agents that might otherwise not be available.
Acknowledgments
This work was supported by the National Institutes of Health research grants AI046629, DP1DA034990, U01DK089572, R24OD016473-0, a Cancer Core Grant CA034196, a grant from the University of Massachusetts Center for AIDS Research, P30 AI042845 and a grant from The Leona M. and Harry B. Helmsley Charitable Trust 2012PG-T1D018. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. MAB and DLG are the consultants for The Jackson Laboratory. We thank Peter Kutny for providing images for microinjection.
Contributor Information
Michael A. Brehm, Email: michael.brehm@umassmed.edu.
Michael V. Wiles, Email: michael.wiles@jax.org.
Dale L. Greiner, Email: dale.greiner@umassmed.edu.
Leonard D. Shultz, Email: lenny.shultz@jax.org.
References
- Abele-Ohl S., Leis M., Wollin M., Mahmoudian S., Hoffmann J., Muller R., Heim C., Spriewald B.M., Weyand M., Stamminger T., Ensminger S.M. Human cytomegalovirus infection leads to elevated levels of transplant arteriosclerosis in a humanized mouse aortic xenograft model. Am. J. Transplant. 2012;12:1720. doi: 10.1111/j.1600-6143.2012.04018.x. [DOI] [PubMed] [Google Scholar]
- Aguzzi A., Kranich J., Krautler N.J. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2013;35:105–113. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Ali N., Flutter B., Sanchez R.R., Sharif-Paghaleh E., Barber L.D., Lombardi G., Nestle F.O. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rgammanull mice display a T-effector memory phenotype. PLoS One. 2012;7:e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C.D., Cyster J.G. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin. Immunol. 2008;20:14. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L., Tyagi R.K., Meija P., Swetman C., Gleeson J., Perignon J.L., Druilhe P. Further improvements of the P. falciparum humanized mouse model. PLoS One. 2011;6:e18045. doi: 10.1371/journal.pone.0018045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma H., Paulk N., Ranade A., Dorrell C., Al-Dhalimy M., Ellis E., Strom S., Kay M.A., Finegold M., Grompe M. Robust expansion of human hepatocytes in Fah −/−/Rag2 −/−/Il2rg −/− mice. Nat. Biotechnol. 2007;25:903. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A.N., Brown M.H. The SIRP family of receptors and immune regulation. Nat. Rev. Immunol. 2006;6:457. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- Baumler A., Fang F.C. Host specificity of bacterial pathogens. Cold Spring Harb. Perspect. Med. 2013;3 doi: 10.1101/cshperspect.a010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart E., Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr. Genet. Ther. 2013;13:73. doi: 10.2174/1566523211313020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J.M., Lehman H., Lis M., Sands A., Wilding G.E., Shultz L., Bankert R., Bobek L. Humanized mouse model used to monitor MUC gene expression in nasal polyps and to preclinically evaluate the efficacy of Montelukast in reducing mucus production. Ann. Otol. Rhinol. Laryngol. 2012;121:307. doi: 10.1177/000348941212100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility M.T., Zhang L., Washburn M.L., Curtis T.A., Kovalev G.I., Su L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat. Protoc. 2012;7:1608. doi: 10.1038/nprot.2012.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig K.D., Wieland S.F., Tran P., Isogawa M., Le T.T., Chisari F.V., Verma I.M. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J. Clin. Invest. 2010;120:924. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Chang H., Sarkis P.T., Fikrig E., Zhu Q., Marasco W.A. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5 + B cells. Immunology. 2011;134:419. doi: 10.1111/j.1365-2567.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bosma G.C., Custer R.P., Bosma M.J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bradfute S.B., Warfield K.L., Bray M. Mouse models for filovirus infections. Viruses. 2012;4:1477. doi: 10.3390/v4091477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D.M., Seung E., Frahm N., Cariappa A., Bailey C.C., Hart W.K., Shin H.S., Brooks S.F., Knight H.L., Eichbaum Q., Yang Y.G., Sykes M., Walker B.D., Freeman G.J., Pillai S., Westmoreland S.V., Brander C., Luster A.D., Tager A.M. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 2009;83:7305. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm M.A., Racki W.J., Leif J., Burzenski L., Hosur V., Wetmore A., Gott B., Herlihy M., Ignotz R., Dunn R., Shultz L.D., Greiner D.L. Engraftment of human HSC in non-irradiated newborn NOD–scid IL2rgammanull mice is enhanced by transgenic expression of membrane-bound human SCF. Blood. 2012;119:2778. doi: 10.1182/blood-2011-05-353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm M.A., Shultz L.D., Luban J., Greiner D.L. Overcoming current limitations in humanized mouse research. J. Infect. Dis. 2013;208(Suppl. 2):S125. doi: 10.1093/infdis/jit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm M.A., Jouvet N., Greiner D.L., Shultz L.D. Humanized mice for the study of infectious diseases. Curr. Opin. Immunol. 2013;25:428–435. doi: 10.1016/j.coi.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezillon N., Brunelle M.N., Massinet H., Giang E., Lamant C., DaSilva L., Berissi S., Belghiti J., Hannoun L., Puerstinger G., Wimmer E., Neyts J., Hantz O., Soussan P., Morosan S., Kremsdorf D. Antiviral activity of Bay 41-4109 on hepatitis B virus in humanized Alb-uPA/SCID mice. PLoS One. 2011;6:e25096. doi: 10.1371/journal.pone.0025096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R.J., Hudson N., Wilson G., Rehman S.U., Jabbari S., Hu K., Tarr A.W., Borrow P., Joyce M., Lewis J., Zhu L.F., Law M., Kneteman N., Tyrrell D.L., McKeating J.A., Ball J.K. Hepatitis C virus envelope glycoprotein fitness defines virus population composition following transmission to a new host. J. Virol. 2012;86:11956. doi: 10.1128/JVI.01079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisine M.P., Aubert J.P., Walker W.A., Savidge T.C. Developmental patterns of mucin gene expression in human fetal small intestinal xenografts maintained in severe-combined immunodeficient mice. Pediatr. Res. 2003;53:898. doi: 10.1203/01.PDR.0000064582.30004.62. [DOI] [PubMed] [Google Scholar]
- Calderon V.E., Valbuena G., Goez Y., Judy B.M., Huante M.B., Sutjita P., Johnston R.K., Estes D.M., Hunter R.L., Actor J.K., Cirillo J.D., Endsley J.J. A humanized mouse model of tuberculosis. PLoS One. 2013;8:e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M.R. Altering the genome by homologous recombination. Science. 1989;244:1288. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Carbery I.D., Ji D., Harrington A., Brown V., Weinstein E.J., Liaw L., Cui X. Targeted genome modification in mice using zinc-finger nucleases. Genetics. 2010;186:451. doi: 10.1534/genetics.110.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateau M., Swanson M.D., Garcia J.V. Inefficient vaginal transmission of tenofovir-resistant HIV-1. J. Virol. 2013;87:1274. doi: 10.1128/JVI.01777-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateau M.L., Denton P.W., Swanson M.D., McGowan I., Garcia J.V. Rectal transmission of transmitted/founder HIV-1 is efficiently prevented by topical 1% tenofovir in BLT humanized mice. PLoS One. 2013;8:e60024. doi: 10.1371/journal.pone.0060024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., He F., Kwang J., Chan J.K., Chen J. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J. Immunol. 2012;189:5223. doi: 10.4049/jimmunol.1201789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., Kim J.S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson S.W., Greiner D.L., Schweitzer I.B., Gott B., Beamer G.L., Schweitzer P.A., Hesselton R.M., Shultz L.D. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6 J-scid mice and in C57BL/6 J-scid bg mice. Cell. Immunol. 1996;171:186. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- Covassin L., Laning J., Abdi R., Langevin D.L., Phillips N.E., Shultz L.D., Brehm M.A. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rgamma(null) H2-Ab1 (tm1Gru) Tg (human leucocyte antigen D-related 4) mice: a mouse model of human allogeneic graft-versus-host disease. Clin. Exp. Immunol. 2011;166:269. doi: 10.1111/j.1365-2249.2011.04462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L., Jangalwe S., Jouvet N., Laning J., Burzenski L., Shultz L.D., Brehm M.A. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin. Exp. Immunol. 2013;174:372. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Mota J., Sukupolvi-Petty S., Diamond M.S., Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J. Virol. 2012;86:7637. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Ji D., Fisher D.A., Wu Y., Briner D.M., Weinstein E.J. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat. Biotechnol. 2011;29:64. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- Dandri M., Burda M.R., Torok E., Pollok J.M., Iwanska A., Sommer G., Rogiers X., Rogler C.E., Gupta S., Will H., Greten H., Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- Danner R., Chaudhari S.N., Rosenberger J., Surls J., Richie T.L., Brumeanu T.D., Casares S. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One. 2011;6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton P.W., Garcia J.V. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol. 2012;20:268. doi: 10.1016/j.tim.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton P.W., Nochi T., Lim A., Krisko J.F., Martinez-Torres F., Choudhary S.K., Wahl A., Olesen R., Zou W., Di Santo J.P., Margolis D.M., Garcia J.V. IL-2 receptor gamma-chain molecule is critical for intestinal T-cell reconstitution in humanized mice. Mucosal Immunol. 2012;5:555–556. doi: 10.1038/mi.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T., Gregg R.G., Maeda N., Hooper M.L., Melton D.W., Thompson S., Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Duchosal M.A., Fuzzati-Armentero M.T., Baccala R., Layer A., Gonzalez-Quintial R., Leturcq D., Ruegg M., Trouillet P., Mauray S., Tissot J.D., Schapira M. Human adult tonsil xenotransplantation into SCID mice for studying human immune responses and B cell lymphomagenesis. Exp. Hematol. 2000;28:177. doi: 10.1016/s0301-472x(99)00137-x. [DOI] [PubMed] [Google Scholar]
- Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat. Biotechnol. 2013;31:822. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C.A., Barbas C.F., III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille C., Orlikowsky T.W., Spring B., Hartwig U.F., Wilhelm A., Wirth A., Goecke B., Handgretinger R., Poets C.F., Andre M.C. Monocytes derived from humanized neonatal NOD/SCID/IL2Rgamma(null) mice are phenotypically immature and exhibit functional impairments. Hum. Immunol. 2012;73:346. doi: 10.1016/j.humimm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Greenblatt M.B., Vbranac V., Tivey T., Tsang K., Tager A.M., Aliprantis A.O. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS One. 2012;7:e44664. doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D.J., Griffiths N.J., Borodina E., Virji M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin. Sci. (Lond.) 2010;118:547. doi: 10.1042/CS20090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D., Wang H., Kiani S., Lai C.S., Gao Q., Cassady J.P., Cost G.J., Zhang L., Santiago Y., Miller J.C., Zeitler B., Cherone J.M., Meng X., Hinkley S.J., Rebar E.J., Gregory P.D., Urnov F.D., Jaenisch R. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011;29:731. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K., Jiang Z., Tabeta K., Du X., Georgel P., Crozat K., Beutler B. Genetic analysis of innate immunity. Adv. Immunol. 2006;91:175. doi: 10.1016/S0065-2776(06)91005-0. [DOI] [PubMed] [Google Scholar]
- Honda K., Nakano H., Yoshida H., Nishikawa S., Rennert P., Ikuta K., Tamechika M., Yamaguchi K., Fukumoto T., Chiba T., Nishikawa S.I. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J. Exp. Med. 2001;193:621. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O., Cradick T.J., Marraffini L.A., Bao G., Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Van R.N., Yang Y.G. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno S., Suzuki M.O., Muhsen M., Ishige M., Kobayashi-Ishihara M., Ohno S., Takeda M., Nakayama T., Morikawa Y., Terahara K., Okada S., Takeyama H., Tsunetsugu-Yokota Y. Sensitive detection of measles virus infection in the blood and tissues of humanized mouse by one-step quantitative RT-PCR. Front. Microbiol. 2013;4:298. doi: 10.3389/fmicb.2013.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain null mice. Blood. 2005;106:1565. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Kurtz J., Shaffer J., Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40–CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J. Immunol. 2001;166:2970. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., Heike T., Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012;9:208. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Diaz M.B., Mulet T., Viera S., Gomez V., Garuti H., Ibanez J., varez-Doval A., Shultz L.D., Martinez A., Gargallo-Viola D., ngulo-Barturen I. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD–scid IL2Rgammanull mice engrafted with human erythrocytes. Antimicrob. Agents Chemother. 2009;53:4533. doi: 10.1128/AAC.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalscheuer H., Danzl N., Onoe T., Faust T., Winchester R., Goland R., Greenberg E., Spitzer T.R., Savage D.G., Tahara H., Choi G., Yang Y.G., Sykes M. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl. Med. 2012;4:125ra30. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato C., Fujii E., Chen Y.J., Endaya B.B., Matsubara K., Suzuki M., Ohnishi Y., Tamaoki N. Spontaneous thymic lymphomas in the non-obese diabetic/Shi-scid, IL-2R gamma (null) mouse. Lab. Anim. 2009;43:402. doi: 10.1258/la.2009.009012. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- King M., Pearson T., Shultz L.D., Leif J., Bottino R., Trucco M., Atkinson M.A., Wasserfall C., Herold K.C., Woodland R.T., Schmidt M.R., Woda B.A., thompson m.j., Rossini A.A., Greiner D.L. A new Hu–PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin. Immunol. 2008;126:303. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- King M.A., Covassin L., Brehm M.A., Racki W., Pearson T., Leif J., Laning J., Fodor W., Foreman O., Burzenski L., Chase T., Gott B., Rossini A.A., Bortell R., Shultz L.D., Greiner D.L. Hu–PBL–NOD–scid IL2rgnull mouse model of xenogeneic graft-versus-host-like disease and the role of host MHC. Clin. Exp. Immunol. 2009;157:104. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkiles-Smith N.C., Harding M.J., Shepherd B.R., Fader S.A., Yi T., Wang Y., McNiff J.M., Snyder E.L., Lorber M.I., Tellides G., Pober J.S. Development of a humanized mouse model to study the role of macrophages in allograft injury. Transplantation. 2009;87:189. doi: 10.1097/TP.0b013e318192e05d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump H., Teichweyde N., Meyer C., Horn P.A. Development of patient-specific hematopoietic stem and progenitor cell grafts from pluripotent stem cells, in vitro. Curr. Mol. Med. 2013;13:815. doi: 10.2174/1566524011313050012. [DOI] [PubMed] [Google Scholar]
- Kosaka K., Hiraga N., Imamura M., Yoshimi S., Murakami E., Nakahara T., Honda Y., Ono A., Kawaoka T., Tsuge M., Abe H., Hayes C.N., Miki D., Aikata H., Ochi H., Ishida Y., Tateno C., Yoshizato K., Sasaki T., Chayama K. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem. Biophys. Res. Commun. 2013;441:230. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Tanaka Y., Tanaka R., Misawa N., Kawano Y., Tanaka T., Miyasaka M., Ito M., Ueyama Y., Yamamoto N. High levels of viremia in hu–PBL–NOD–scid mice with HIV-1 infection. Leukemia. 1997;11(Suppl. 3):109. [PubMed] [Google Scholar]
- Krautler N.J., Kana V., Kranich J., Tian Y., Perera D., Lemm D., Schwarz P., Armulik A., Browning J.L., Tallquist M., Buch T., Oliveira-Martins J.B., Zhu C., Hermann M., Wagner U., Brink R., Heikenwalder M., Aguzzi A. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34 + cell transplantation. Blood. 2006;108:487. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Lapidot T., Pflumio F., Doedens M., Murdoch B., Williams D.E., Dick J.E. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- Lavender K.J., Pang W.W., Messer R.J., Duley A.K., Race B., Phillips K., Scott D., Peterson K.E., Chan C.K., Dittmer U., Dudek T., Allen T.M., Weissman I.L., Hasenkrug K.J. BLT-humanized C57BL/6 Rag2 −/− gammac −/− CD47 −/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood. 2013;122:4013. doi: 10.1182/blood-2013-06-506949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Brehm M.A., Greiner D., Shultz L.D., Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol. 2013;14:53. doi: 10.1186/1471-2172-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepus C.M., Gibson T.F., Gerber S.A., Kawikova I., Szczepanik M., Hossain J., Ablamunits V., Kirkiles-Smith N., Herold K.C., Donis R.O., Bothwell A.L., Pober J.S., Harding M.J. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD–scid/gammac −/−, BALB/c-Rag1 −/− gammac −/−, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 2009;70:790. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby S.J., Brehm M.A., Greiner D.L., Shultz L.D., McClelland M., Smith K.D., Cookson B.T., Karlinsey J.E., Kinkel T.L., Porwollik S., Canals R., Cummings L.A., Fang F.C. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella typhi infection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15589. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A., Plaisier C.L., Carroll D., Drews G.N. Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2232. doi: 10.1073/pnas.0409339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge J.L., Zhou Y., Becker Y.A., Ma S., Kenney S.C., Hematti P., Capitini C.M., Burlingham W.J., Gendron-Fitzpatrick A., Gumperz J.E. Mice engrafted with human fetal thymic tissue and hematopoietic stem cells develop pathology resembling chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2013;19:1310. doi: 10.1016/j.bbmt.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B.E., Krebs M.P., Joung J.K., Tsai S.Q., Nishina P.M., Wiles M.V. Correction of the Crb1rd8 allele and retinal phenotype in C57BL/6 N mice via TALEN-mediated homology-directed repair. Invest. Ophthalmol. Vis. Sci. 2014;55:387. doi: 10.1167/iovs.13-13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgehetmann M., Bornscheuer T., Volz T., Allweiss L., Bockmann J.H., Pollok J.M., Lohse A.W., Petersen J., Dandri M. Hepatitis B virus limits response of human hepatocytes to interferon-alpha in chimeric mice. Gastroenterology. 2011;140(2074):2083. doi: 10.1053/j.gastro.2011.02.057. [DOI] [PubMed] [Google Scholar]
- Mackay F., Majeau G.R., Lawton P., Hochman P.S., Browning J.L. Lymphotoxin but not tumor necrosis factor functions to maintain splenic architecture and humoral responsiveness in adult mice. Eur. J. Immunol. 1997;27:2033. doi: 10.1002/eji.1830270830. [DOI] [PubMed] [Google Scholar]
- Mashimo T., Takizawa A., Voigt B., Yoshimi K., Hiai H., Kuramoto T., Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS One. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T., Murata Y., Okazawa H., Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Kametani Y., Ando K., Hirano Y., Katano I., Ito R., Shiina M., Tsukamoto H., Saito Y., Tokuda Y., Kato S., Ito M., Motoyoshi K., Habu S. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp. Hematol. 2003;31:789. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- McCune J.M., Namikawa R., Kaneshima H., Shultz L.D., Lieberman M., Weissman I.L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mebius R.E., Rennert P., Weissman I.L. Developing lymph nodes collect CD4 + CD3 − LTbeta + cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Medine C.N., Lucendo-Villarin B., Storck C., Wang F., Szkolnicka D., Khan F., Pernagallo S., Black J.R., Marriage H.M., Ross J.A., Bradley M., Iredale J.P., Flint O., Hay D.C. Developing high-fidelity hepatotoxicity models from pluripotent stem cells. Stem Cells Transl. Med. 2013;2:505. doi: 10.5966/sctm.2012-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melican K., Michea V.P., Martin T., Bruneval P., Dumenil G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathog. 2013;9:e1003139. doi: 10.1371/journal.ppat.1003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A., Wege A.K., Haase A.T., Garcia J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006;12:1316. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- Mercer D.F., Schiller D.E., Elliott J.F., Douglas D.N., Hao C., Rinfret A., Addison W.R., Fischer K.P., Churchill T.A., Lakey J.R., Tyrrell D.L., Kneteman N.M. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001;7:927. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Morosan S., Hez-Deroubaix S., Lunel F., Renia L., Giannini C., Van Rooijen N., Battaglia S., Blanc C., Eling W., Sauerwein R., Hannoun L., Belghiti J., Brechot C., Kremsdorf D., Druilhe P. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J. Infect. Dis. 2006;193:996. doi: 10.1086/500840. [DOI] [PubMed] [Google Scholar]
- Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Mosier D.E., Gulizia R.J., Baird S.M., Wilson D.B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mosier D.E., Gulizia R.J., Baird S.M., Wilson D.B., Spector D.H., Spector S.A. Human immunodeficiency virus infection of human-PBL– SCID mice. Science. 1991;251:791. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I.L., McCune J.M. Infection of the SCID– hu mouse by HIV-1. Science. 1988;242:1684. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Nauseef W.M. Biological roles for the NOX family NADPH oxidases. J. Biol. Chem. 2008;283:16961. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe T., Kalscheuer H., Danzl N., Chittenden M., Zhao G., Yang Y.G., Sykes M. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J. Immunol. 2011;187:3895. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando S.J., Santiago Y., DeKelver R.C., Freyvert Y., Boydston E.A., Moehle E.A., Choi V.M., Gopalan S.M., Lou J.F., Li J., Miller J.C., Holmes M.C., Gregory P.D., Urnov F.D., Cost G.J. Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology. Nucleic Acids Res. 2010;38:e152. doi: 10.1093/nar/gkq512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V., Lin S., Guilinger J.P., Ma E., Doudna J.A., Liu D.R. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013;31:839. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault B., Tirouvanziam R., Sombardier M.N., Chen S., Perricaudet M., Gaillard D. Gene transfer to human fetal pulmonary tissue developed in immunodeficient SCID mice. Hum. Gene Ther. 1994;5:1131. doi: 10.1089/hum.1994.5.9-1131. [DOI] [PubMed] [Google Scholar]
- Porteus M.H., Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- Racki W.J., Covassin L., Brehm M., Pino S., Ignotz R., Dunn R., Laning J., Rossini A.A., Shultz L.D., Greiner D.L. NOD– scid IL2rgnull (NSG) mouse model of human skin transplantation and allograft rejection. Transplantation. 2010;89:527. doi: 10.1097/TP.0b013e3181c90242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C., Poueymirou W.T., Rojas J., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Rongvaux A., Eynon E.E., Manz M.G., Flavell R.A. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118:3119. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- Rochman Y., Spolski R., Leonard W.J. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano G., Morales F., Marino I.R., Giordano A. A commentary on iPS cells: potential applications in autologous transplantation, study of illnesses and drug screening. J. Cell. Physiol. 2014;229:148. doi: 10.1002/jcp.24437. [DOI] [PubMed] [Google Scholar]
- Rongvaux A., Willinger T., Takizawa H., Rathinam C., Auerbach W., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Eynon E.E., Stevens S., Manz M.G., Flavell R.A. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2378. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A., Takizawa H., Strowig T., Willinger T., Eynon E.E., Flavell R.A., Manz M.G. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu. Rev. Immunol. 2013;31:635. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J.D., Ramirez C.L., Linder S.J., Pattanayak V., Shoresh N., Ku M., Foden J.A., Reyon D., Bernstein B.E., Liu D.R., Joung J.K. In silico abstraction of zinc finger nuclease cleavage profiles reveals an expanded landscape of off-target sites. Nucleic Acids Res. 2013;41:e181. doi: 10.1093/nar/gkt716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva A., Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.R., Appel M.C., Giassi L.J., Greiner D.L., Shultz L.D., Woodland R.T. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R.K., Beltran P., Smith N.H., Helmuth R., Rubin F.A., Kopecko D.J., Ferris K., Tall B.D., Cravioto A., Musser J.M. Evolutionary genetic relationships of clones of Salmonella serovars that cause human typhoid and other enteric fevers. Infect. Immun. 1990;58:2262. doi: 10.1128/iai.58.7.2262-2275.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla V.K., Doyon Y., Miller J.C., DeKelver R.C., Moehle E.A., Worden S.E., Mitchell J.C., Arnold N.L., Gopalan S., Meng X., Choi V.M., Rock J.M., Wu Y.Y., Katibah G.E., Zhifang G., McCaskill D., Simpson M.A., Blakeslee B., Greenwalt S.A., Butler H.J., Hinkley S.J., Zhang L., Rebar E.J., Gregory P.D., Urnov F.D. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature. 2009;459:437. doi: 10.1038/nature07992. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Schweitzer P.A., Christianson S.W., Gott B., Schweitzer I.B., McKenna S., Mobraaten L., Rajan T.V., Greiner D.L., Leiter E.H. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995;154:180. [PubMed] [Google Scholar]
- Shultz L.D., Banuelos S.J., Leif J., Appel M.C., Cunningham M., Ballen K., Burzenski L., Greiner D.L. Regulation of human short-term repopulating cell (STRC) engraftment in NOD/SCID mice by host CD122+ cells. Exp. Hematol. 2003;31:551. doi: 10.1016/s0301-472x(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S., Gillies S.D., King M., Mangada J., Greiner D.L., Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rγnull mice engrafted with mobilized human hematopoietic stem cell. J. Immunol. 2005;174:6477. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Shultz L.D., Saito Y., Najima Y., Tanaka S., Ochi T., Tomizawa M., Doi T., Sone A., Suzuki N., Fujiwara H., Yasukawa M., Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13022. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L.D., Brehm M.A., Garcia-Martinez J.V., Greiner D.L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 2012;12:786. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidler A.L., Terenzi O., Soichot J., Levashina E.A., Marois E. Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS One. 2013;8:e74511. doi: 10.1371/journal.pone.0074511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Willinger T., Rongvaux A., Eynon E.E., Stevens S., Manz M.G., Flavell R.A., Galan J.E. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D.S., Farley M.M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev. Infect. Dis. 1991;13:22. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- Stoddart C.A., Maidji E., Galkina S.A., Kosikova G., Rivera J.M., Moreno M.E., Sloan B., Joshi P., Long B.R. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD–scid IL-2Rgamma(−/−) (NSG) BLT mice. Virology. 2011;417:154. doi: 10.1016/j.virol.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T., Gurer C., Ploss A., Liu Y.F., Arrey F., Sashihara J., Koo G., Rice C.M., Young J.W., Chadburn A., Cohen J.I., Munz C. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J. Exp. Med. 2009;206:1423. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T., Rongvaux A., Rathinam C., Takizawa H., Borsotti C., Philbrick W., Eynon E.E., Manz M.G., Flavell R.A. Transgenic expression of human signal regulatory protein alpha in Rag2 −/−{gamma}c −/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13218. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y.H., Baek I.J., Kim D.H., Jeon J., Lee J., Lee K., Jeong D., Kim J.S., Lee H.W. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 2013;31:23. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Takahashi T., Katano I., Ito R., Ito M., Harigae H., Ishii N., Sugamura K. Induction of human humoral immune responses in a novel HLA-DR-expressing transgenic NOD/Shi-scid/gammacnull mouse. Int. Immunol. 2012;24:243. doi: 10.1093/intimm/dxs045. [DOI] [PubMed] [Google Scholar]
- Tager A.M., Pensiero M., Allen T.M. Recent advances in humanized mice: accelerating the development of an HIV vaccine. J. Infect. Dis. 2013;208(Suppl. 2):S121. doi: 10.1093/infdis/jit451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S., Saito Y., Hijikata A., Tanaka S., Watanabe T., Hasegawa T., Mochizuki S., Kunisawa J., Kiyono H., Koseki H., Ohara O., Saito T., Taniguchi S., Shultz L.D., Ishikawa F. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K., Prasolava T.K., Wang J.C., Mortin-Toth S.M., Khalouei S., Gan O.I., Dick J.E., Danska J.S. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 2007;8:1313. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Tan C.S., Koralnik I.J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.S., Broge T.A., Jr., Seung E., Vrbanac V., Viscidi R., Gordon J., Tager A.M., Koralnik I.J. Detection of JC virus-specific immune responses in a novel humanized mouse model. PLoS One. 2013;8:e64313. doi: 10.1371/journal.pone.0064313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Saito Y., Kunisawa J., Kurashima Y., Wake T., Suzuki N., Shultz L.D., Kiyono H., Ishikawa F. Development of mature and functional human myeloid subsets in hematopoietic stem cell-engrafted NOD/SCID/IL2rgammaKO mice. J. Immunol. 2012;188:6145. doi: 10.4049/jimmunol.1103660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer T.C., Delano M., Liu C., Chen J., Padgett L.E., Tse H.M., Annamali M., Piganelli J.D., Moldawer L.L., Mathews C.E. Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes. Diabetes. 2011;60:2144. doi: 10.2337/db10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A., Manz M.G. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Tsai R.K., Discher D.E. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 2008;180:989. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja R. Malaria — an overview. FEBS J. 2007;274:4670. doi: 10.1111/j.1742-4658.2007.05997.x. [DOI] [PubMed] [Google Scholar]
- Urnov F.D., Miller J.C., Lee Y.L., Beausejour C.M., Rock J.M., Augustus S., Jamieson A.C., Porteus M.H., Gregory P.D., Holmes M.C. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- Vallet V., Mauray S., Kindler V., Aubry D., Ruegg M., Cherpillod J., Waridel F., Schapira M., Duchosal M.A. Human tonsil implants xenotransplanted in SCID mice display broad lymphocytic diversity and cellular activation profile similar to those in the original lymphoid organ. Xenotransplantation. 2005;12:38. doi: 10.1111/j.1399-3089.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Vaughan A.M., Aly A.S., Kappe S.H. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A.M., Mikolajczak S.A., Wilson E.M., Grompe M., Kaushansky A., Camargo N., Bial J., Ploss A., Kappe S.H. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J. Clin. Invest. 2012;122:3618. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges M., Bachmann V., Naujoks J., Kopp K., Hauck C.R. Extracellular IgC2 constant domains of CEACAMs mediate PI3K sensitivity during uptake of pathogens. PLoS One. 2012;7:e39908. doi: 10.1371/journal.pone.0039908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waern J.M., Yuan Q., Rudrich U., Becker P.D., Schulze K., Strick-Marchand H., Huntington N.D., Zacher B.J., Wursthorn K., DiSanto J.P., Guzman C.A., Manns M.P., Ott M., Bock M. Ectopic expression of murine CD47 minimizes macrophage rejection of human hepatocyte xenografts in immunodeficient mice. Hepatology. 2012;56:1479. doi: 10.1002/hep.25816. [DOI] [PubMed] [Google Scholar]
- Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C.F. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005;23:787. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Washburn M.L., Bility M.T., Zhang L., Kovalev G.I., Buntzman A., Frelinger J.A., Barry W., Ploss A., Rice C.M., Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Takahashi T., Okajima A., Shiokawa M., Ishii N., Katano I., Ito R., Ito M., Minegishi M., Minegishi N., Tsuchiya S., Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu–HSC NOG mice) Int. Immunol. 2009;21:843. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- Willinger T., Rongvaux A., Takizawa H., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Auerbach W., Eynon E.E., Stevens S., Manz M.G., Flavell R.A. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2390. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T., Ando K., Sato T., Miyatake H., Nakamura Y., Muguruma Y., Kato S., Hotta T. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101:2905. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- Yamanaka N., Yamamoto Y., Kuki K. Engraftment of tonsillar mononuclear cells in human skin/SCID mouse chimera-validation of a novel xenogeneic transplantation model for autoimmune diseases. Microbiol. Immunol. 2001;45:507. doi: 10.1111/j.1348-0421.2001.tb02651.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Takenaka K., Urata S., Shima T., Kikushige Y., Tokuyama T., Iwamoto C., Nishihara M., Iwasaki H., Miyamoto T., Honma N., Nakao M., Matozaki T., Akashi K. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood. 2013;121:1316. doi: 10.1182/blood-2012-06-440354. [DOI] [PubMed] [Google Scholar]
- Yanagida A., Ito K., Chikada H., Nakauchi H., Kamiya A. An in vitro expansion system for generation of human iPS cell-derived hepatic progenitor-like cells exhibiting a bipotent differentiation potential. PLoS One. 2013;8:e67541. doi: 10.1371/journal.pone.0067541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wang H., Shivalila C.S., Cheng A.W., Shi L., Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Honda K., Shinkura R., Adachi S., Nishikawa S., Maki K., Ikuta K., Nishikawa S.I. IL-7 receptor alpha + CD3(−) cells in the embryonic intestine induces the organizing center of Peyer's patches. Int. Immunol. 1999;11:643. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Takayama K., Kondoh M., Sakurai F., Tani H., Sakamoto N., Matsuura Y., Mizuguchi H., Yagi K. Use of human hepatocyte-like cells derived from induced pluripotent stem cells as a model for hepatocytes in hepatitis C virus infection. Biochem. Biophys. Res. Commun. 2011;416:119. doi: 10.1016/j.bbrc.2011.11.007. [DOI] [PubMed] [Google Scholar]


