Abstract
Objective:
To analyze the potential impact of aspirin on outcome at hospital discharge after acute stroke in resource-limited settings without access to neuroimaging to distinguish ischemic stroke from intracerebral hemorrhage (ICH).
Methods:
A decision analysis was conducted to evaluate aspirin use in all patients with acute stroke of unknown type for the duration of initial hospitalization. Data were obtained from the International Stroke Trial and Chinese Acute Stroke Trial. Predicted in-hospital mortality and stroke recurrence risk were determined across the worldwide reported range of the proportion of strokes caused by ICH. Sensitivity analyses were performed on aspirin-associated relative risks in patients with ICH.
Results:
At the highest reported proportion of strokes due to ICH from a large epidemiologic study (34% in sub-Saharan Africa), aspirin initiation after acute stroke of undetermined etiology is predicted to reduce in-hospital mortality (from 85/1,000 without treatment to 81/1,000 with treatment), in-hospital stroke recurrence (58/1,000 to 50/1,000), and combined risk of in-hospital mortality or stroke recurrence (127/1,000 to 114/1,000). Benefits of aspirin therapy remained in sensitivity analyses across a range of plausible parameter estimates for relative risks associated with aspirin initiation after ICH.
Conclusion:
Aspirin treatment for the period of initial hospitalization after acute stroke of undetermined etiology is predicted to decrease acute stroke-related mortality and in-hospital stroke recurrence even at the highest reported proportion of acute strokes due to ICH. In the absence of clinical trials to test this approach empirically, clinical decisions require patient-specific evaluation of risks and benefits of aspirin in this context.
The burden of stroke-related disability and mortality falls disproportionately on low-income countries, where access to acute stroke care, rehabilitation, and preventive medications are most limited.1–3 Aspirin is an inexpensive and effective medication for secondary stroke prevention in both the acute4 and chronic5,6 settings, but only 3.8% of patients with prior stroke in low-income countries take antiplatelet agents, compared to 53.1% in high-income countries.7 These lower rates of aspirin use may contribute in part to the burden of potentially preventable stroke-related mortality in high-incidence, low-resource settings.
One of us (A.L.B.) has worked in such settings, where colleagues have described that one reason that aspirin is used less often for patients with acute stroke is because CT is often unavailable to distinguish ischemic stroke (IS) from intracerebral hemorrhage (ICH). Clinical predictors to differentiate IS from ICH are not sufficiently reliable to guide clinical decision-making.8 Clinicians must therefore balance potential benefits of secondary prevention in patients with possible IS against presumed risks of aspirin administration in patients with potential ICH when stroke etiology is unknown.
In scenarios of clinical uncertainty in which clinical trial data are unavailable and unlikely to be collected, decision analysis represents a powerful tool to assess the risks and benefits of treatment interventions.9,10 In order to assist clinicians practicing in resource-limited settings, we conducted a decision analysis to determine the expected outcomes of administering aspirin to all patients with acute stroke when it is unknown whether the etiology is IS or ICH.
METHODS
Model.
We conducted our decision analysis using TreeAge Software (Williamstown, MA). The decision tree is displayed in figure 1. We evaluated 2 strategies for treatment of acute stroke of undetermined etiology during the period of initial hospitalization: to administer aspirin to all patients for this period vs not to administer aspirin to any patients. During hospitalization for acute stroke, several outcomes were possible: patients could have a second stroke (after which they could either survive or die), survive without further stroke, or die without further stroke.
Figure 1. Schematic of decision tree.
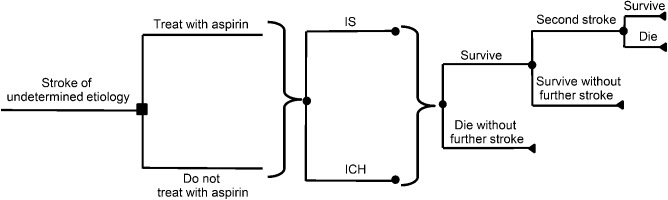
The decision node (aspirin vs no aspirin) is represented by a square. Each chance node is designated by a circle. A bracket indicates that the subtree to the right applies to each of the branches leading to it. Patients receiving aspirin or no aspirin may have had an initial ischemic stroke (IS) or an initial intracerebral hemorrhage (ICH). During hospitalization, patients may die without further stroke, survive without further stroke, or have a second stroke after which they may either die or survive.
Our model assessed the effect of aspirin administration on overall mortality at hospital discharge, in-hospital stroke recurrence, and mortality at discharge without recurrent stroke. We defined the optimal treatment strategy as the one leading to the highest rate of survival to hospital discharge.
Data.
Data on the effects of aspirin on outcomes after acute stroke were obtained from the meta-analysis4 of the International Stroke Trial (IST)11 and the Chinese Acute Stroke Trial (CAST)12 (table 1). Together, these trials randomized over 40,000 patients with acute stroke to aspirin therapy or placebo, and measured the effect of aspirin on recurrent IS, hemorrhagic conversion or new ICH, and mortality during initial hospitalization. The meta-analysis of these trials demonstrated a modest but significant benefit of aspirin in reducing the rate of early recurrent in-hospital IS and a trend toward reduction of in-hospital death in patients without in-hospital stroke recurrence, despite a small increase in the risk of ICH after IS.
Table 1.
Data from the meta-analysis of IST and CAST4
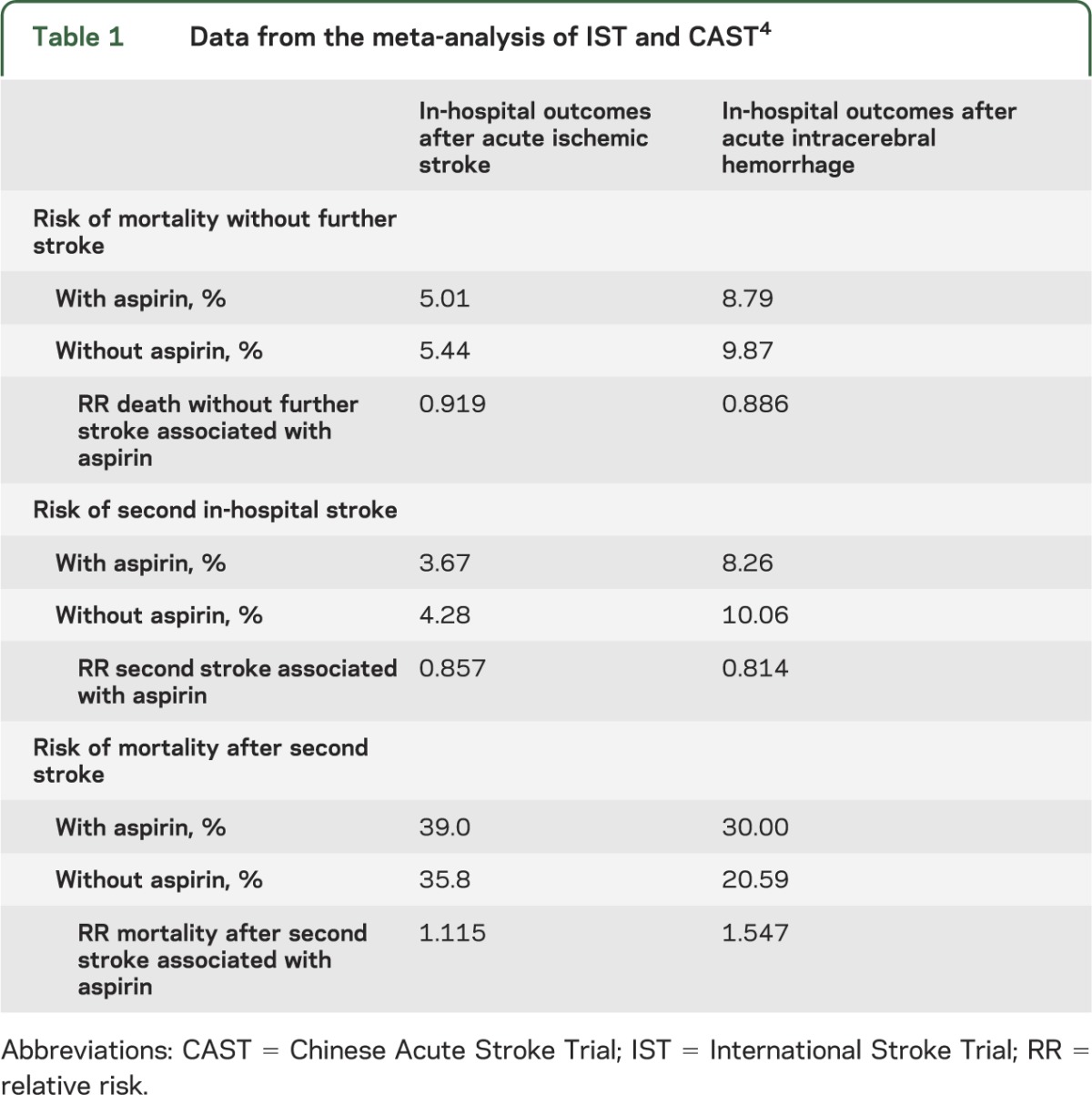
Across the 2 trials, 773 patients whose initial stroke was due to ICH were inadvertently included and randomized to receive aspirin (398 patients) or placebo (375 patients). There appeared to be no statistically significant adverse effects of aspirin in these patients. There was a small but not statistically significant increase in recurrent ICH in patients given aspirin, but a trend toward overall reduction in total secondary strokes of other types (ischemic and “stroke of unknown type”).
Since both IS and ICH groups in IST/CAST had a proportion of second strokes classified as “of unknown type,” precise numbers of acute stroke recurrences due to IS and ICH could not be determined. Therefore, all in-hospital strokes following the initial stroke were grouped together in our model as “second stroke” (i.e., IS, ICH, and stroke of unknown type). When grouped in this way, aspirin actually increased mortality in patients who had in-hospital stroke recurrence both for patients whose initial stroke was IS and for patients whose initial stroke was ICH. The overall mortality benefit of aspirin in the acute setting of IS reported in the meta-analysis of IST and CAST is therefore likely driven by the benefit to patients who had no recurrent stroke events in the acute period.
In a study of stroke risk factors in 3,000 patients in 22 countries, the proportion of first strokes due to ICH ranged from 9% in high-income counties to as high as 34% in parts of Africa.13 Smaller studies in individual countries in sub-Saharan Africa have reported the proportion of stroke due to ICH to be as high as 60%.14–16 However, ICH prevalence may be overrepresented in these small series due to the severity of illness required for presentation to the referral centers with the capacity to conduct such studies.2 The relative risks and benefits of aspirin were modeled across this range of proportions of first stroke due to ICH.
Assumptions within the model.
Our model considered patients with acute stroke presenting to clinics or hospitals in low-income countries where neuroimaging is not available to distinguish between ischemic and hemorrhagic causes of acute stroke. We modeled only the time period from presentation to discharge from initial hospitalization, as these are the data available in IST/CAST.
We made the following assumptions: thrombolysis with IV tissue plasminogen activator was not available or unable to be administered without CT scan to determine whether radiologic contraindications to thrombolysis were present17,18; aspirin was the only antiplatelet agent available, given the expense of clopidogrel and dipyridamole; anticoagulation was not feasible given the lack of access to monitoring of prothrombin time and partial thromboplastin time in resource-limited settings; and patients presenting with acute stroke had had either IS or ICH, but not subarachnoid hemorrhage (SAH). We made the latter assumption because the incidence of SAH is far less than that of IS or ICH (accounting for 7% of strokes in low-income countries),19 SAH generally presents with a clinical syndrome that is distinct from IS or ICH, and SAH would be expected to be almost uniformly fatal in resource-limited settings without timely access to advanced neurocritical care.
It is widely believed that aspirin initiation in patients with acute ICH could worsen outcome by causing ICH extension. However, IST and CAST provide the only empiric data on the initiation of aspirin at the time of acute ICH, and meta-analysis of these data suggests that aspirin initiation at the time of acute ICH may actually be protective with respect to the risks of second in-hospital stroke (relative risk [RR] 0.814) and in-hospital mortality among patients who do not have a second in-hospital stroke (RR 0.884) in spite of an increased risk of death in patients who have an acute stroke recurrence after initial ICH (table 1). These apparent protective effects of aspirin initiation at the time of acute ICH may be due to reduction in secondary ischemic stroke, myocardial infarction, or other thromboembolic events.20 (It should be noted that this scenario is different from that in which patients develop ICH while already on aspirin,21 and also distinct from the long-term relative risks and benefits of aspirin use months and years after ICH.22)
With respect to the risk of death without further in-hospital stroke after initial stroke and the risk of second in-hospital stroke, aspirin appeared to be slightly more protective after ICH than after IS. However, IST and CAST data were not specifically designed to study aspirin initiation in patients with acute ICH, and the differences in RRs between ICH and IS were small. Therefore, to avoid biasing our model toward aspirin use after acute stroke of unknown etiology, we conservatively assumed that the acute mortality and secondary stroke prevention benefits of aspirin initiation at the time of acute ICH were the same as those reported after acute IS (i.e., 0.919 and 0.857, respectively).
Analyses.
The base case was a hypothetical adult with an acute stroke of undetermined etiology in a low-income country with 34% of strokes caused by ICH, as this is the highest reported proportion of ICH in a large epidemiologic study of incident stroke.13 Base case analyses also incorporated the increases in estimates of RRs of second stroke after ICH and death without further stroke after ICH discussed above. We conducted sensitivity analyses across the worldwide range of reported proportions of stroke caused by ICH to determine the threshold proportion below which aspirin would yield benefit in patients presenting with acute stroke of undetermined etiology. We also performed sensitivity analyses on all RRs related to aspirin administration after ICH (RR of death without further stroke after initial ICH, RR of second stroke after ICH, and RR of death after second stroke after initial ICH). Additionally, we conducted two-way sensitivity analyses for each aspirin-associated RR after ICH across the range of proportion of strokes due to ICH to assess how simultaneous variation of 2 risk parameters would affect the balance of risk and benefit of aspirin therapy for acute stroke of undetermined etiology.
RESULTS
Across the entire reported range of the proportion of first strokes due to ICH (9%–60%), compared to no treatment, aspirin treatment for the period of initial hospitalization after acute stroke of undetermined etiology is predicted to increase overall survival at discharge from initial hospitalization (figure 2A), decrease the risk of second in-hospital stroke (figure 2B), and increase survival to hospital discharge in patients who do not have in-hospital stroke recurrence (figure 2C).
Figure 2. One-way sensitivity analyses of expected outcomes at hospital discharge after acute stroke of undetermined etiology with and without aspirin administration in the acute setting over the range of proportion of initial stroke due to intracerebral hemorrhage.
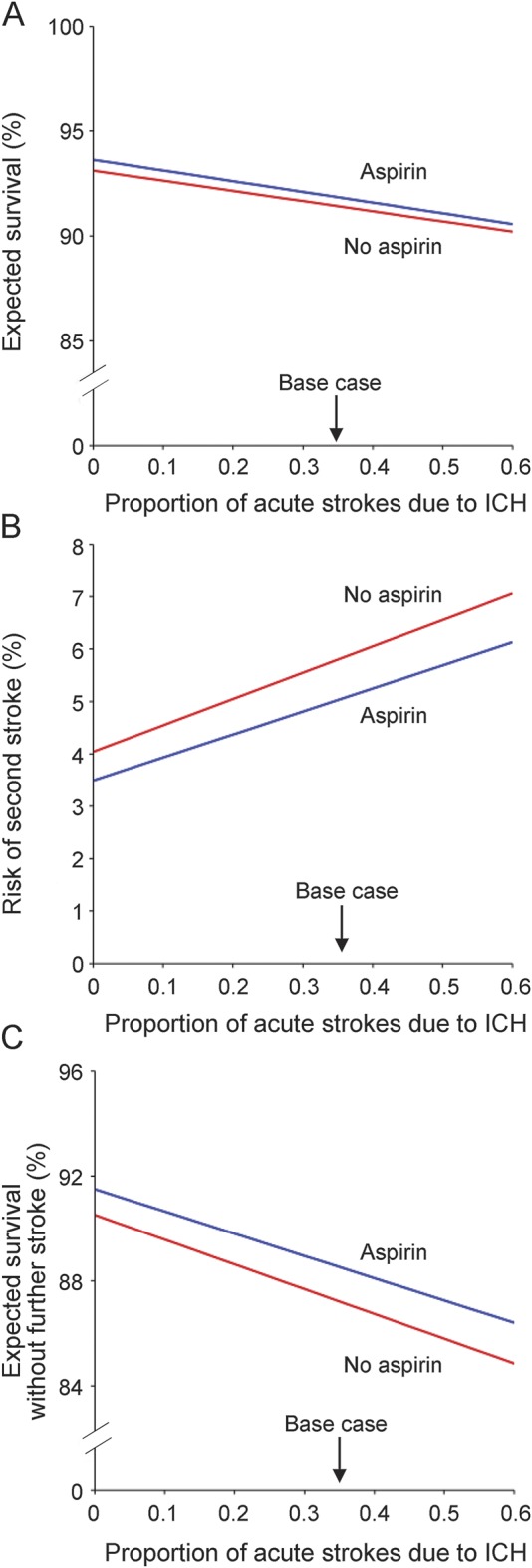
The x-axis indicates the proportion of initial strokes due to intracerebral hemorrhage (ICH). The y-axes represent (A) survival at hospital discharge (%); (B) risk of second in-hospital stroke (%); and (C) survival to hospital discharge without further stroke (%). The base case (34% of initial strokes due to ICH) is marked with an arrow. Treatment with aspirin is the preferred strategy for all outcome measures across the entire range of values for proportion of initial strokes due to ICH.
For the base case scenario in which ICH is the etiology of initial stroke in 34% of cases, aspirin is predicted to decrease in-hospital mortality by 4 per 1,000 (number needed to treat [NNT] 250), second in-hospital stroke by 8 per 1,000 (NNT 125), and the combined risk of in-hospital mortality or second stroke by 13 per 1,000 (NNT 77) (table 2).
Table 2.
Predicted outcomes at hospital discharge after acute stroke of undetermined etiology (per 1,000 patients) at base case values (proportion of first stroke due to ICH of 34%)

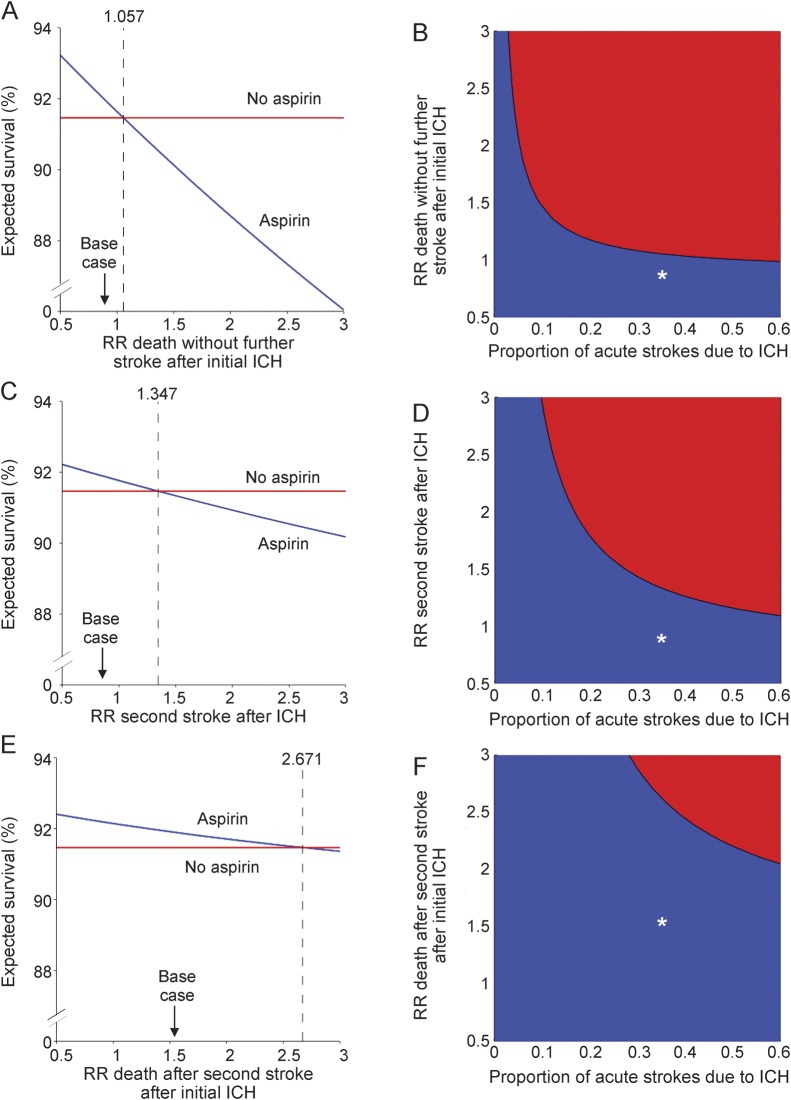
We conducted one-way sensitivity analyses in which we varied each RR for which aspirin could cause harm in the setting of acute ICH (figure 3, A, C, and E; figure e-1, A, C, and E, on the Neurology® Web site at www.neurology.org). Thresholds for RRs under which aspirin is predicted to yield benefit in the acute setting for acute stroke of unknown etiology are reported in table e-1. These analyses demonstrated that aspirin is predicted to reduce acute mortality (figure 3, A, C, and E) and the risk of second in-hospital stroke (figure e-1, A, C, and E) up to RR values beyond base case values. Two-way sensitivity analyses of these RRs across the worldwide range of the proportion of strokes due to ICH demonstrated the benefit of aspirin therapy for the acute period after stroke of undetermined etiology up to the highest reported proportion of acute strokes due to ICH worldwide and across a plausible range of RRs conferred by aspirin (figure 3, B, D, and F; figure e-1, B, D, and F). As the proportion of acute strokes due to ICH increases, the threshold RRs up to which aspirin for acute stroke of unknown etiology is preferred to no therapy decrease, but remain above base case values.
Figure 3. One-way sensitivity analyses and two-way sensitivity analyses of the relative risks associated with aspirin after intracerebral hemorrhage for the outcome of survival to discharge after acute stroke of unknown etiology.
Base case values for relative risk (RR) assessed are denoted with an arrow in one-way sensitivity analyses (A, C, E). The intersection of base case RR and base case proportion of initial strokes due to intracerebral hemorrhage (ICH) are denoted with an asterisk (*) in two-way sensitivity analyses (B, D, F). In one-way sensitivity analyses, the threshold at which the preferred strategy changes is marked by a vertical dashed line; aspirin treatment is preferred to no treatment to the left of this threshold value. In two-way sensitivity analyses, the region favoring aspirin treatment is shaded blue and the region favoring no treatment is shaded red. The y-axis for the one-way sensitivity analyses (A, C, and E) represents the expected percent survival to hospital discharge. The x-axes represent (A) aspirin-associated RR of in-hospital mortality with no further strokes after initial acute ICH; (C) aspirin-associated RR of second in-hospital second stroke after initial acute ICH; (E) aspirin-associated RR of death after second in-hospital stroke after initial ICH. The x-axis for the two-way sensitivity analyses represents the proportion of initial strokes due to ICH. The y-axes indicate (B) aspirin-associated RR of in-hospital mortality with no further strokes after initial acute ICH; (D) aspirin-associated RR of second in-hospital second stroke after initial acute ICH; (F) aspirin-associated RR of death after second in-hospital stroke after initial acute ICH.
DISCUSSION
Our decision analysis suggests that in resource-limited settings without access to neuroimaging, administration of aspirin to all patients presenting with acute stroke of undetermined etiology for the period of initial hospitalization could result in improved outcomes at hospital discharge. In the absence of a clinical trial to test this approach empirically, clinical decisions require patient-specific assessment of risks and benefits. Although clinical features cannot reliably distinguish IS from ICH, several clinical signs increase the likelihood of ICH rather than IS, such as coma, neck stiffness, seizures at onset of neurologic deficit, diastolic blood pressure greater than 110 mm Hg, vomiting, and headache.8 One strategy based on our results would be to consider aspirin administration to patients with acute stroke of undetermined etiology only in the absence of one or more of these signs. However, the widely held assumption that aspirin cannot be safely administered to patients with acute stroke of unknown etiology without neuroimaging to assess for ICH is not supported by our model, which favors aspirin administration across a range of plausible parameter estimates.
This conclusion is supported by the finding from the meta-analysis of IST and CAST that a subgroup of 8,889 patients who did not undergo CT scanning prior to randomization had no statistically significant difference in the favorable risk–benefit profile for aspirin demonstrated for the entire cohort.4 Additionally, in a cohort study of 148 patients with stroke of undetermined etiology in the Gambia, aspirin treatment was associated with improved outcome, despite estimation that 46% of patients had hemorrhagic strokes based on clinical evaluation.23
The strengths of our study include availability of a large dataset on which to base the decision analysis model. Additionally, we used as our base case the highest reported proportion of strokes due to ICH from a large epidemiologic study (34%),13 and ran sensitivity analyses up to the highest reported proportion of strokes due to ICH from smaller case series (60%).14–16 Two-way sensitivity analyses demonstrate that aspirin use after acute stroke is predicted to improve outcomes in regions where the proportion of strokes due to ICH is lower than these extreme values up to substantially higher potential post-ICH RRs of aspirin therapy than those reported in the literature.
Our study has several limitations. First, the time period under evaluation included only the initial hospitalization after acute stroke. Further studies should examine whether the longer-term benefits of ischemic stroke prevention with aspirin outweigh the potential risks of subsequent ICH in patients whose initial stroke type is unknown. Second, IST and CAST were randomized controlled trials, and outcomes may therefore be better than what one would expect in community settings in low-income countries. However, it should be noted that the data from IST and CAST include patients from countries classified at the time of the study (1997) as low income (China, India, Sri Lanka) and lower-middle income (Slovak Republic, Turkey),24 which is the population for whom our analysis is aimed. Third, it is likely that nonthromboembolic etiologies of acute stroke are more common in lower-income countries as compared to higher-income countries (e.g., complications of CNS infections),2,25 and the risks and benefits of aspirin are less clear in these clinical scenarios. However, epidemiologic data from low- and middle-income countries suggest that the majority of strokes are caused by the same risk factors for atherosclerotic disease as in high-income countries.2,13 Fourth, the randomization of patients to receive aspirin after ICH in IST and CAST was inadvertent, thus limiting the statistical robustness of the data on which to base assumptions of risk and benefit in this population compared to those with IS as the index event. We balanced this limitation by using conservative estimates for RRs where aspirin appeared protective after ICH and by performing sensitivity analyses on each of these RRs. Additionally, patients with ICH in IST and CAST who were deemed clinically likely to have IS and thus inadvertently randomized to receive aspirin ostensibly did not have severe ICH, which could bias our results. The clinical predictors of ICH described above should therefore lead to caution when considering empiric aspirin therapy for stroke of unknown etiology, as many of these clinical signs are suggestive of elevated intracranial pressure, which is more likely to occur hyperacutely in ICH than in IS. Finally, while we did not include patients with SAH in our model since we assumed that patients with SAH would be clinically distinct from those with IS or ICH, this may not always be the case. Again, in settings of clinical uncertainty with respect to the presentation of an acute-onset neurologic deficit, our results should be interpreted with caution.
An additional limitation is that it is unclear what the appropriate dosage and timing of aspirin administration should be in the setting of acute stroke of undetermined etiology. Although there appears to be no change in efficacy in long-term secondary prevention at varying dosages of aspirin from 50 to 1,500 mg,5 our model is based on the data from IST and CAST, which utilized 300 mg/day and 160 mg/day, respectively. IST and CAST randomized patients presenting within 48 hours of acute stroke and continued therapy for the duration of hospitalization (maximum duration 2 to 4 weeks). Aspirin administration at 160 or 320 mg within 24–48 hours for 2 to 4 weeks after stroke of undetermined etiology could be considered based on the data incorporated in our model. However, given that the risk of ICH expansion is greatest within the first 24 hours after ICH,26 and given that approximately 40% of patients across IST and CAST were randomized between 25 and 48 hours after stroke onset,4 one could consider beginning aspirin therapy 25–48 hours after acute stroke onset when the etiology of the stroke is unknown.
Although administering aspirin to patients with acute ICH is not without potential risk, our decision analysis and the data on which it is based4 suggest that such risks may be less than commonly perceived. Additionally, the strategy of withholding aspirin from all patients with acute stroke increases the risk of worse outcomes in patients with ischemic stroke. While awaiting improved access to CT for optimal management of acute stroke in resource-limited settings, our model suggests that clinicians practicing in such settings can consider aspirin administration to patients with acute stroke of unknown etiology for the period of initial hospitalization. This may lead to decreased acute stroke-related mortality in regions of the world where the burden of cerebrovascular disease–related deaths is greatest.
Supplementary Material
ACKNOWLEDGMENT
Dr. Berkowitz thanks Dr. Wendell Blaise of Hôpital St Boniface in Fond-des-Blancs, Haiti, who first alerted him to this clinical conundrum. All authors thank Dr. Steven M. Greenberg, Dr. Steven K. Feske, and Dr. Allan H. Ropper for comments on earlier versions of the manuscript.
GLOSSARY
- CAST
Chinese Acute Stroke Trial
- ICH
intracerebral hemorrhage
- IS
ischemic stroke
- IST
International Stroke Trial
- NNT
number needed to treat
- RR
relative risk
- SAH
subarachnoid hemorrhage
Footnotes
Editorial, page 778
Supplemental data at Neurology.org
NOTE ADDED IN PROOF
A forthcoming study examines the risks and benefits of aspirin for long-term secondary prevention after stroke of unknown etiology.27
AUTHOR CONTRIBUTIONS
Aaron L. Berkowitz: study design, data collection, data analysis, data interpretation, literature search, figures, drafting and revising the manuscript. M. Brandon Westover: study design, data analysis, data interpretation, figures, manuscript revision. Matt T. Bianchi: study design, data analysis, data interpretation, manuscript revision. Sherry H.-Y. Chou: study design, data analysis, data interpretation, manuscript revision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Berkowitz receives royalties from Clinical Pathophysiology Made Ridiculously Simple (Medmaster, Inc.) and The Improvising Mind (Oxford University Press). M. Brandon Westover and M. Bianchi report no disclosures relevant to the manuscript. S. Chou has been funded by the American Heart Association and the NIH, is currently funded by National Institute of Neurological Disorders and Stroke, and serves on the endpoint committee of a clinical trial funded by Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brainin M, Teuschl T, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol 2007;6:553–561 [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modeling. Lancet Neurol 2009;8:345–354 [DOI] [PubMed] [Google Scholar]
- 4.Chen ZM, Sandercock P, Pan HC, Counell C, Collins R, Liu LS. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. Stroke 2000;31:1240–1249 [DOI] [PubMed] [Google Scholar]
- 5.Johnson ES, Lanes S, Wentworth C, Satterfield M, Abebe BL, Dicker LW. A metaregression analysis of the dose-response effect of aspirin on stroke. Arch Intern Med 1999;159:1248–1253 [DOI] [PubMed] [Google Scholar]
- 6.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA 1998;280:1930–1935 [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet 2011;378:1231–1243 [DOI] [PubMed] [Google Scholar]
- 8.Runchey S, McGee S. Does this patient have a hemorrhagic stroke? Clinical findings distinguishing hemorrhagic stroke from ischemic stroke. JAMA 2010;303:2280–2286 [DOI] [PubMed] [Google Scholar]
- 9.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med 1987;316:250–258 [DOI] [PubMed] [Google Scholar]
- 10.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716 [DOI] [PubMed] [Google Scholar]
- 11.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. Lancet 1997;349:1569–1581 [PubMed] [Google Scholar]
- 12.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomized placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641–1649 [PubMed] [Google Scholar]
- 13.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–123 [DOI] [PubMed] [Google Scholar]
- 14.Zenebe G, Alemayehu M, Asmera J. Characteristics and outcomes of stroke at Tikur Anbessa Teaching Hospital, Ethiopia. Ethiop Med J 2005;43:251–259 [PubMed] [Google Scholar]
- 15.Matuja W, Janabi M, Kazema R, Mashuke D. Stroke subtypes in black Tanzanians: a retrospective study of computerized tomography scan diagnoses at Muhimbili National Hospital, Dar es Salaam. Trop Doct 2004;34:144–146 [DOI] [PubMed] [Google Scholar]
- 16.Nyame PK, Jumah KB, Adjei S. Computerised tomographic scan of the head in evaluation of stroke in Ghanians. East Afr Med J 1998;75:637–639 [PubMed] [Google Scholar]
- 17.Durai Pandian J, Padma V, Vijaya P, Sylaja PN, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke 2007;2:17–26 [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz AL, Mittal M, Mclane HC, et al. Worldwide reported use of IV-tissue plasminogen activator for acute ischemic stroke. Int J Stroke 2014;9:349–355 [DOI] [PubMed] [Google Scholar]
- 19.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369 [DOI] [PubMed] [Google Scholar]
- 20.Ogata T, Yasaka M, Wakugawa Y, Inoue T, Ibayashi S, Okada Y. Deep venous thrombosis after acute intracerebral hemorrhage. J Neurol Sci 2008;272:83–86 [DOI] [PubMed] [Google Scholar]
- 21.Thompson BB, Bejot Y, Caso V, et al. Prior antiplatelet use and outcome following intracerebral hemorrhage. Neurology 2010;75:1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn R, MacDonald TM, Murray GD, MacWalter RS, Doney ASF. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606–2611 [DOI] [PubMed] [Google Scholar]
- 23.Garbusinski JM, van der Sande MA, Bartholome EJ, et al. Stroke presentation and outcome in developing countries: a prospective study in the Gambia. Stroke 2005;36:1388–1393 [DOI] [PubMed] [Google Scholar]
- 24.The World Bank. World Development Indicators 1997. Washington, DC: IEC Information Center, Development Data Group; 1997 [Google Scholar]
- 25.Chow FC, Marra CM, Cho TA. Cerebrovascular disease in central nervous system infections. Semin Neurol 2011;31:286–306 [DOI] [PubMed] [Google Scholar]
- 26.Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage incidence and time course. Stroke 1996;27:1783–1787 [DOI] [PubMed] [Google Scholar]
- 27.Berkowitz AL, Westover MB, Bianchi MT, Chou SH. Aspirin for secondary prevention after stroke of unknown etiology in resource-limited settings: a decision analysis. Neurology (in press 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.