Abstract
This brief review is based on a President’s Lecture presented at the Annual Meeting of the American College of Sports Medicine in 2013. The purpose of this review is to assess the effects of climate change and consequent increases in environmental heat stress on the aging cardiovascular system. The earth’s average global temperature is slowly but consistently increasing, and along with mean temperature changes come increases in heat wave frequency and severity. Extreme passive thermal stress resulting from prolonged elevations in ambient temperature, as well as prolonged physical activity in hot environments, creates a high demand on the left ventricle to pump blood to the skin to dissipate heat. Even healthy aging is accompanied by altered cardiovascular function, which limits the extent to which older individuals can maintain stroke volume, increase cardiac output, and increase skin blood flow when exposed to environmental extremes. In the elderly, the increased cardiovascular demand during heat waves is often fatal due to increased strain on an already compromised left ventricle. Not surprisingly, excess deaths during heat waves 1) occur predominantly in older individuals and 2) are overwhelmingly cardiovascular in origin. Increasing frequency and severity of heat waves coupled with a rapidly growing at-risk population dramatically increases the extent of future untoward health outcomes.
Keywords: Heat stress, climate change, cardiovascular strain, heat wave, cutaneous blood flow, age, cardiovascular health
Introduction
The earth’s climate is warming, with global mean temperature increasing by 0.74°C from between the years of 1906 and 2005 (34). Humans are tropical animals, and therefore capable of surviving in, and adapting to, such relatively small changes in mean ambient temperatures. However, as mean global temperature rises, the frequency, severity, and relative length of heat waves increase (53). Heat waves can be functionally defined as an extended period of days with higher than normal temperatures. Prolonged exposure to high ambient temperatures induces a substantial stress on the human cardiovascular system. And while human beings are capable of withstanding extremely high temperatures for short periods of time, the cardiovascular strain induced by prolonged heat exposure contributes negatively to health outcomes. Indeed during a heat wave, most of the excess morbidity and mortality are not directly heat-related, but are cardiovascular in origin, brought about by the increased cardiovascular challenge associated with thermoregulatory responses to heat stress (65).
Elderly individuals, even in the absence of overt cardiovascular disease, are the most vulnerable population during prolonged environmental heat exposure, experiencing significantly worse health outcomes than any other age cohort. Individuals older than 65 years comprise a majority of the extra emergency room visits and deaths during heat waves (16, 58). The global population of aged individuals is rapidly growing (1), meaning that an increasingly larger subset of the population will be susceptible to illness and death as climatic temperature continues to rise.
To defend against increasing core temperature, humans increase skin blood flow and sweat rate to dissipate heat. These effector responses are necessary for thermoregulation but place a great demand on the cardiovascular system by necessitating a relatively large increase in cardiac output (47, 57). Decrements in skin blood flow are also observed and compounded with pathologies including hypertension (9, 30, 31) and hypercholesterolemia (27, 29), as well as with common medications used in the primary and secondary prevention of cardiovascular disease (26, 28). Even healthy aging is associated with an attenuated rise in skin blood flow (39) and decreased sweat gland output (2) in response to heat stress, but the integrated response to heat stress still places a great burden on a compromised (decreased β-adrenergic responsiveness) left ventricle.
The purpose of this review is to discuss the effect of heat stress on the aging cardiovascular system and, within that context, the projected effects of global warming on human cardiovascular health. This brief review is based on a President’s Lecture presented at the 60th Annual Meeting of the American College of Sports Medicine in 2013.
Climate Change
Climate change and global warming are (pardon the pun) hot topics, with their fair share of political controversy with respect to causation. There is little controversy, however, that over the past several decades the average temperature of the earth has been steadily increasing (34). The year 2012 was the hottest year on record in the United States and one of the 10 warmest in global history (50). In fact, all 10 of the warmest years on record have occurred within the last 15 years (18). October 2012 was the 332nd consecutive month with above-average global temperatures (50). This means that, as of the end of 2013, anyone born after April of 1985 has never experienced a month with below-average temperatures (18).
Summers categorized as “hot,” which occurred 33% of the time from 1950 to 1981, now occur 75% of the time (20). James Hansen, of the NASA Goddard Institute for Space Studies and an adjunct professor in the Department of Earth and Environmental Sciences at Columbia University, has used the term “climate dice” to describe this change in the frequency of hot weather. In this analogy, each side of a die represents a temperature range. In a normally distributed climate, average weather, cold weather, and hot weather would each be represented by two sides of the die. As the outcome of rolling a die is random, the probability of average, warm, or cool weather would be equal over the long term. With climate change, the sides of the die have changed. Only one side now represents cool weather, one side represents average weather and four sides signify warmer than average weather; Hansen describes this as “loaded dice”. With climate change there will still be variability in temperature and cool weather will still occur, but it will occur much less frequently than warm weather (21). Evaluation of seasonal temperatures supports the increased frequency of warm weather. In the United States from 1990 to 2010, 16 of 20 winters and 15 of 20 summers were warmer than the 1951–1980 average. For the same time period in Europe, 16 of 20 winters and 19 of 20 summers have been above average in temperature (19). Along with proportionally warmer weather, more record high temperatures are also being set. Since the year 2000, record high temperatures have occurred twice as often as record low temperatures (46). As these data clearly illustrate, the earth’s climate is warming well above normal historical temperatures. But what does that mean for human health, especially among the elderly?
Heat Wave Frequency and Severity
Accompanying the rise in average global temperatures is a rise in the frequency and severity of heat waves. Over the past several decades, the frequency, duration, and severity of heat waves has increased (53). From 1951 to 1980, environmental heat waves covered on average 0.1 percent to 0.2 percent of the earth’s surface at any given time; from 1981 to 2010, 10 percent of the planet experiences a heat wave at a given point in time (20). This means that the occurrence of a heat wave has been 50 to 100 times more likely over the last three decades. To account for this increase in heat wave frequency, one side of Hansen’s climate die has now changed from “warm” to “extremely hot” weather (21).
Numerous computer models predict that the frequency, intensity, and duration of heat waves will continue to increase over the course of the century. Meehl and Tebaldi predict a 25–30% increase in the number of heat waves per year along, accompanied by an increased heat wave duration (45). Nakano et al. predict an increase of more than 22 extra heat wave days per year in Japan (48). Hayhoe et al. predict that by the end of the 21st century, extremely deadly heat waves will occur in the city of Chicago as frequently as every three months (23, 59).
Our Aging Population
The population of the world is rapidly aging. The number of people age 65 and over in the United States has increased from 35 million in 2000 to over 41 million in 2011 and this segment of the population is projected to increase to nearly 80 million by the year 2040 (1). By 2050, there will be more people over 60 years old than under 15 years old in the world for the first time ever (64). To go along with the increase in the number of aged people, the “very old” population is also increasing. The number of people 80 years old and above is increasing at the rate of 3.8% per year, which makes them the fastest growing age group across the world (64).
The increasing number of aged people increases health concerns during periods of elevated ambient temperatures. People over the age of 65 years exhibit disproportionately larger increases in mortality during heat waves than younger individuals, with the majority of excess deaths during heat waves occurring in the elderly (10). With the rapidly growing number of older individuals on the planet, the number of people at risk of dying during a heat wave increases. With an expanded at-risk population there exists the potential for a greater number of casualties during any single heat wave.
Daily Heat, Heat Waves, and Mortality
Humans evolved from tropical climates and are capable of withstanding even extremely high environmental temperatures – temperatures exceeding 200 °F -- (6) provided that (1) they can produce enough sweat, (2) the environment permits evaporation of that sweat, and (3) there is no direct contact with hot surfaces. However, physiological homeostasis in such extreme temperatures is only sustainable for short periods of time.
Despite the ability to tolerate extreme heat stress for short periods of time, prolonged moderate heat stress, such as that observed during a heat wave, represents a different set of stressors, and prolonged warm environmental temperatures are associated with an increased risk of mortality. For example, temperatures above the 90th percentile in California were found to increase risk of excess mortality by 4.3% for every 5.6°C increase in apparent temperature (4). [Note: Apparent temperature is a temperature index that combines the effects of air temperature, relative humidity and wind speed.] In 15 European cities, an increase in apparent temperature of 1°C above a threshold temperature unique to each city was associated with a 3.12% increase in mortality in Mediterranean cities and a 1.84% increase in mortality in northern European cities (3).
While elevated daily temperatures increase the mortality rate, severe heat waves – several consecutive days of hot weather -- cause much greater increases in mortality. Two different heat waves within the past 20 years received considerable attention due to innovations in recording morbidity and mortality statistics. In the summer of 1995, the Chicago heat wave was notable because minimum apparent temperatures remained above 31.5°C for two days (36). This very high minimum temperature prevented recovery from heat stress at night. Instead, the heat stress was continuous throughout the day and night. There were approximately 700 excess deaths during the Chicago heat wave compared to the same period of time in the previous year (67).
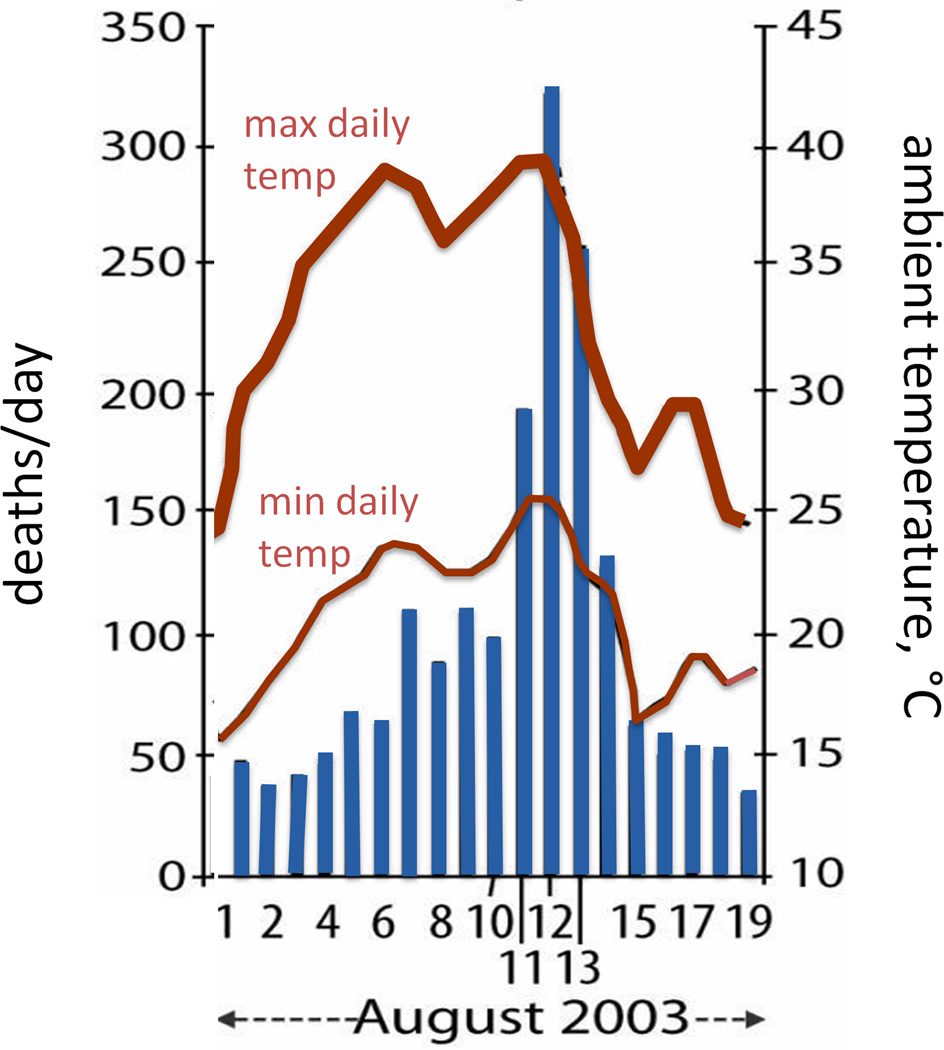
In the European heat wave centered around France in August 2003, temperatures were elevated by 11°C above the seasonal average for nine consecutive days. In total, the French heat wave is estimated to have caused almost 15,000 excess deaths over approximately one month (17, 24, 63). Excess deaths began to occur three days after the start of the heat wave and mortality returned to normal four days after conclusion of the heat wave (17), demonstrating that is takes a prolonged period of time with elevated temperatures to increase mortality (Fig. 1).
Figure 1.
Daily deaths (left axis) and minimum and maximum air temperatures (right axis) during the 2003 French heat wave. The abnormally hot daily temperatures in early August 2003 were followed by a consequent dramatic increase in daily mortality, mostly among the elderly. Redrawn from Dousset et al. International Journal of Climatology 2011, 31:313–323; Royal Meteorological Society (14).
It is hypothesized that the increase in mortality during heat waves, such as those in Chicago and France, may be reflect a harvesting effect. Harvesting, or mortality displacement, means that the excess deaths during a heat wave occur in frail individuals whose death was only slightly expedited by the heat wave. The excess mortality is then counteracted by periods of reduced mortality following the heat wave. No clear consensus has been reach on the magnitude of the harvesting effect during heat waves. Some have reported that nearly all deaths during times of extreme temperature are accounted for by mortality displacement (7) while others have reported no mortality displacement (4, 8). It is possible that the wide variation between studies is due to differences in preparedness for managing public health during heat waves among the cities studied. Differences in intensity and duration of heat waves also likely determine the degree of harvesting. A moderate harvesting effect of approximately 30% is often observed, especially when examining very severe heat waves or looking at large, varied populations (3, 35, 63). This suggests that some deaths during heat waves occur in those who were in poor health and had only a brief period left to live but that a majority of deaths during heat waves occur in those with a normal remaining life expectancy.
Clearly, determinants of mortality during heat waves are not all physiological. Socio-economic status and living conditions undoubtedly play a major role in mortality during heat waves. Odds of death during a heat wave are increased if one is elderly, is confined to bed, sleeps on the top floor of a building, lacks thermal insulation, or resides inside an urban heat island (49, 59, 65). In the French heat wave of 2003, excess mortality rates in deprived areas of Paris were twice as high as those in privileged areas of Paris (55). While being frail, sick, or living in an underprivileged region during a heat wave increase risk of death, there are countermeasures that decrease the risk of mortality during a heat wave. Dressing in light clothing, hydrating, owning air conditioning, having access to transportation, and having nearby social contacts are all associated with decreased mortality (49, 59, 65). Preparedness of residents or of a city to deal with severe heat waves can also affect mortality. Chicago experienced a heat wave in 1999 that was meteorologically very similar to the 1995 heat wave; however, in 1999 there were only 114 excess deaths compared to 700 in 1995 (52). Part of the drop in mortality is attributed to increased public awareness and an improved municipal response (52). Other interventions such as cooling centers have not been shown to be protective due to underutilization (49). Regardless, steps can be taken to protect the most vulnerable populations, such as the old and sick, during heat waves to limit mortality.
Cause of Death during Heat Waves
Direct heat related fatalities, such as death from heat stroke, number approximately 120 per year in the United States, which is more than any other individual environmental causes including death from cold, flooding, tornados, hurricanes, and lightning (51). However, the number of deaths linked to periods of excess heat or heat waves is much greater than those attributable to heat stroke. For example, the August 2003 heat wave in Europe resulted in 15,000 excess deaths in France alone and as many as 70,000 excess deaths across all of the European continent (17, 24, 56, 63). The Chicago 1995 heat wave registered an average of 241 excess deaths per day, far outnumbering the average annual United States heat fatalities in a single day (35).
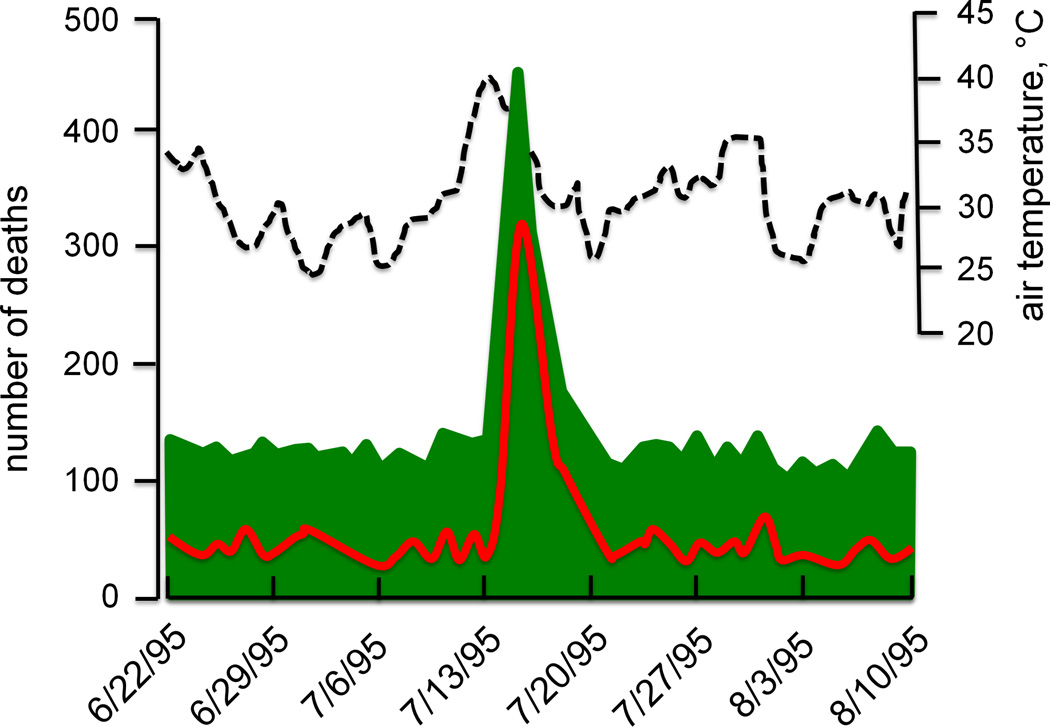
The reason for this difference between direct heat deaths and overall heat wave mortality is that direct effects of heat alone are not the primary cause of death for most of the excess deaths during heat waves. Of the excess deaths in the Chicago heat wave, only 4.7% listed excessive heat (i.e., heat stroke or hyperpyrexia) as the primary cause of death on death certificates, another 28.1% listed heat as a contributing cause, while 93.7% of excess deaths documented an underlying cardiovascular cause (Fig. 2) (35).
Figure 2.
Mortality (green shaded area and red line) and maximum daily temperatures (dashed line) during the summer 1995 Chicago heat wave. Dates are shown along the x-axis. Mortality data in green represent the total death toll, while the superimposed red line depicts deaths from cardiovascular causes alone or from death certificates that mention combined cardiovascular and heat causes. Redrawn from Kaiser et al., American Journal of Public Health 2007, 97 Suppl 1:S158-62; APHA Press (35).
Evaluation of deaths during two heat waves in Milwaukee likewise revealed cardiovascular disease as the primary cause of death in 51% and 64% of the deaths in the two heat waves, respectively (66). Although the vast majority of excess deaths during a heat wave are cardiovascular in origin, associated physiological strain on other systems contributes to the elevated mortality too. An increase in respiratory deaths (12, 33) and cerebrovascular deaths (12) also contribute to the increased mortality during heat waves that were not directly attributed to hyperthermia. Overall, there is a substantial increase excess death during heat waves, but heat-related illness only contributes moderately to the increased mortality. Cardiovascular causes form the majority of contributing factors to excess deaths, especially so in the elderly.
Heat Stress and Cardiovascular Strain in the Elderly
Older humans have a much greater mortality risk during heat waves (59, 65). Death from excessive environmental heat affects adults above age 50 at a higher rate than adults below 50, with the rate increasing exponentially beyond that point (15). In some instances, over 90% of excess deaths during heat waves occur in the elderly (10). As most deaths during heat waves are cardiovascular in origin, the complex reasons for higher mortality in the aged can, at least in part, be understood by examining the cardiovascular responses of older and young apparently healthy human subjects to passive heat stress.
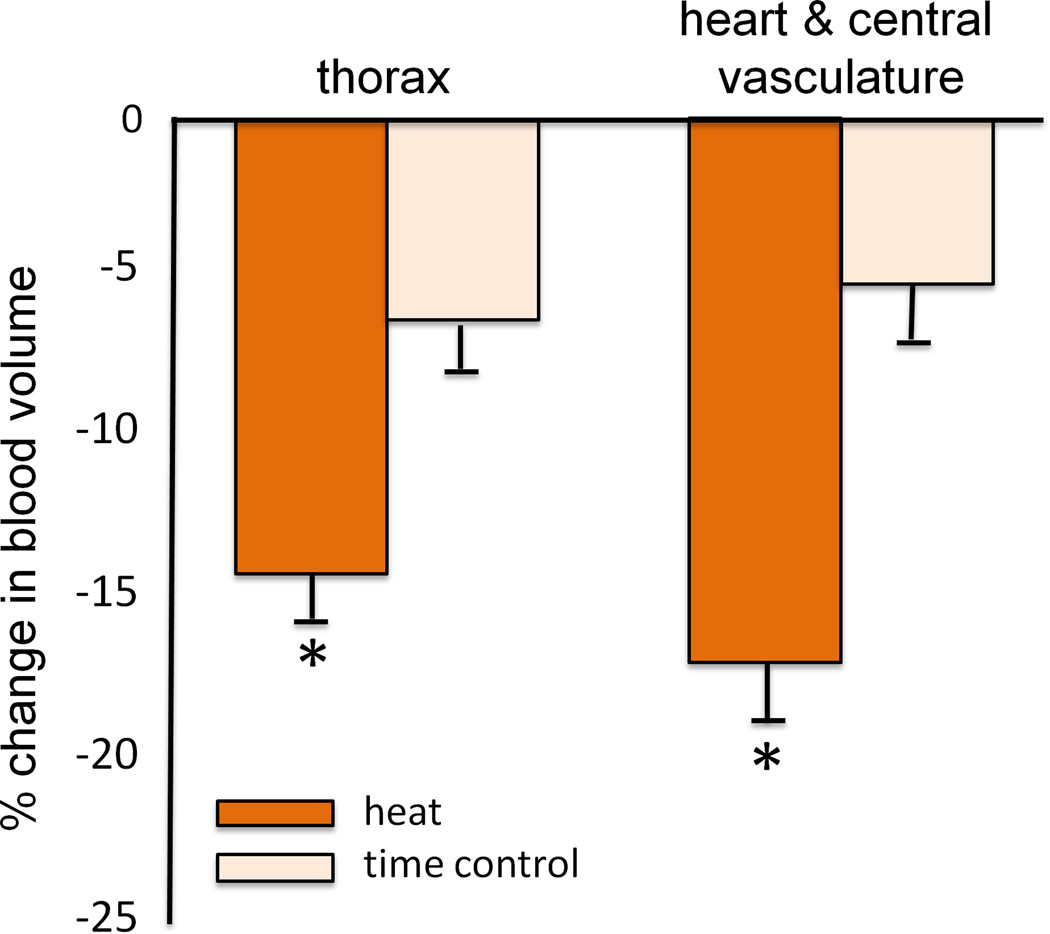
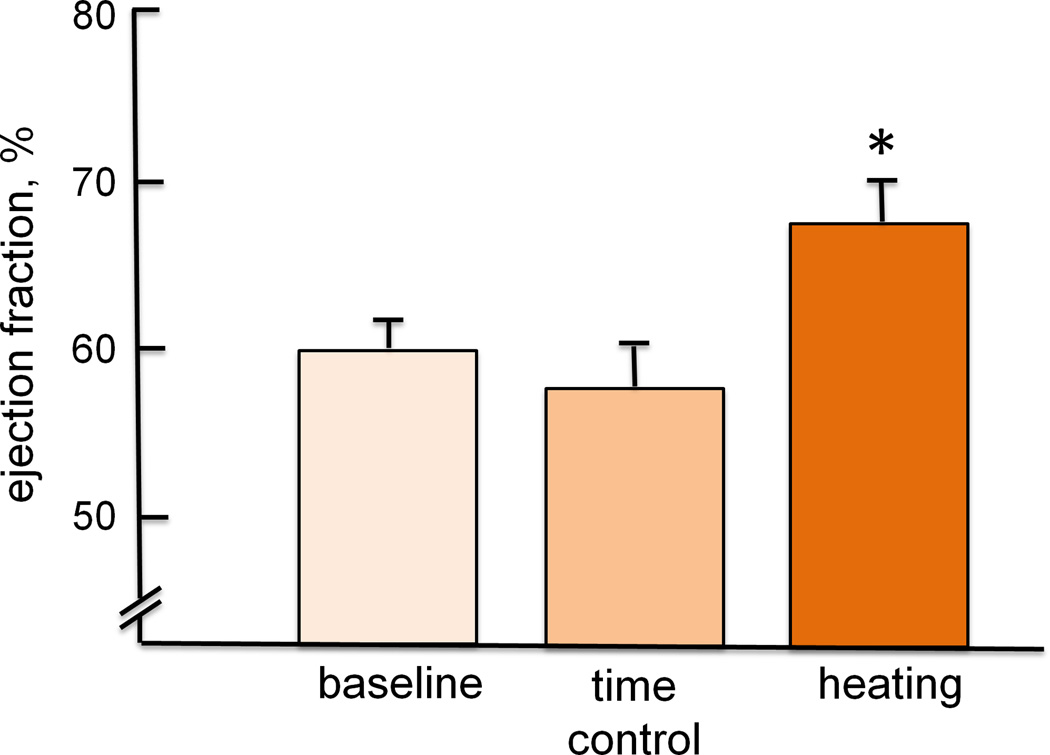
The integrated cardiovascular responses of young, healthy men to passive supine heat stress were eloquently described by Rowell in the 1960s. By increasing skin temperature to 40.5°C using a water-perfused suit, Rowell saw a doubling of resting cardiac output, slightly increased stroke volume, and a redistribution of blood flow from the splanchnic and renal circulations to the skin. Interestingly, despite a profound reduction in right atrial mean pressure to almost zero, mean arterial pressure was well maintained and inotropic function of the heart increased (57). Together these data demonstrated that blood volume is redistributed from the central to peripheral circulations to aid in thermoregulation (11) at the expense of increased ventricular work to pump blood in light of a profoundly reduced filling pressure (Fig. 3).
Figure 3.
(A) The change in central blood volume, measured by technetium-99 scanning, with passive heat stress compared to a time control. Blood volume in the overall thorax and centered around the heart and central vasculature are shown. Heat stress significantly lowered blood volume compared to time control. (B) Ejection fraction at baseline, with passive heat stress and during a time control trial. Passive heat stress significantly increased ejection fraction compared to baseline and time control. Collectively, these data reflect the added strain on the left ventricle during passive heat stress, as contractility increased in light of a falling central venous pressure, to pump blood to the skin. Redrawn from Crandall et al., The Journal of Physiology 2008, 586(1): 293–301; The Physiological Society (11).
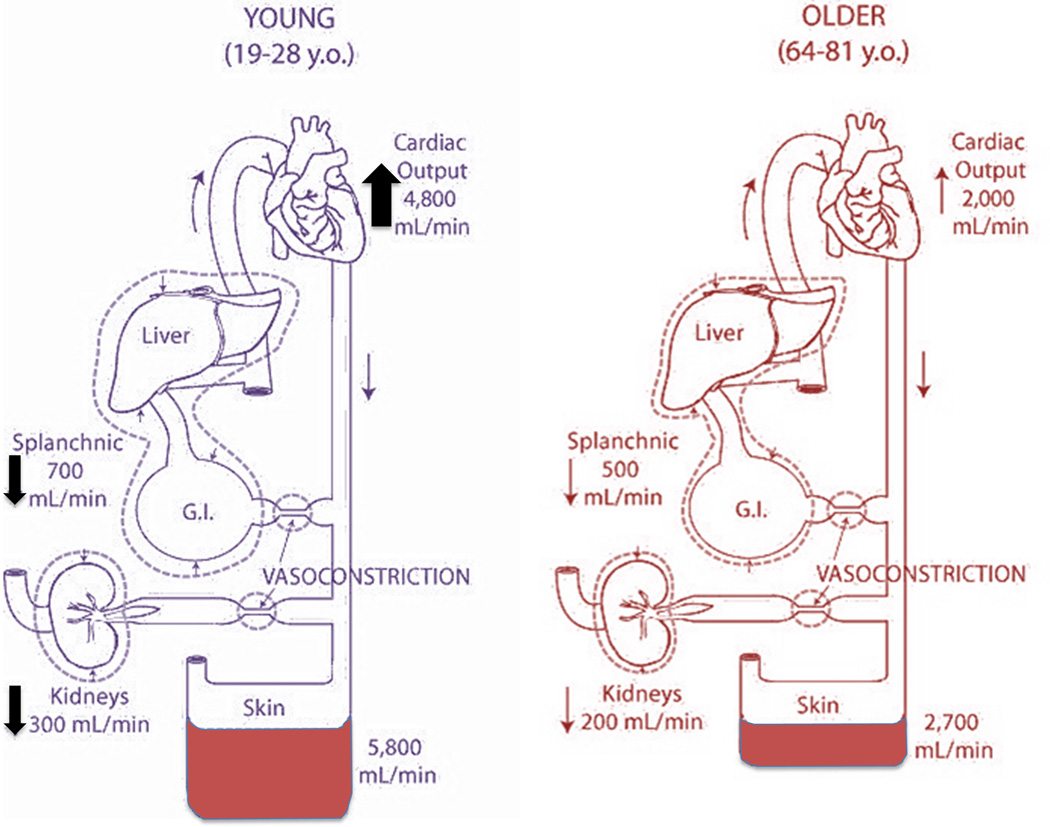
Healthy older humans have an altered cardiovascular response to heat stress compared to their younger counterparts. During passive heating to the limits of thermal tolerance, young subjects increased cutaneous blood flow by ~5,800 ml/min compared to an increase of only 2,700 ml/min in older subjects (47). The increase in skin blood flow in young subjects came from both increased cardiac output (4,800 ml/min) and a redistribution of blood from both the splanchnic and renal circulations (1,000 ml/min). In contrast, 70 year-old subjects had an attenuated increase in cardiac output (2,000 ml/min) coupled with a reduction in the ability to redistribute blood flow from the splanchnic and renal circulations (700 ml/min) (47) (Fig. 4).
Figure 4.
Changes in cardiac output, renal, splanchnic and cutaneous blood flow with passive heating to thermal tolerance (water-perfused suit) in young and older men. Young subjects increased cutaneous blood flow to a larger extent than did older subjects. The larger increase in cutaneous blood flow in the young men was accomplished by both raising cardiac output significantly more and by reducing renal and splanchnic blood flow to a higher degree compared with the older subjects. Redrawn from data published by Minson et al., Journal of Applied Physiology 1998, 84(4):1323-32; American Physiological Society (47).
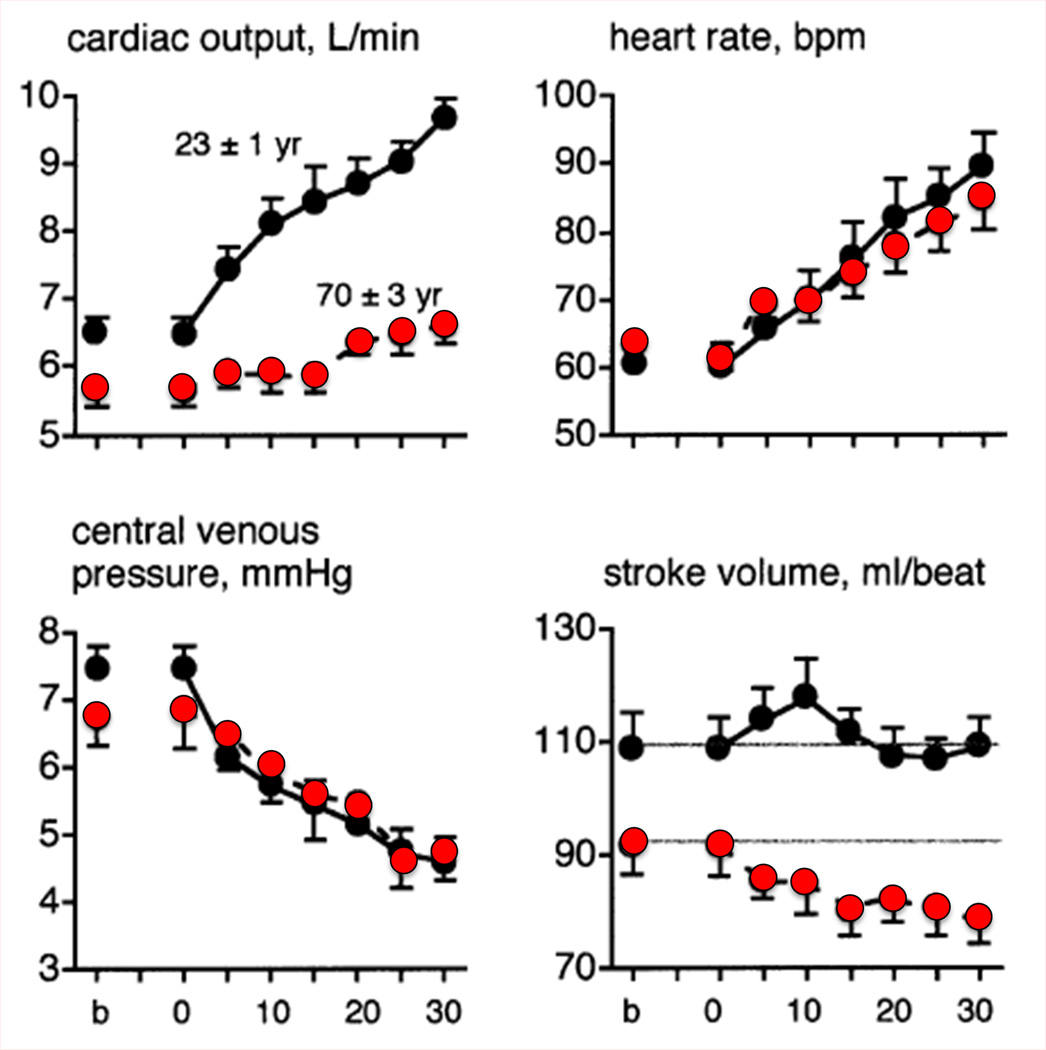
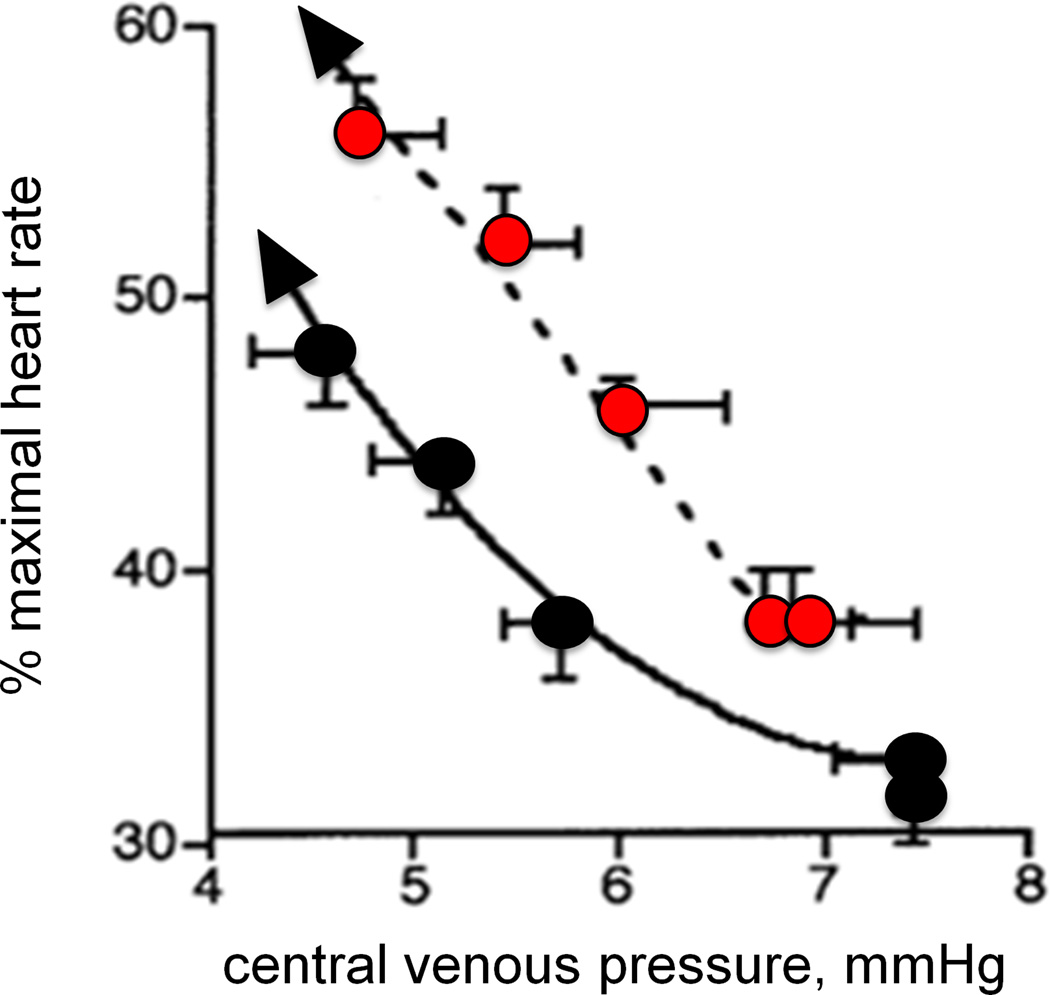
One reason for the attenuated increase in cardiac output during passive heating in older subjects is because of their lack of ability to maintain stroke volume. With prolonged passive heating, central venous pressure (CVP) falls similarly in aged and young subjects (Fig. 5A) (47). The young subjects were able to maintain or slightly increase stroke volume by increasing contractility, whereas stroke volume declined progressively in the older subjects. While the absolute heart rate response was similar in both age groups, heart rate as a percentage of maximum was higher in the older individuals at any given CVP (Fig. 5B). Thus, the older subjects relied on a greater percentage of their heart rate reserve to increase cardiac output during whole body heat stress (47). In the aged there is excess central cardiovascular strain as well as an attenuated increase in thermoregulatory skin blood flow. With such changes evident in apparently healthy older men free from overt heart disease, such increases in myocardial oxygen demand may prompt untoward events in those with clinical or subclinical disease.
Figure 5.
Cardiac responses to prolonged passive heating as a function of (A) time and (B) central venous pressure in young (19–28 yrs; black circles) and older (64–81 yrs; red circles) men. Only the initial 30 min of heating are shown in panel A. Older subjects had a significantly attenuated rise in cardiac output and a decrease in stroke volume compared with young subjects, despite a similar fall in central venous pressure. Stroke volume was well maintained in the young men. As shown in panel B, the fall in central venous pressure (CVP) (right to left along the x-axis) due to venous blood pooling caused a similar increase in absolute heart rate (see panel A) but a larger rise in HR as a percent of maximal heart rate. Heart rate reserve is consequently lower in the older men at any given level of heat stress (and CVP). Redrawn from data published by Minson et al., Journal of Applied Physiology 1998, 84(4):1323-32; American Physiological Society (47).
Along with a drop in cutaneous perfusion, aging is associated with a decreased sweat rate (60) and decreased sweat output per gland (2). Regional sweating patterns also change with aging, with the largest reductions in sweating occurring in the abdomen and less significant reductions occurring in the lower back, thigh, and arm (60). An attenuated evaporative heat loss results in greater heat storage in older men and women (41, 42) which can exacerbate the cardiovascular strain described above. Even though the sweating response in the aged is often attenuated, prolonged sweating during long duration heat stress causes a significant reduction in plasma volume. This fall precipitates increases in red blood cell and neutrophil counts as well as increased plasma viscosity. Heat stress also causes the release of extra platelets into the circulation. These changes in blood properties contribute to increased susceptibility to cardiovascular death due to acute coronary events (37).
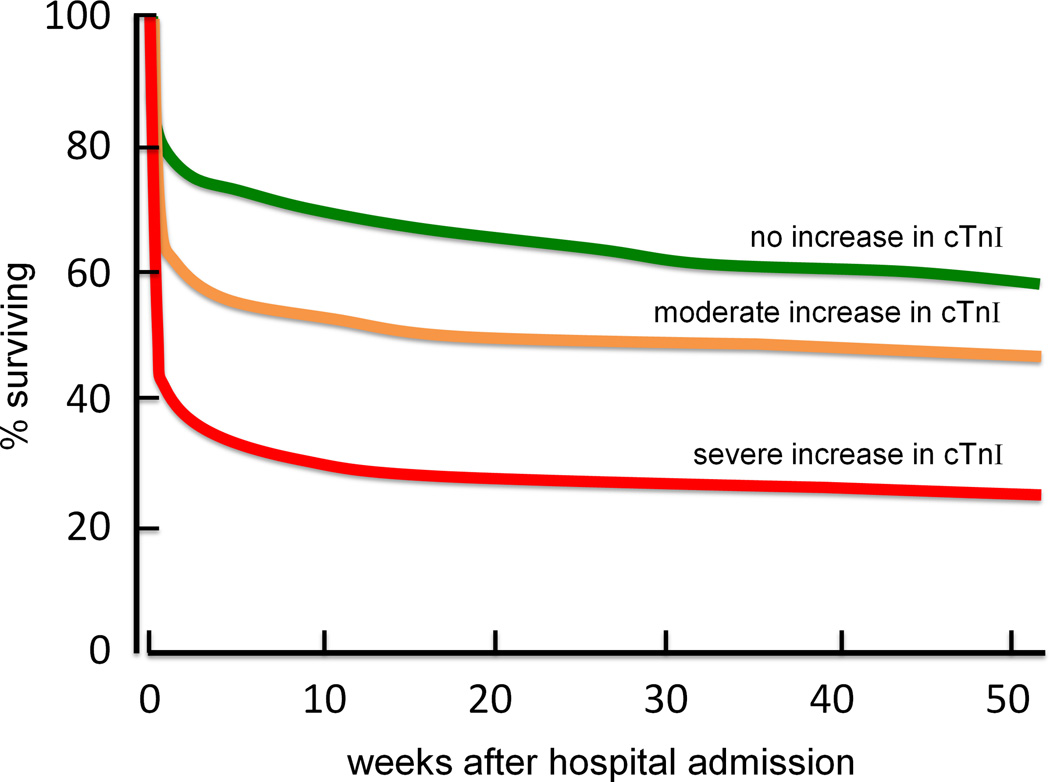
One method for assessing cardiovascular strain and damage during heat stress is by measuring the enzyme cardiac troponin I (cTnI). When myocardial cell are damaged, cTnI is released into the bloodstream (43). Cardiac troponin I is elevated when examined postmortem in hyperthermia-related mortalities (68), indicating myocardial damage associated with the severe heat stress. cTnI has also been found to be elevated in aged subjects with non-exertional heat related illnesses (13, 22). An increase in cTnI is associated with decreased survival from a heat illness and is an independent prognostic factor for survival (Fig. 6) (13, 22). This association provides evidence for myocardial damage being a contributing factor in deaths in the elderly during heat waves. Even a modest increase in core temperature (below the clinical criteria for heat stroke 40°C) can cause myocardial damage.
Figure 6.
Patient survival curves following emergency admission to hospitals during the weeks after the 2003 heat wave in France. Separate curves are drawn for patients (mean age = 84 yrs) who had no elevation in cardiac troponin I (cTnI) (n = 252; green line), a moderate increase (up to 1.5 ng.mL−1, n = 165; orange line), and a severe increase in cTnI (>1.5 ng.mL−1, n = 97; red line). These data support the association between heat stress and cardiac strain in the elderly during environmental heat waves. Redrawn from data originally published by Hausfater et al., Critical Care 2010, 14:R99; BioMed Central publishing (22).
Changes in Aged Skin
The ability to maintain core temperature during heat stress is in part dependent upon increasing cutaneous blood flow. When examining the integrated cardiovascular response to passive whole body heating, skin blood flow has been calculated to increase to 7–8 L/min in young subjects (57). With healthy aging, there is an attenuated skin blood flow response to passive, whole-body heating (40, 47). While central cardiovascular changes with aging contribute to the reduction in skin blood flow (47), there are also peripheral limitations to reflex cutaneous vasodilation. Nitric oxide (NO) is a potent vasodilator that is required for full expression of reflex vasodilation and contributes directly to approximately 30–40% of the cutaneous vasodilatory response in young skin (25, 38). Even though NO bioavailability is decreased in aged vasculature, aged humans rely primarily on compromised NO-mediated mechanisms to increase skin blood flow during heat stress (25). The reason for the increased dependency on NO-mediated vasodilation in aged skin is the decreased contribution of cholinergic co-transmitter(s) to reflex vasodilation (25).
Many peripheral factors contribute to the decreased NO bioavailability in aged human vasculature. In human skin there is upregulation of the enzyme arginase which preferentially utilizes the common NO-synthase (NOS) substrate, L-arginine, to produce urea and L-ornithine (31). Either localized supplementation of L-arginine or inhibition on arginase augments reflex cutaneous vasodilation in aged skin (31). Aging is also associated with increased oxidative stress including increased superoxide production from a variety of enzymatic and non-enzymatic sources. Superoxide reacts with NO to form peroxynitrite (OONO–) than is degraded by superoxide dismutase (5). This reaction decreases NO bioavailability and functionally reduces vasodilation. Moreover free radical species can also uncouple the NOS dimer which further decreases NO production and increases superoxide synthesis (30). Short term antioxidant supplementation (ascorbate) serves to quench free radicals and increase reflex vasodilation in aged skin (30). However, ascorbate is a generalized antioxidant and can either decrease oxidant species and/or improve the availability of the essential NOS cofactor tetrahydrobiopterin (BH4). Aging in general is associated with reduced bioavailability of BH4, which potentiates NOS uncoupling and contributes to increased oxidant stress (54). Localized supplementation of BH4 increases NO-dependent vasodilation in aged human skin (61, 62).
Along with NO-mediated vasodilation, cyclooxygenase (COX) pathways contribute to reflex vasodilation in young, healthy subjects (44). However with aging there is an increase in COX derived vasoconstrictors and attenuated prostanoid-dependent vasodilation which together may increase cutaneous vasoconstriction (32). Together, the attenuation of NO-, COX-, and co-transmitter mediated vasodilation with aging demonstrates an overall loss in redundancy in vasodilator mechanisms with aging.
While reflex cutaneous vasodilation is attenuated in aged skin, local pharmacological interventions can largely restore skin blood flow in the aged to match that of young people. However, it is currently not known if the aged cardiovascular system, including the compromised left ventricle, could support the increase in skin blood flow afforded by these pharmacological intervention.
Summary
The earth’s climate is rapidly warming and the increased average daily temperature is accompanied by an increased frequency and severity of environmental heat waves. Passive thermal stress places a large demand on the cardiovascular system to pump blood to the skin. The increased cardiovascular demand of high ambient temperatures, with or without the added strain of physical exertion, can be especially challenging in aged humans who exhibit altered cardiovascular function and thermoregulatory ability. For these individuals, heat waves can often be fatal as demonstrated by the large number of excess deaths – primarily of cardiovascular origin -- in elderly individuals during heat waves.
With the number of older people rapidly increasing and the climate warming, cardiovascular deaths precipitated by prolonged periods of high ambient temperatures are of increasing concern. The integrated cardiovascular response to heat stress is an example of elegant homeostasis. Yet alterations in the aged cardiovascular system limit the requisite increase in cardiac output while putting increased strain on a potentially compromised left ventricle. The result is an increased mortality among the elderly during environmental extremes of heat, not from heat stroke but due to cardiovascular strain.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report. No funding was received for this work. The contents of this review do not constitute endorsement by ACSM.
References
- 1. [cited 8/26/2013];A profile of older Americans: 2012. 2012 Available from: http://www.aoa.gov/AoARoot/Aging_Statistics/Profile/2012/2.aspx.
- 2.Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. Journal of applied physiology. 1987;63(3):1089–1094. doi: 10.1152/jappl.1987.63.3.1089. [DOI] [PubMed] [Google Scholar]
- 3.Baccini M, Biggeri A, Accetta G, Kosatsky T, Katsouyanni K, Analitis A, et al. Heat effects on mortality in 15 European cities. Epidemiology. 2008;19(5):711–719. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- 4.Basu R, Malig B. High ambient temperature and mortality in California: exploring the roles of age, disease, and mortality displacement. Environmental research. 2011;111(8):1286–1292. doi: 10.1016/j.envres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chemical research in toxicology. 1996;9(5):836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 6.Blagden C. Experiments and Observations in an Heated Room. Philosophical Transactions. 1775;65:111–123. [Google Scholar]
- 7.Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology. 2001;12(6):662–667. doi: 10.1097/00001648-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Bustinza R, Lebel G, Gosselin P, Belanger D, Chebana F. Health impacts of the July 2010 heat wave in Quebec, Canada. BMC public health. 2013;13:56. doi: 10.1186/1471-2458-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carberry PA, Shepherd AM, Johnson JM. Resting and maximal forearm skin blood flows are reduced in hypertension. Hypertension. 1992;20(3):349–355. doi: 10.1161/01.hyp.20.3.349. [DOI] [PubMed] [Google Scholar]
- 10.Conti S, Meli P, Minelli G, Solimini R, Toccaceli V, Vichi M, et al. Epidemiologic study of mortality during the Summer 2003 heat wave in Italy. Environmental research. 2005;98(3):390–399. doi: 10.1016/j.envres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. The Journal of physiology. 2008;586(1):293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Ippoliti D, Michelozzi P, Marino C, de'Donato F, Menne B, Katsouyanni K, et al. The impact of heat waves on mortality in 9 European cities: results from the EuroHEAT project. Environmental health : a global access science source. 2010;9:37. doi: 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davido A, Patzak A, Dart T, Sadier MP, Meraud P, Masmoudi R, et al. Risk factors for heat related death during the August 2003 heat wave in Paris, France, in patients evaluated at the emergency department of the Hopital Europeen Georges Pompidou. Emergency medicine journal : EMJ. 2006;23(7):515–518. doi: 10.1136/emj.2005.028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dousset B, Gourmelon F, Laaidi K, Zeghnoun A, Giraudet E, Bretin P, et al. Satellite monitoring of summer heat waves in the Paris metropolitan area. Int J Climatol. 2011;31(2):313–323. [Google Scholar]
- 15.Ellis FP. Mortality from heat illness and heat-aggravated illness in the United States. Environmental research. 1972;5(1):1–58. doi: 10.1016/0013-9351(72)90019-9. [DOI] [PubMed] [Google Scholar]
- 16.Ellis FP, Nelson F, Pincus L. Mortality during heat waves in New York City July, 1972 and August and September, 1973. Environmental research. 1975;10(1):1–13. doi: 10.1016/0013-9351(75)90069-9. [DOI] [PubMed] [Google Scholar]
- 17.Fouillet A, Rey G, Laurent F, Pavillon G, Bellec S, Guihenneuc-Jouyaux C, et al. Excess mortality related to the August 2003 heat wave in France. International archives of occupational and environmental health. 2006;80(1):16–24. doi: 10.1007/s00420-006-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillis J Not Even Close: 2012 Was Hottest Ever in U.S. The New York Times. 2013;Sect. A:1. [Google Scholar]
- 19.Hansen J, Ruedy R, Sato M, Lo K. Global Surface Temperature Change. Rev Geophys. 2010;48 [Google Scholar]
- 20.Hansen J, Sato M, Ruedy R. Perception of climate change. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(37):E2415–E2423. doi: 10.1073/pnas.1205276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen JE. Climate change is here - and worse than we thought. Sect. A. Washington Post; 2012. p. 14. [Google Scholar]
- 22.Hausfater P, Doumenc B, Chopin S, Le Manach Y, Santin A, Dautheville S, et al. Elevation of cardiac troponin I during non-exertional heat-related illnesses in the context of a heatwave. Critical care. 2010;14(3):R99. doi: 10.1186/cc9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayhoe K, Sheridan S, Kalkstein L, Greene S. Climate change, heat waves, and mortality projections for Chicago. J Great Lakes Res. 2010;36:65–73. [Google Scholar]
- 24.Hemon D, Jougla E. The heat wave in France in August 2003. Revue d'epidemiologie et de sante publique. 2004;52(1):3–5. doi: 10.1016/s0398-7620(04)99017-7. [DOI] [PubMed] [Google Scholar]
- 25.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, et al. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. American journal of physiology Heart and circulatory physiology. 2003;284(5):H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 26.Holowatz LA, Jennings JD, Lang JA, Kenney WL. Systemic low-dose aspirin and clopidogrel independently attenuate reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol (1985) 2010;108(6):1575–1581. doi: 10.1152/japplphysiol.01362.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. The Journal of physiology. 2011;589(Pt 19):4787–4797. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holowatz LA, Kenney WL. Chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol (1985) 2009;106(2):500–505. doi: 10.1152/japplphysiol.91215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. The Journal of physiology. 2011;589(Pt 8):2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. American journal of physiology Heart and circulatory physiology. 2006;291(6):H2965–H2970. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- 31.Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. The Journal of physiology. 2006;574(Pt 2):573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. The Journal of physiology. 2005;563(Pt 3):965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynen MM, Martens P, Schram D, Weijenberg MP, Kunst AE. The impact of heat waves and cold spells on mortality rates in the Dutch population. Environmental health perspectives. 2001;109(5):463–470. doi: 10.1289/ehp.01109463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pachauri RK, Resinger A, editors. IPCC CCSRCoWGI, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change. 2007. p. 104. Core Writing Team. [Google Scholar]
- 35.Kaiser R, Le Tertre A, Schwartz J, Gotway CA, Daley WR, Rubin CH. The effect of the 1995 heat wave in Chicago on all-cause and cause-specific mortality. American journal of public health. 2007;97(Suppl 1):S158–S162. doi: 10.2105/AJPH.2006.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karl TR, Knight RW. The 1995 Chicago heat wave: How likely is a recurrence. B Am Meteorol Soc. 1997;78(6):1107–1119. [Google Scholar]
- 37.Keatinge WR, Coleshaw SR, Easton JC, Cotter F, Mattock MB, Chelliah R. Increased platelet and red cell counts, blood viscosity, and plasma cholesterol levels during heat stress, and mortality from coronary and cerebral thrombosis. The American journal of medicine. 1986;81(5):795–800. doi: 10.1016/0002-9343(86)90348-7. [DOI] [PubMed] [Google Scholar]
- 38.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. Journal of applied physiology. 1998;85(3):824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 39.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. The American journal of physiology. 1997;272(4 Pt 2):H1609–H1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- 40.Kenney WL, Tankersley CG, Newswanger DL, Hyde DE, Puhl SM, Turner NL. Age and hypohydration independently influence the peripheral vascular response to heat stress. Journal of applied physiology. 1990;68(5):1902–1908. doi: 10.1152/jappl.1990.68.5.1902. [DOI] [PubMed] [Google Scholar]
- 41.Larose J, Wright HE, Sigal RJ, Boulay P, Hardcastle S, Kenny GP. Do Older Females Store More Heat than Younger Females during Exercise in the Heat. Medicine and science in sports and exercise. 2013;45(12):2265–2276. doi: 10.1249/MSS.0b013e31829d24cc. [DOI] [PubMed] [Google Scholar]
- 42.Larose J, Wright HE, Stapleton J, Sigal RJ, Boulay P, Hardcastle S, et al. Whole body heat loss is reduced in older males during short bouts of intermittent exercise. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(6):R619–R629. doi: 10.1152/ajpregu.00157.2013. [DOI] [PubMed] [Google Scholar]
- 43.Lazzeri C, Bonizzoli M, Cianchi G, Gensini GF, Peris A. Troponin I in the intensive care unit setting: from the heart to the heart. Intern Emerg Med. 2008;3(1):9–16. doi: 10.1007/s11739-008-0089-3. [DOI] [PubMed] [Google Scholar]
- 44.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291(3):R596–R602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- 45.Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305(5686):994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 46.Meehl GA, Tebaldi C, Walton G, Easterling D, McDaniel L. Relative increase of record high maximum temperatures compared to record low minimum temperatures in the U. S. Geophys Res Lett. 2009:36. [Google Scholar]
- 47.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. Journal of applied physiology. 1998;84(4):1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 48.Nakano M, Matsueda M, Sugi M. Future projections of heat waves around Japan simulated by CMIP3 and high-resolution Meteorological Research Institute atmospheric climate models. J Geophys Res-Atmos. 2013;118(8):3097–3109. [Google Scholar]
- 49.Naughton MP, Henderson A, Mirabelli MC, Kaiser R, Wilhelm JL, Kieszak SM, et al. Heat-related mortality during a 1999 heat wave in Chicago. American journal of preventive medicine. 2002;22(4):221–227. doi: 10.1016/s0749-3797(02)00421-x. [DOI] [PubMed] [Google Scholar]
- 50.NOAA National Climatic Data Center SotCGAfO. [8/26/2013]. Available from: http://www.ncdc.noaa.gov/sotc/gobal/2012/10.
- 51.NOAA Office of Climate W, and Weather Services. Natural Hazard Statistics [cited 8/20/2013] Available from: http://www.nws.noaa.gov/om/hazstats.shtml.
- 52.Palecki MA, Changnon SA, Kunkel KE. The nature and impacts of the July 1999 heat wave in the midwestern United States: Learning from the lessons of 1995. B Am Meteorol Soc. 2001;82(7):1353–1367. [Google Scholar]
- 53.Perkins SE, Alexander LV, Nairn JR. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys Res Lett. 2012:39. [Google Scholar]
- 54.Raman CS, Li H, Martasek P, Kral V, Masters BS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95(7):939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 55.Rey G, Fouillet A, Bessemoulin P, Frayssinet P, Dufour A, Jougla E, et al. Heat exposure and socio-economic vulnerability as synergistic factors in heat-wave-related mortality. European journal of epidemiology. 2009;24(9):495–502. doi: 10.1007/s10654-009-9374-3. [DOI] [PubMed] [Google Scholar]
- 56.Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, Michel JP, et al. Death toll exceeded 70,000 in Europe during the summer of 2003. Comptes rendus biologies. 2008;331(2):171–178. doi: 10.1016/j.crvi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Rowell LB. Human Cardiovascular Adjustments to Exercise and Thermal-Stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 58.Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. American journal of preventive medicine. 1999;16(4):269–277. doi: 10.1016/s0749-3797(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 59.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, et al. Heat-related deaths during the July 1995 heat wave in Chicago. New Engl J Med. 1996;335(2):84–90. doi: 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- 60.Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(8):R877–R885. doi: 10.1152/ajpregu.00290.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol (1985) 2013;115(7):972–978. doi: 10.1152/japplphysiol.00481.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. Journal of applied physiology. 2012;112(5):791–797. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toulemon L, Barbieri M. The mortality impact of the August 2003 heat wave in France: investigating the 'harvesting' effect and other long-term consequences. Population studies. 2008;62(1):39–53. doi: 10.1080/00324720701804249. [DOI] [PubMed] [Google Scholar]
- 64.United Nations. Department of Economic and Social Affairs., Population Division. World population ageing, 1950–2050. New York: United Nations; p. 483. xlix. [Google Scholar]
- 65.Vandentorren S, Bretin P, Zeghnoun A, Mandereau-Bruno L, Croisier A, Cochet C, et al. August 2003 heat wave in France: Risk factors for death of elderly people living at home. Eur J Public Health. 2006;16(6):583–591. doi: 10.1093/eurpub/ckl063. [DOI] [PubMed] [Google Scholar]
- 66.Weisskopf MG, Anderson HA, Foldy S, Hanrahan LP, Blair K, Torok TJ, et al. Heat wave morbidity and mortality, Milwaukee, Wis, 1999 vs 1995: an improved response. American journal of public health. 2002;92(5):830–833. doi: 10.2105/ajph.92.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitman S, Good G, Donoghue ER, Benbow N, Shou W, Mou S. Mortality in Chicago attributed to the July 1995 heat wave. American journal of public health. 1997;87(9):1515–1518. doi: 10.2105/ajph.87.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu BL, Ishikawa T, Michiue T, Li DR, Zhao D, Bessho Y, et al. Postmortem cardiac troponin I and creatine kinase MB levels in the blood and pericardial fluid as markers of myocardial damage in medicolegal autopsy. Leg Med (Tokyo) 2007;9(5):241–250. doi: 10.1016/j.legalmed.2007.01.010. [DOI] [PubMed] [Google Scholar]