Abstract
Inflammation is a facilitating process for multiple cancer types. It is believed to affect cancer development and progression through several etiologic pathways including increased levels of DNA adduct formation, increased angiogenesis and altered anti-apoptotic signaling. This review highlights the application of inflammatory biomarkers in epidemiologic studies and discusses the various cellular mediators of inflammation characterizing the innate immune system response to infection and chronic insult from environmental factors. Included is a review of six classes of inflammation-related biomarkers: cytokines/chemokines, immune-related effectors, acute phase proteins, reactive oxygen and nitrogen species, prostaglandins and cyclooxygenase-related factors, and mediators such as transcription factors and growth factors. For each of these biomarkers we provide a brief overview of the etiologic role in the inflammation response and how they have been related to cancer etiology and progression within the literature. We provide a discussion of the common techniques available for quantification of each marker including strengths, weaknesses and potential pitfalls. Subsequently, we highlight a few under-studied measures to characterize the inflammatory response and their potential utility in epidemiologic studies of cancer. Finally, we suggest integrative methods for future studies to apply multi-faceted approaches to examine the relationship between inflammatory markers and their roles in cancer development.
Keywords: Inflammation, biomarkers, neoplasm, cancer epidemiology
INTRODUCTION
The role of inflammation in the development and progression of cancer is of great scientific and public health interest and has drawn much attention of late. Several excellent reviews have described the likely cellular and molecular roles of inflammation in the development of cancer (1-13) and have outlined the consistent associations between chronic inflammatory conditions (14-19) and inflammation-inducing risk factors (such as tobacco (20-22)) in the development of cancer at various sites. As the burden of cancer increases globally (23, 24) so does the value of identifying therapeutic targets. The role of inflammation in carcinogenesis requires additional research to clarify the mediators, pathways and steps through which increased or altered inflammation leads to neoplastic development or progression. Ultimately, continued research is required to identify the key points of potential intervention to successfully improve outcomes. In order to proceed, epidemiologic studies will require integrative techniques across many platforms to elucidate meaningful mechanisms and improve outcomes.
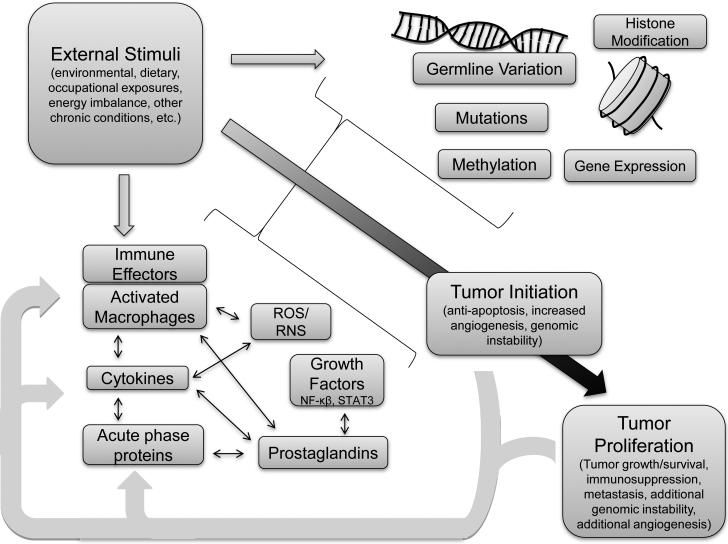
In this review we provide an overview of several effectors of inflammation involving the response of the immune system to infection and to chronic insult from environmental factors. We summarize the commonly used measurements to evaluate inflammatory status or alteration in the development of cancer, including the strengths and weaknesses of the common techniques available for each marker. We have provided recent evidence and findings to date regarding associations with cancer etiology and progression. Our literature summaries are not meant to be exhaustive due to the extent of this field and where possible we refer readers to relevant meta-analyses and literature reviews for concision. Subsequently, we highlight several under-studied measures of the inflammatory response. A general framework for the biomarkers of interest and their inter-relationships with cancer risk is depicted in Figure 1. We suggest integrative multi-faceted approaches for future studies seeking to examine the relationship between the markers and their roles in cancer development. Per definition, we refer to all of the biological measures of the immune and inflammation responses included in this paper as ‘inflammation biomarkers’ for simplicity although there is certainly great variety in the measures discussed.
Figure 1.
The complex interactions involved in the role of inflammation in the cancer progression spectrum.
Methodological Issues in Epidemiologic Studies on Inflammation and Cancer
In the evaluation of the associations between inflammation and cancer risk, careful consideration must be taken to address the potential for biased associations due to the well-known pro-inflammatory potential of tumors and, thus, their microenvironment (6). Prospective studies are, therefore, preferable due to lower risk of presenting temporally biased associations. Prospective designs also allow for latency analyses to determine whether the inflammatory marker associations are causal drivers of carcinogenesis or simply pre-diagnosis manifestation of tumor-related inflammation. Therefore, ideal prospective research designs investigating inflammatory markers should be conducted on samples taken many years, perhaps even decades, prior to diagnosis. Repeat sampling is useful, because of the different mechanisms by which inflammation can drive carcinogenesis, e.g., DNA damage (in earlier years) vs. enhancement of angiogenesis (later). Samples collected only a few years prior to diagnosis may no longer reflect an evaluation of causality of the biomarker, instead becoming an evaluation of an early disease prediction marker, which although still clinically relevant, reflects a different hypothesis. In this context, large population-based cohorts with biobanking initiatives are extremely valuable in evaluating associations of inflammatory biomarkers. In our presentation of the literature we emphasized large, prospective studies over retrospective designs. We also emphasize evidence from biomarkers measured in blood and to a lesser degree urine samples in large epidemiologic studies. Although several protocols exist for measuring target organ-specific inflammation-related compounds in several media including exhaled breath and its condensate (25), sputum (26, 27) brochioalveolar lavage (28), and feces (29) among others (30) at present these biospecimens are prohibitively expensive to feasibly collect in large population-based initiatives.
The inflammation responses under investigation may be due to a multiplicity of factors which have been consistently linked to cancer risk including, but not limited to tobacco consumption (31), overweight and obesity (32, 33), physical inactivity (34, 35), persistent and or transient infection (36) and immunosuppression (37-39). Thus, in a well-designed epidemiologic evaluation of the causality of inflammatory biomarkers during carcinogenesis these factors should be accounted for, depending on which specific biomarkers are being evaluated, in both the design and analysis stages in order to best isolate the causal associations being examined.
In studies of inflammation and subsequent cancer-related clinical outcomes and survival, pre-surgical or pre-treatment blood samples should be collected to avoid the impact of treatment on levels of inflammatory and immune markers. In population-based studies examining inflammatory biomarkers on survival outcomes through active follow-up or passive cancer registry linkage, attention must be taken to collect detailed staging information for adjustment in analysis. Failure to do so invites the possibility of confounding due to a third factor related to inflammation and advanced stage at diagnosis, therefore affecting survival. Below we review six main classes of inflammation-related biomarkers: cytokines/chemokines, immune-related effectors, acute phase proteins (C-reactive protein, Serum Amyloid A), reactive oxygen and nitrogen species, prostaglandins and cyclooxygenase-related factors, and mediators such as transcription factors and growth factors.
INFLAMMATION BIOMARKERS
Cytokines/Chemokines
Background
During both acute and chronic inflammatory processes, a variety of soluble factors known as cytokines are involved in leukocyte recruitment through increased expression of cellular adhesion molecules and chemoattraction (40-43). To a large extent they orchestrate the inflammatory response, i.e. they are major determinants of the make-up of the cellular infiltrate, the state of cellular activation, and the systemic responses to inflammation (44). Cytokines are central in extensive networks that involve synergistic as well as antagonistic interactions and exhibit both negative and positive regulatory effects on various target cells (42). Although produced by a wide variety of cell types, macrophages and T lymphocytes (T-cells) are the primary producers of cytokines which may have predominantly pro-inflammatory (inflammation-promoting)(IL-1α, IL-1β, IL-2, IL-6, Il-8, IL-12, TNF-α, IFN-γ(45)) or anti-inflammatory (inflammation-suppressive)(IL-4, IL-5, IL-10, TGF-β) abilities.
Measurement
The measurement of cytokines as an indicator of inflammatory status in population-based initiatives is an area of great promise, yet provides several challenges due to the biochemistry of the molecules, particularly their short half-life (46, 47). Considering the immediate response of the body to injury, it can be advisable to draw the blood tube that is dedicated for cytokine measurements first during a blood draw. Cytokines can be measured in serum and plasma samples however, measurements from the different sample types cannot be used interchangeably (48, 49). They can also be measured in tissues or as supernatant from cultured peripheral blood mononuclear cell (PBMC) preparations (50). Cytokine measurements can be multiplexed to simultaneously assess multiple targets (51), presenting the opportunity to broaden the scope of investigation or test for possible interactions between the mediators. These techniques are, however, limited by differential concentrations of the varying cytokines. In addition cytokine quantification can be affected by degradation through freeze/thaw cycles over longitudinal storage (52). Also, issues of standardized sample collection, processing and study design must be carefully considered or sensitivities in the protein measurements may create artifactual associations if care is not taken (46). Concentrations of cytokines are known to vary in different tissues and a standard blood draw may not adequately reflect tissue-specific levels of inflammation (53). However, measurements of circulating cytokines may provide a general sense of an individual's inflammatory state. Additional advantages and disadvantages to measurement of cytokines are summarized in Table 1.
Table 1.
Summary of inflammatory markers, associated techniques, tissue requirements with corresponding advantages and disadvantages of their application.
| Inflammatory Marker | Explanation | Techniques* | Advantages | Disadvantages | Tissue Requirement** | Current Evidence of Cancer Association*** |
|---|---|---|---|---|---|---|
| Cytokines and Chemokines | ||||||
| Cytokines/ Chemokines | Small secreted proteins which mediate as well as regulate immunity, inflammation, and hematopoiesis. Cytokines generally act at very low concentrations over short distances and short time spans. | ELISA, multiplex bead assays | Simultaneous measurement of several cytokines possible | No strong evidence to predict progression or survival. Blood draw and processing conditions can affect levels. Degradation over time when samples stored improperly or over multiple freeze/thaw cycles. Costly Tumors create an inflammatory milieu and can produce cytokines. Difficult to assess reverse causality without longitudinal data. Lack of standardization of assays Function in multiple pathways (lack of specificity) |
Serum/plasma/tissue/cell culture supernatant Depends on assay used (5-100uL) |
Direct measurement in several cancers with correlation with tumor stage and disease extent. Related to cancer risk in prospective collected data. |
| Immune-Related Effectors | ||||||
| White cell count | A measure of the total white blood content, generally indicative of infection (neutrophils and monocytes-bacteria, lymphocytes - viral, eosinophils - parasitic). | FACS | Routinely measured and used in clinical practice (useful for prediction and maximization of currently available data and employed clinical algorithms). Stable over time when frozen. Blood neutrophilia and thrombocytosis established as indicators of systemic inflammatory response. |
Levels may be altered due to transient infection not-related to chronic inflammation. | Serum Portable kits available that need only 10uL |
Associated with lung cancer risk in prospectively collected data. Associated with cancer-related mortality in prospective studies. |
| Glasgow Prognostic score | A combination of Albumin and C-reactive protein measurements into a 3 level predictive score. 2 when both C-RP>10mgl-1 and albumin <35gl-1. 1 if only one abnormality present. 0 if both not. | Combined C-RP and albumin tests. | Inexpensive if C-RP and albumin already measured. Standardized score |
Levels may be altered due to transient infection not-related to chronic inflammation same issues for C-RP. | Serum | Not related to risk of cancer development. Evidence suggests use as a prognostic score independent of tumor stage and treatment. |
| Neutrophil/Lymphocyte ratio | The ratio of neutrophils to lymphocytes, where higher values reflect states of dramatic inflammation. | Same as white cell count | Potential as a simple, cost effective, and readily available test. | Different cutoff levels reported across studies. Additional value obtained beyond normal white cell counts is of question. |
Serum | Shown to be related to survival in many cancer sites after diagnosis and various treatment modalities. |
| Platelet/Lymphocyte ratio Th17 lymphocytes |
The ratio of platelets to lymphocytes, where higher values reflect states of dramatic inflammation. Recently discovered inflammatory T-cell subset with associations to autoimmune diseases and potential role in cancer risk and progression. |
Same as white cell count IHC, FACS |
Routinely measured and used in clinical practice (useful for prediction and maximization of currently available data and employed clinical algorithms). Potential as a simple, cost-effective, and readily available test. All three cell types give insight into the T-cell functional status and thus immune responses. A combined analysis can potentially advance the current analysis focusing on one of the cell types. |
Levels may be altered due to transient infection not-related to chronic inflammation. Not well studied with risk of disease. Heterogeneous results are assumable between cancer entities. Material acquisition might be problematic. |
Serum Tissue (TMAs), Peripheral blood cells (requires fresh cells for FC) |
Predicts outcomes in colorectal cancer. Not as of yet extensively studied with risk. |
| Acute Phase Proteins | ||||||
| C-reactive Protein | An acute phase protein produced by hepatocytes in response to pro-inflammatory cytokines. Produced in times of inflammation to age damaged cells for excretion by the liver. | Fluorescence polarization-immunoassay, Nephelometry, ELISA | Quantitative and sensitive measurement | Non-specific marker of inflammation | Serum or plasma | Large meta-analysis indicates poor evidence to support use as a diagnostic marker; may be useful in colorectal and lung cancers. |
| Easily measured | Levels may be altered due to transient infection not-related to chronic inflammation (CRP rises drastically in acute inflammation, such as infection, therefore several measurements over time are encourage for a better characterization of chronic states. | |||||
| Serum Amyloid A (SAA) | Similar to CRP an acute phase protein, but levels may be even more responsive to inflammation. | Fluorescence polarization-immunoassay, Nephelometry, ELISA | Easily measured Rather novel marker |
Limited evidence for use to predict treatment response. | Serum or plasma | Strongly associated with worse long-term survival from breast cancer. |
| Reactive Oxygen & Nitrogen Species | ||||||
| Reactive Oxygen & Nitrogen Species | Chemically-reactive molecules produced as by-products of normal metabolic processes in all aerobic organisms. Characterized by the presence of unpaired electrons. | Would provide a direct estimate of ROS burden and would be beneficial for prediction of risk and carcinogenicity of lifestyle patterns. | No standardized methods to capture actual ROS levels in humans to date Compounds have very short half-life in systems. Questionable quality due to the volatility of compounds. Levels affected by lifestyle factors i.e. Nutritional status |
Tissue Serum |
Direct evidence and measurement in prospective studies lacking Signaling Pathways regulated by ROS in cancer models. Association with expression/enzyme activity of ROS/RNS producers shown in various cancers. Weak evidence of variants in ROS/RNS production genes and cancer risk. |
|
| Oxidatively/Nitrosatively modified DNA, or proteins | The product of excess ROS/RNS in tissue | |||||
| 3-Nitrotyrosine | The product of nitrosylated proteins | HPLC | Not well standardized | Healthy or tumor tissue | Higher level observed in the tumors of never smoking lung cancer cases suggesting a marker for inflammation-related carcinogenesis. | |
| 8-hydroxy-2′-deoxyguanosine (8-oxodg or 8-OHdG) | 8-oxodG is a sensitive surrogate biomarker for in vivo oxidative stress. | ELISA, HPLC methods | Provides a measure of DNA damage due to ROS. | Not tissue specific | Plasma, Urine | Elevated levels observed in several cancers included esophageal, colon and breast |
| 8-Iso-PGF2_α | Lipid peroxidation product. | ELISA, HPLC methods | Provides a measure of DNA damage due to ROS. | Not tissue specific | Plasma, Urine | Related to breast and colon cancer risk |
| Malondialdehyde(MDA) | Lipid peroxidation product. | ELISA, HPLC methods | Provides a measure of DNA damage due to ROS. | Not tissue specific | Plasma, Urine | Associated with lung and colon cancer risk |
| Trans-4-hydroxy-2-noneal (HNE) | Lipid peroxidation product. | ELISA, HPLC methods | Provides a measure of DNA damage due to ROS. | Not tissue specific | Plasma, Urine | Not as well studied as other peroxidation products |
| Prostaglandins, Cyclooxygenases, Lipoxygenases and Related Factors | ||||||
| Prostaglandin levels | Lipid compounds containing 20 carbon ring including a 5-carbon group. Produced by the sequential oxidation of Prost. By COX-1 &2. COX-1 is believed to control baseline levels of prosts, while COX-2 increases levels of PGE by response to stimulation | ELISA Liquid chromatography/tandem mass spectrometry |
Levels may be tissue-specific difficult to measure. Affected by several pharmacologic interventions which could complicate association modeling. Some prostaglandins are rapidly degraded |
Saliva, Urine, Serum, EDTA and Heparin Plasma | ||
| COX-2 Expression | Enzymes integral to prostaglandin synthesis. | IHC | Interest as target for chemo-prevention | Levels may be tissue-specific difficult to measure. Affected by several pharmacologic interventions which could complicate association modeling. |
Tissue Culture Media TMA |
COX-2 expression observed in nearly every tumor type examined. |
| Transcription Factors and Growth Factors | ||||||
| NF-κB Activation | A transcription factor that functions in inflammatory pathways by inducing the expression of inflammatory cytokines, adhesion molecules, cyclooxygenases, NO synthase and angiogenic factors. | ELISA RtPCR to measure mRNA |
Can measure quantity, activation, translocation and transcriptional potential. | Clinical evaluation of NF-κB requires cell culture. | Serum, plasma, peripheral blood lymphocytes | Predictive of outcomes in BC and CRC. |
| STAT3 Activation | A transcription factor activated in response to various factors including inflammatory cytokines. Mediates the expression of several key cell growth and apoptosis genes. | ELISA | Also regulated by other non-inflammatory growth factors. | Serum or plasma | Less well studied than NF-κB. | |
Most commonly cited techniques although others may exist
Quantity of sample will depend on quality of material extraction, processing and type
For additional details see text
Cancer Associations
Risk
Systemic cytokine concentrations have been associated with both cancer risk (54-57) and cancer progression (58-62) suggesting a pivotal role in carcinogenesis. For example, in the Health, Aging and Body Composition cohort circulating IL-6 and TNF-α were associated with lung cancer (LC), IL-6 was also associated colorectal cancer (CRC), however, neither were associated breast (BC) and prostate (PC) (62). Investigation of serum IL-6 and IL-8 levels in the Prostate Lung Colon and Ovarian (PLCO) Cancer Screening Trial showed associations with LC (IL-6, Odds Ratio (OR)=1.48, 95% Confidence Interval (CI)=1.04-2.10; IL-8, OR=1.57, 95% CI=1.10-2.24), compared with the lowest quartile. However, increased IL-6 levels were only associated with cancers diagnosed within two years of blood collection, whereas increased IL-8 levels were associated with cancers diagnosed more than two years after blood collection (OR = 1.57, 95% CI = 1.15-2.13) (54). Whether this difference in association is due to cytokine degradation over time or a real association remains to be determined.
IL-10 has been investigated in the development of non-Hodgkin's lymphoma (NHL) in a prospective study with a significant positive association observed (63) as well as with pre-diagnostic levels of IL-10, TNF-α and sTNF-R2 in a separate prospective investigation (64).
Progression
Several studies have observed negative prognosis of various cancers associated with IL-6 level including PC (65), renal cell carcinoma (RCC) (66), non-small cell LC (67), OC (68), lymphoma (69, 70), chronic lymphocytic leukemia (CLL) (71), esophageal (ESOC) (72), CRC and BC (73).
Investigation of prognosis with IL-6 serum concentrations (≥4.0 pg/mL) in the Multiethnic Cohort Study showed associations with significantly poorer survival in both African Americans (hazard ratio (HR)=2.71, 95% CI=1.26-5.80) and Caucasians (HR=1.71, 95% CI=1.22-2.40). IL-10 (HR=2.62, 95% CI=1.33-5.15) and IL-12 (HR=1.98, 95% CI=1.14-3.44) were associated with LC survival only in African Americans (74). Serum levels of IL-6 have also been associated with tumor proliferative activity among patients with CRC (75).
An examination of clinical outcomes among hepatocellular carcinoma (HCC) patients after potentially curative hepatectomy reported that higher pre-therapy serum levels of IL-17 and lower levels of IL-1 were associated with early recurrence. After adjustment for general tumor clinic-pathological factors, elevated serum levels of IL-17 (≥ 0.9 pg/ml) were found to be an independent risk factor for HCC early recurrence with an HR of 2.46 (95%CI=1.34-4.51). Patients with larger tumors (>5 cm in diameter) and elevated serum levels of IL-17 had the highest risk of early recurrence as compared to those with only one of these factors (P = 0.009) or without any (P<0.001). The authors suggest that these factors showed similar effects on the HCC patient overall survival (76).
Immune-Related Effectors
Background
Leukocytes comprise an integral portion of the innate, as well as of the adaptive immune system, and include granulocytes (neutrophils, basophils, eosinophils) monocytes, macrophages, dendritic cells and lymphocytes (B&T cells), which can exert immune-stimulating or immune-suppressive functions (77). In cancer patients, several pathways can be activated to suppress the effective adaptive immune response, triggered to avoid the destruction of the tumor by immune cells (78). Leukocytes also activate to release cytokines and growth factors, which support tumor growth. The activities of the immune system lead to a change of blood leukocytes profile, which serves as a marker of the systemic inflammatory response. Based on this principle, several measurements of inflammation and a shift in number or ratios of immune cells have been investigated for the association with cancer risk or outcome of the disease such as the modified Glasgow Prognostic Score (mGPS), (79) neutrophil to lymphocyte ratio (NLR) (80) and platelet to lymphocyte ratio (PLR) (81).
Tumor infiltrating lymphocytes (TILs) are white-blood cells found within the tumor which presumably reflect an immune response against the tumor (82). It is thought that TILs work in combination with chemotherapies which can promote cytotoxic T-lymphocytes that can produce anti-tumor immunity and thus lead to improved outcomes (83). However, a role in supporting tumor-growth cannot be excluded. T Helper 17 (Th17) cells are a CD4+ T cell subset in addition to Th1 and Th2 that lead to increased levels of IL-17, IL-22 and IL21 production (84-86). IL-6 and other cytokines including IL-23 are thought to play a key role in the production of the Th17 cells (84, 87, 88). They mediate host defensive mechanisms to various infections through provide anti-microbial immunity at epithelial/mucosal barriers and are involved in the pathogenesis of many autoimmune diseases (86).
Measurement
The measurement of lymphocytes can be performed using tissue, or peripheral blood samples and is based on standard clinical routines (white blood cell [(WBC]) counts). Flow cytometry has also become a widely used tool to quantify phenotypic subsets of immune cells and thus provides a snapshot that allows for some understanding of the current immune response (89-91). Flow cytometry can also be used to quantify T cell proliferation using dyes (92).
Despite great promise, flow cytometry is limited in its epidemiologic application as the experiments are sensitive to issues of standardization particularly from differences reagents, sample handling, instrument setup and data analysis. These differences across study sites are known to effect outcome measurements (91, 93, 94) and have been shown to affect results in multi-centered projects (95). Attention to standardization of procedures along the project pipeline may aid in alleviating these concerns and promote cross-project collaborations which is one of the goals of the human immunology project (96). In addition, the quantification of immune cells generally requires fresh biospecimen, which limits its use in epidemiologic studies.
TIL can be measured by immunohistochemistry (97) using different stains including hematoxylin and eosin and through the use of multicolor flow cytometry (98). TIL can be quantified using tissue microarrays and whole tissue sections (99). Th17 can be measured by multicolor flow cytometry (100) can be evaluated in peripheral blood and other body fluids (101, 102).
Cancer Associations
Etiology
A comprehensive review of the associations between these immunological markers and outcomes is beyond the scope of this review. Briefly, various measures of leukocyte quantities such as WBC count, platelet to lymphocyte ratio (PLR) and the neutrophil to lymphocyte ratio (NLR) have been associated with increased risk of several types of cancer including BC, CRC and EC), but also with tumor progression (81, 103). The WHI (Women's Health Initiative) observed a significant association between WBC count and increased risk of invasive BC, CRC, EC, and LC in more than 140,000 postmenopausal women (103).
Prognosis
Likewise, a study investigating the association between several inflammation-based prognostic scores such as mGPS, NLR and PLR and cancer survival observed strong prognostic values of all three scores for cancer survival independent of tumor site (BC, BLC, OC, PC, gastro-esophageal, hematological, RCC, CRC, NHC, hepatopancreaticobiliary and LC) in more than 27,000 patients (104).
TILs have been the focus of many studies and were shown to be positively associated with improved survival among cancer patients, including CRC (105), LC (106), and others sites (107-109). In addition, the assessments of TIL densities at the margin of liver metastasis of CRC patients were predictive for chemotherapy response (110). CD8+ TILs were independently predictive of improved BC survival however results vary by molecular subtype (improved in basal, but not in triple-negative) (111) and by estrogen receptor status and histological grade (112).
The presence of Th17 cells in OC (113), PC (114), LC (115) and PANCC (116) as well as in melanoma (117) were repeatedly associated with better survival of patients (118).
Acute Phase Proteins
C-reactive Protein (CRP)
Background
CRP is an acute phase protein found in blood, which is synthesized in the liver in response to inflammation. Physiologically the protein activates the complement system via the Q1 complex (119). Once activated, the complement system aids in clearing the injured or dead cells from tissues. CRP has been related to systemic levels of inflammation in various inflammatory conditions as well as chronic diseases such as cardiovascular disease and type II diabetes (120). CRP is also highly related to obesity (generally measured using body mass index in population studies) across genders and study populations (121), although most research has been done on Caucasian populations, as obesity is a chronic inflammatory state/condition (122). Obesity has been associated with cancer risk and progression at various sites with one of the suggested mechanisms to be operating through chronic altered inflammation (32, 33, 123-127). Therefore, CRP may act not as a causal protein but as a marker of systemic inflammation. Elevated CRP levels have also been correlated with other elevated inflammatory markers (128).
Measurement
CRP measurements can be performed in whole blood, plasma and serum using various immunoassays (129) with high sensitivity nephrelometry being the gold standard. As with other inflammatory markers, CRP has a relatively short half-life, and thus proper sample processing is essential (130). Transient conditions such as a common cold or mild injury/trauma can drastically alter individual CRP levels (131). Thus, variability of CRP levels may lead to issues in analyses and or biased statistical estimates. It is possible to recognize and eliminate infection-induced very high values by reviewing CRP levels against age- and BMI-standardized rates and excluding individuals with a certain level of variability above. Nevertheless, single studies with single measurements can be affected by transient conditions. One investigation into the effects of a single CRP measurement in epidemiologic studies suggested that conducting a single measurement could largely attenuate observed effect sizes from true effect sizes (132). Multiple measurements would therefore be optimal to track changes in levels over time. However, an analyses with repeated measurements has shown that a small index of individuality was observed in healthy individuals with relative rankings over a six month interval differing minimally (133).
Cancer Associations
Etiology
Several prospective analyses have shown that CRP is associated with risk of cancer at various sites (54, 134, 135). An investigation of risk at multiple sites in the Healthy Aging and Body Composition Study showed that baseline levels were associated with LC, CRC and BC risk. A nested case-control study also suggested associations for HCC, LC, skin, RCC, and bladder cancer (BLC) (136). Prospective investigations have observed null associations for BC (137, 138) but increased risk for OC (139).
The association results for CRP and CRC risk, however, are contradictory, as a previous meta-analysis of eight prospective studies suggested that increased CRP levels collected at baseline was related to a modest increase in CRC risk (RR=1.12, 95% CI=1.01-1.25) (134), while, a recent nested case–control conducted in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, observed a 15% reduction in risk of developing colorectal adenoma (OR=0.85, 95% CI=0.75–0.98, P-trend=0.01) (140). A study by Toriola et al. utilizing repeat assessments of CRP in the Women's Health Initiative Observational Study Cohort among 980 women and controls demonstrated that CRP was associated with an increased risk of CRC, however, that the change in CRP over time was not predictive, thus, suggesting little value as an early detection marker (141).
For LC, the associations appear to be consistent across studies. A meta-analysis of 10 studies involving 1918 LC cases showed a pooled RR of 1.28 (95%CI=1.17–1.41) for LC for one unit change in natural logarithm (ln) CRP (142).
Prognosis
CRP has also been shown to be associated with cancer progression (143) and survival (144, 145). Clinical investigations have shown that CRP levels of patients with pancreatic (PANCC), (146) ESOC, (147) PC (148) and NHL had advanced staging (149), higher disease recurrence (150, 151) and shorter and shorter survival, which was also observed for CRC (152).
A meta-analysis of 10 BC studies that involved 4,502 patients observed significantly decreased overall (HR=1.62, 95%CI=1.20-2.18) and disease-free survival (HR=1.81, 95%CI=1.44-2.26) when CRP levels were elevated. For cancer-specific survival, the pooled HR in higher CRP expression in BC was 2.08 (95%CI=1.48-2.94), which could strongly predict poorer survival in BC (153).
Serum Amyloid A
Background
Serum Amyloid A (SAA) is another acute phase protein similar to CRP. However, circulating SAA levels are thought to be more responsive to inflammation as levels drop off more rapidly following an inflammatory stimulus (154). Unlike CRP, which activates the complement system, to eliminate target cells and induce inflammatory cytokines and tissue factor in monocytes (155, 156), the physiologic effects of SAA are far less understood.
Measurement
SAA is measured in serum, similarly to CRP, using high-sensitivity nephelometry often with micro-latex agglutination tests as the gold standard. Levels can also be detected in saliva using different techniques including fluorescent immunoassays (157).
Cancer Associations
Etiology
SAA has been related to risk at several cancer sites including colon OR=1.5, 95% CI=1.12-2.00 among women (141). Elevated SAA levels have also been highly related to LC risk in the PLCO study as well as gastric cancer in the Japan Public Health Center-based prospective study (158). These analyses have also shown a strong correlation between SAA and CRP, suggesting that measurement of both is essential to control for possible confounded associations and that any independent predictive ability remains to be determined.
Prognosis
SAA is related to stage of disease (159) and strongly associated with reduced long-term survival of BC (160), LC (161) and esophageal squamous cell carcinoma (162). SAA may represent a link between inflammation and metastasis, thereby reducing survival outcomes in CRC (163).
Reactive Oxygen & Nitrogen Species
Background
Reactive oxygen (ROS) and reactive nitrogen species (RNS) are free radicals that are produced as part of the normal metabolic cycle. ROS generation is based on the reduction of molecular oxygen, catalyzed by NAD(P)H oxidases and xanthine oxidase or in a non-enzymatic reaction by redox-reactive compounds of the mitochondrial electron transport chain (164). RNS are produced as by-products of the conversion of arginine to citrulline by nitric oxide synthase (NOS). Both, ROS and RNS are important signaling molecules and involved in metabolism, cell cycle and intercellular signaling cascades, especially in inflammation processes (41), as their formation is stimulated by cytokines and chemokines through activation of protein kinase signaling cascades (165). In a vicious cycle, ROS and RNS recruit additional inflammatory cells, leading to further generation of free radicals. An overproduction of ROS or RNS and limited antioxidative capacities can result in unbalanced metabolism and consequently lead to oxidative or nitrosative stress (166). This is accompanied by damage of DNA, protein, lipids carbohydrates and small metabolites and can be deleterious for cells, tissues and organisms (14, 165, 167). DNA damage through nitrosative deamination of nucleobases or guanosine peroxidation results in 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), the addition of a hydroxyl radical to the c8 position of the guanine ring. This alteration can subsequently lead to single- or double-stranded breaks, deoxynucleotide or deoxyribose modifications and DNA crosslinks (168). These genomic alterations can exert oncogenic effects through altered replication, transcription and translation (169, 170). Oxidation of the guanine base is the most abundant DNA lesion and can be a highly mutagenic miscoding lesion (171). Measurement of oxidatively generated DNA damage products in urine has been shown to be useful for epidemiologic studies to quantify inflammatory exposures (172). 8-oxodG is often referred to as 8-hydroxy-2′deoxyguanosine (8-OHdG), however, for consistency in the review we refer henceforth only to 8-oxodG even for those studies who have used the term 8-OHdG. For a discussion of this nomenclature see Cooke et al (173) who recommend this term as it conforms with the International Union of Pure and Applied Chemistry. Furthermore, this is the more appropriate term as the oxidised nucleobase (8-oxoGua) is a tautomer that at physiological pH is mainly present in the oxo-form and not the hydroxy-form.
ROS also leads to lipid peroxidation (LPO) whose products are genotoxic and mutagenic and can react with protein and DNA (174). Two LPO products generated by ROS which have been investigated in cancer etiology are DNA-reactive aldehyde byproducts trans-4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA). These molecules react with DNA bases to form exocyclic DNA adducts (175, 176). Reaction of DNA bases with these LPO end-products yield five-membered rings (etheno-DNA adducts) attached to DNA including 1,N6-etheno-2′-deoxyadenosine (εdA) and 3,N4-etheno-2′-deoxycytidine (εdC) (177). εdA and εdC appear to be promising tools for quantifying pro-mutagenic DNA damage in early, premalignant stages of the carcinogenesis process (178). These etheno-DNA adducts can be directly quantified in tissues and urine. They have been implicated in clinical studies (179) and may serve as potential risk markers for associations between inflammatory diseases and cancer (180).
A F2-isoprostane isomer, 8-isoprostaglandin F2α (8-Iso-PGF2α), has also been found to be a sensitive and reliable index of in vivo oxidative stress reflective of DNA damage through lipid peroxidation (181).
In addition, ROS play a crucial role in angiogenesis by triggering the release of angiogenic factors such as vascular endothelial growth factor (VEGF). Thus, it is hypothesized that ROS are involved not only in developing cancer but also in cancer progression (13, 182).
Measurement
The measurement of reactive oxygen species presents an interesting and challenging possibility to directly quantify the oxidative burden within tissues. ROS/RNS can be measured either directly in several different tissue types (182-184) or indirectly by measuring the product of ROS/RNS reactions. The main limitation to the direct measurement of ROS/RNS is the extremely short half-life with an estimated lifespan of OH component of < 1ns in blood (185). Consequently, most blood and tissue storage protocols used in observational study designs are not feasible. Measurement of H2O2 can be measured directly in urine as a proxy of whole body oxidative stress (186), however, as dietary factors can also raise urinary H2O2 (187) associations may be confounded. H2O2 can also be measured in exhaled air and breath condensate (188), this however, may not be feasible on a large-scale for population-based studies. Probes such as Dichloro-dihydro-fluorescein diacetate (DCFH-DA) can also be used to detect ‘cellular peroxides’ in cells (189).
Methods have been developed that assess oxidative DNA and protein damage that results from ROS/RNS using tissue-specific measures of protein residues (190). Oxidative DNA damage can be measured by gas chromatography-mass spectrometry (GC-MS), high performance liquid chromatography coupled to electrochemical detection (HPLC-ECD), HPLC--mass spectrometry (MS) as well as immunoassays and enzymatic assays among others (167, 191).
8-oxodG in urine serve as a reliable measures of ‘whole-body’ oxidative stress (192, 193) and can be quantified using HPLC-MS (194-196) or HPLC-ECD (197). 8-oxodG and 8-Iso-PGF2_α can also both be measured in Plasma using commercially available ELISA protocols. Of concern for epidemiologic studies is the poor agreement between ELISA and tandem-mass spectrometric HPLC-MS in methodological comparisons of measurements from urine (198-200).
MDA can be quantified in plasma, urine and tissue using several methods including HPL-ECD, GC-MS, LS-ES-MS/MS (201). MDA quantification by HPLC has shown good inter-laboratory validity in replicate human EDTA treated plasma samples sent to multiple labs (202). HNE can also be reliably detected in plasma and urine using both HPLC (203) methods and ELISAs (204).
Etheno-DNA adducts can be directly quantified in tissues and urine (179). εdA can be quantified in using immunoprecipitation/HPLC/fluorescence detection methods (205) and εdC can be quantified using modified thin layer chromatographic protocols (206). HPLC-MS protocols have also been developed to quantify εdA and εdC from a single DNA sample using purified DNA from cells or tissues (207). A recent population-based application of immunoaffinity/32P-postlabeling (208) successfully quantified εdA and εdC from buffy coat collected in a population-based study (EPIC-Heidelberg) suggesting potential utility in larger population-based investigations as a direct measure of exposure related DNA alterations from oxidative stress (209).
Nitrotyrosine, a byproduct of reactions with nitrogen radicals and reactive nitrogen species can be measured in various tissues. 3-Nitrotyrosine can be assayed in serum or from tissue sample using commercially available ELISA kits, however, commercially available kits have provided low reproducibility and conflicting results (210). It can also be measured using electron spin resonance (211), polychromatic flow cytometry (FACS)(212) and GC-MS (213). These techniques have been limited in their application in population-based studies, however, these biomarkers present as an interesting avenue for inflammation quantification projects.
Cancer Associations
Etiology
Epidemiological studies have shown that serum 8-oxodG levels were significantly increased in patients with CRC compared with controls. A Japanese study suggested that levels of 8-oxodG and fibrosis were significant risk factors for HCC, especially in patients with hepatitis C virus infection (214). Several studies have observed either elevated blood (215), urinary or salivary levels of 8-oxodG in oral cancer compared to controls. For example, an investigation of salivary 8-oxodG levels in oral squamous cell carcinoma patients showed a 65% increase compared with controls (216). Urinary 8-oxodG levels were also significantly higher among breast (217, 218) and colorectal (219) cancer patients than among controls subjects in adjusted analyses. Elevated 8-oxodG levels have also been observed in blood from patients with squamous cell carcinoma of the esophagus (220-222) . Elevated 8-oxodG has been associated with a modestly increased risk of BC (IRR: 1.08; 1.00-1.17 per nmol/mmol creatinine excretion) increase) (223) and LC among never smokers (IRR= 1.17 (1.03–1.31)) (224).
Epidemiologic data examining DNA damage using LPO suggest increased 8-Iso-PGF2α is positively associated with risk of breast cancer (225, 226) and colorectal cancer (227). MDA levels have been associated with lung cancer (228, 229). In a prospective investigation of pre-diagnostic serum levels of reactive oxygen metabolites (ROM), specifically hydroperoxides, in the European Prospective Investigation into Cancer and Nutrition cohort (EPIC) ROM were associated with overall CRC risk when comparing highest tertile vs. lowest (adjusted incidence rate ratio (IRR)=1.91, 95%CI=1.47- 2.48). This association was, however, seen only in subjects with relatively short follow-up, suggesting that the association results from production of ROS by preclinical tumors (230). In a study of oral cavity cancer patients lipid peroxidation products such as lipid hydroperoxide (LHP) and malondialdehyde (MDA) and nitric oxide products like nitrite (NO2 –), nitrate (NO3 –) and total nitrite (TNO2 –) were significantly elevated, whereas enzymatic and non-enzymatic antioxidants were significantly lowered in cancer patients when compared to healthy subjects (231).
Progression
Reactive oxygen species have been much less well studied in regards to disease progression, likely because of the related difficulties in collecting appropriate materials for measurement and the influence of cancer treatment modalities on ROS generation and subsequent bi-products. Expression of nitrotyrosine and inducible nitric oxide synthase has, however, been associated with poor survival in stage III melanoma patients (232).
Prostaglandins, Cyclooxygenases, Lipoxygenases and Related Factors
Background
Prostaglandins (PGs) have a wide range of strong physiological effects and can be found in most tissues and organs (233). PGs constitute a group of lipid compounds that are enzymatically derived from essential fatty acids (EFAs) and have important functions in different cell types (234). EFAs are modified by either of two pathways: the prostaglandin H synthase-cyclooxygenase (COX) pathway or the lipoxygenase (ALOX) pathway. The COX pathway produces thromboxane, prostacyclin and prostaglandins D, E & F. The COX pathway includes two rate-limiting enzymes, COX-1 & COX-2 (235). COX-1 has been traditionally characterized as constitutionally expressed and thus responsible for baseline PGs levels, while COX-2 is more easily inducible, including through IL-6 and peroxides. The ALOX pathway is inactive in leukocytes and synthesizes leukotrienes in macrophages (236). Both of these pathways, their intermediates or end products are involved in the inflammation response, although COX-2 has been given more attention in the investigation of cancer etiology in population-based research (235).
Measurement
The role of disruption in PG synthesis in cancer development can be evaluated at several points in the various pathways using several techniques. The most frequently used methods to measure levels of PGs in a variety of liquid biospecimens are chromatography-based methods, i.e. GC-MS, and antibody-based methods such as ELISAs and RIAs (237, 238). While GC-MS provides high sensitivity and specificity, the method also involves labor-intensive sample preparation and is not suitable for high throughput analysis. In contrast, antibody-based methods enable the measurements of multiple samples simultaneously; however, these assays frequently lack specificity (238). At present, the most precise, informative and reliable method, with a reasonable throughput is LC-MS/MS (239), which was recently optimized for the measurement of PGE2 and PGD2, by incorporating special standards in the samples (240). However, this approach is not high-throughput. COX-2 expression can also be measured by quantitative immunohistochemistry in tissue.
Cancer Associations
Etiology
Direct measurement of urinary PGE metabolites (PGE-M) has been associated with increased cancer risk for BC among postmenopausal women who did not regularly use non-steroidal anti-inflammatory drugs (NSAIDs) HR=2.1 (95% CI: 1.0-4.3); 2.0 (95% CI=1.0-3.9); and 2.2 (95% CI=1.1-4.3) for the second, third, and highest quartiles of PGE-M (241). Increasing quartiles of urinary PGE-M levels were also associated with risk of gastric cancer (statistically significant test for trend (P = 0.04)) (242).
Prognosis
A meta-analysis of 23 studies evaluating COX-2 expression from immunohistochemistry suggested that COX-2 over-expression in tumor tissues had an unfavorable impact on overall survival (OS) in CRC patients (HR=1.19, 95% CI=1.02-1.37)(243). COX-2 correlates with poor prognostic markers in BC (large tumor size and high tumor grade), but not with outcome (244) and with reduced survival in cervical and OC (245-247). In an investigation of COX-2 expression in bladder cancer, a weak association with recurrence in non-muscle invasive bladder tumors was observed (p-value = 0.048). In the multivariable analyses, COX2 expression did not independently predict any of the considered outcomes (248).
Transcription Factors and Growth Factors as Mediators of an Inflammation and Cancer Association
Background
Several substances created by the cellular mediators of inflammation propagate the inflammation response and their actions elicit a cellular growth response. These growth factors/transcription factors are proteins that bind to cellular and nuclear receptors to elicit a downstream response. Nuclear factor-kappaB (NF-κB) one such transcription factor has been suggested to play a strong molecular role linking inflammation and cancer development (249, 250). NF-κB is activated downstream through 1) the toll-like receptor (TLR)-MYD88 pathway responsible for sensing microbes and tissue damage, as well as the inflammatory cytokines TNF-α and IL-1B (251). NF-κB activation can also be the result of cell-autonomous genetic alterations (252). NF-κB functions in inflammatory pathways by inducing the expression of inflammatory cytokines, adhesion molecules, cyclooxygenases, NO synthase and angiogenic factors, all propagating an exacerbated inflammation response (253). It also promotes tumor survival by inducing anti-apoptotic genes (BCL2) (254). A lack of checkpoint for growth factors such as NF-κB activation leads to increased proinflammatory cytokine and chemokine secretion as well prostaglandin release downstream of NF-κB signaling which were shown to promote neoplasia (255).
Another transcription factor also believed to play a pivotal role in linking inflammation and cytokines to cancer development and progression is STAT3. Most inflammatory signals affect tumorigenesis by activating STAT3 in a similar method to those described for NF-κB (256, 257). Persistent STAT3 activation in malignant cells stimulates proliferation, survival, angiogenesis, invasion and tumor-promoting inflammation (258, 259).
Measurement
Transcription factors such as NF-κB activity can be measured on various levels including: 1) quantity in fluids 2) levels of activation and 3) translocation. Quantification can be completed using ELISA and other high sensitivity assays; however, stability in blood samples is an issue. Levels of NF-κB activation in stimulated normal peripheral blood lymphocytes can be completed using a real-time PCR to measure of Iκβα mRNA levels as a rapid, sensitive, and powerful method to quantify the transcriptional power of NF-κB. It can be used for clinical evaluation of NF-κB status, but requires cell culture and is thus not easily adaptable in epidemiologic studies (260). NF-κB translocation to the nucleus, where it regulates cytokine and immunoglobulin expression, can be measured by both confocal microscopy and flow cytometry (261).
Cancer Associations
In comparison to the other markers discussed in this review, comparatively fewer studies directly quantifying cancer risk and prognosis related to changes in NF-κB and STAT3 quantity in fluids, levels of activation and translocation have been completed, perhaps due to the difficulty of appropriate biospecimen collection. Several investigations have examined polymorphisms in NF-κB genes in the development of OC (262) and CRC (263, 264). Examination of NF-κB activation has suggested an association with a high-risk subset of hormone-dependent BC (263) with increased expansion of cancer stem cells in basal-like BCs (265). Results also suggest that NF-κB activation maybe be predictive of response to treatment (266) and survival (267) in CRC. Proteasome inhibitors used for treatment of various cancers including multiple myeloma and NHL (268) elicit their effects partially reducing NF-κB activity (269).
DISCUSSION
The Potential for Prevention and Therapeutic Intervention
Prevention
Several of the biomarkers discussed in this review presently have the potential to be used for cancer prevention. From a primary and tertiary prevention perspective, the use of non-steroidal anti-inflammatory drugs has been related to reduced cancer risk at several sites including BC, CRC, OC, GC and LC (270-276) and improved disease outcomes (277). These data suggest that blocking inflammatory pathways, in this case the prostaglandin-related and subsequent downstream pathways (278) can prevent the development of cancer at the population level. Evaluations of whether intervention at different points across the inflammation spectrum can prevent cancer are an interesting area of developing research (279-281) that could yield great impact in the prevention of cancer.
From a secondary prevention perspective, several research groups have and continue to evaluate the utility of inflammatory biomarkers in the development of risk prediction models. For example, Pine et al. observed that 10-year predicted risk for LC was highest among those smokers with elevated CRP and IL-8 in the PLCO study (54). Extensions of these methods and models with other types of inflammatory markers and other cancer sites may help to identify those at greatest risk for developing cancer, and therefore refine the population that would most benefit from increased screening based on their inflammatory profiles.
Therapeutic intervention
Several developing avenues of immune based therapies including tumor vaccine approaches, immune-checkpoint inhibitors and antagonists of immunosuppressive molecules such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) among others which seem promising in early stages of development and trials (282). This topic is beyond the scope of this review, but it is a rapidly emerging area in cancer therapeutics and early results appear quite promising with cancer immunotherapy heralded as the scientific breakthrough of 2013 (283).
The Need for Integrative Targeted Approaches to Examine Inflammation in Cancer
As the inflammation response and its role in carcinogenesis is vastly complex in nature, novel approaches to characterize the roles of inflammatory markers in cancer development and progression to identify elevated risk profiles and subsequent intervention targets are needed. An approach limited to single markers will yield ineffective and fragmented results suboptimal for the reduction of cancer burden. Three overarching principles should be the goal of future research in this area.
First, investigations should aim to be as comprehensive as possible in order to examine multiple exposures as well as their interactions (Figure 1). For example, assessing a comprehensive set of biomarkers will provide a better picture and will enable more-pathway-based analyses. In addition, evaluating germ-line variation, epigenetic modification, expression and protein product levels in a comprehensive pathway-based analytic approach would provide a more resolute image of the relevant associations. This will, however, require substantial funding and access to a diverse set of biospecimens.
Second, the use of existing data platforms, such as large prospective studies and bio-repositories should be targeted for the evaluation of these integrative hypotheses to be able to better address the issue of causality. This approach will require focusing on analytes that are less sensitive to degradation over time. Integration with lifestyle factors in these data platform would be also important as several of them (per Introduction) are correlated with the inflammatory markers of interest. Mediation analyses or structural equation modeling/path analyses may be necessary to adequately unravel the complex associations of interest.
Lastly, etiologic and prognostic associations should be evaluated across cancer sites where possible. As discussed, imbalances in the markers of altered inflammation have been associated differently with cancers at multiple sites, yet it is clear that inflammatory imbalances play a role to some degree across the majority of solid tumor sites. Comprehensively evaluating associations similarly across cancer sites where sample availability permits, for example in a large cohort setting, dramatically increases the potential benefit of identifying chemopreventive or therapeutic targets. Several initiatives are underway to advance this cross-cancer inflammation hypothesis, yet more research is needed.
Acknowledgments
The scientific development and funding of this project were, in part supported by the Genetic Associations and Mechanisms in Oncology (GAME-ON), a NCI Cancer Post-GWAS Initiative, and U19 CA148127 (Principal Investigator: Amos). L. Le Marchand is supported by NIH funding: National Cancer Institute (R01 CA129063; Principal Investigator: L. Le Marchand). A.T. Chan is supported by NIH funding: R01 CA137178 (Principal Investigator: A.T. Chan) and K24 DK098311 (Principal Investigator: A.T. Chan). E.L. Goode is supported by NIH funding: R01-CA-122443 (Principal Investigator: E.L. Goode) and P50-CA-136393 (Principal Investigator: E.L. Goode). R.J. Hung is supported by Canadian Cancer Society Research Institute (no. 020214, Principal Investigator: Hung)
Cancer Site Abbreviations
- LC
Lung Cancer
- CRC
Colorectal Cancer
- BC
Breast Cancer
- PC
Prostate Cancer
- EC
Endometrial Cancer
- NHL
Non-Hodgkin's Lymphoma
- RCC
Renal Cell Carcinoma
- OC
Ovarian Cancer
- CLL
Chronic Lymphocytic Leukemia
- ESOC
Esophageal Cancer
- HCC
Hepatocellular Carcinoma
- BLC
Bladder Cancer
- PANCC
Pancreatic Cancer
- GC
Gastric Cancer
Footnotes
Conflict of Interest: The authors have none to declare.
References
- 1.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int Immunopharmacol. 2001;1:1651–67. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Pierotti MA. Cancer and inflammation: a complex relationship. Cancer Lett. 2008;267:180–1. doi: 10.1016/j.canlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Peek RM, Jr., Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a national cancer institute-sponsored meeting. Cancer Res. 2005;65:8583–6. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 9.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 12.Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–63. [PubMed] [Google Scholar]
- 13.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 14.O'Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–83. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontos M, Fentiman IS. Systemic lupus erythematosus and breast cancer. The breast journal. 2008;14:81–6. doi: 10.1111/j.1524-4741.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 16.Parikh-Patel A, White RH, Allen M, Cress R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer causes & control : CCC. 2008;19:887–94. doi: 10.1007/s10552-008-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh-Patel A, White RH, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer causes & control : CCC. 2009;20:1001–10. doi: 10.1007/s10552-009-9298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. European journal of cancer. 1996;32A:1753–7. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 19.Brenner DR, McLaughlin JR, Hung RJ. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS One. 2011 doi: 10.1371/journal.pone.0017479. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffetta P. Involuntary smoking and lung cancer. Scand J Work Environ Health. 2002;28(Suppl 2):30–40. [PubMed] [Google Scholar]
- 21.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–45. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 22.Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120:1577–83. doi: 10.1378/chest.120.5.1577. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 24.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 25.Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther Adv Respir Dis. 2007;1:5–23. doi: 10.1177/1753465807082373. [DOI] [PubMed] [Google Scholar]
- 26.Prieto L. [Induced sputum as a method for the study of bronchial inflammation]. Arch Bronconeumol. 2011;47:323–4. doi: 10.1016/j.arbres.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Aaron SD, Vandemheen KL, Ramsay T, Zhang C, Avnur Z, Nikolcheva T, et al. Multi analyte profiling and variability of inflammatory markers in blood and induced sputum in patients with stable COPD. Respir Res. 2010;11:41. doi: 10.1186/1465-9921-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bargagli E, Mazzi A, Rottoli P. Markers of inflammation in sarcoidosis: blood, urine, BAL, sputum, and exhaled gas. Clin Chest Med. 2008;29:445–58. viii. doi: 10.1016/j.ccm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755–62. doi: 10.1111/j.1572-0241.2002.05837.x. [DOI] [PubMed] [Google Scholar]
- 30.Pitrez PM, Brennan S, Turner S, Sly PD. Nasal wash as an alternative to bronchoalveolar lavage in detecting early pulmonary inflammation in children with cystic fibrosis. Respirology. 2005;10:177–82. doi: 10.1111/j.1440-1843.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 31.Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect. 1997;105(Suppl 4):875–82. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 33.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 34.Courneya KS, Friedenreich CM. Physical Activity and Cancer. Springer-Verlag; Berlin: 2011. [Google Scholar]
- 35.Ulrich C, Steindorf K, Berger NA. Exercise, Energy Balance, and Cancer. Spinger; New York, NY: 2013. [Google Scholar]
- 36.Brenner DR, Boffetta P, Duell EJ, Bickeboller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176:573–85. doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirk GD, Merlo C, P OD, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–10. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 39.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 40.Rankin JA. Biological mediators of acute inflammation. AACN Clin Issues. 2004;15:3–17. doi: 10.1097/00044067-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–7. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 42.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 43.Mak TW, Saunders ME. In: The Immune Response. Basic and Clinical Principles. Press EA, editor. San Diego: 2006. pp. 464–516. [Google Scholar]
- 44.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 45.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13:541–7. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brower V. Researchers attempting to define role of cytokines in cancer risk. Journal of the National Cancer Institute. 2005;97:1175–7. doi: 10.1093/jnci/dji269. [DOI] [PubMed] [Google Scholar]
- 48.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. Journal of immunological methods. 1992;153:125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 49.Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev. 2008;17:3450–6. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- 50.Bienvenu JAD, Monneret G, Gutowski MC, Fabien N. Cytokine assays in human sera and tissues. Toxicology. 1998;129:55–61. doi: 10.1016/s0300-483x(98)00063-8. [DOI] [PubMed] [Google Scholar]
- 51.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 52.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan KE, Cutilli J, Piliero LM, Ghavimi-Alagha D, Starr SE, Campbell DE, et al. Measurement of cytokine secretion, intracellular protein expression, and mRNA in resting and stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2000;7:920–4. doi: 10.1128/cdli.7.6.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. Journal of the National Cancer Institute. 2011;103:1112–22. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–77. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–9. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Yao Y, Gong C, Yu F, Su S, Liu B, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. British journal of cancer. 2004;90:2312–6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Street ME, Miraki-Moud F, Sanderson IR, Savage MO, Giovannelli G, Bernasconi S, et al. Interleukin-1beta (IL-1beta) and IL-6 modulate insulin-like growth factor-binding protein (IGFBP) secretion in colon cancer epithelial (Caco-2) cells. J Endocrinol. 2003;179:405–15. doi: 10.1677/joe.0.1790405. [DOI] [PubMed] [Google Scholar]
- 62.Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 63.Conroy SM, Maskarinec G, Morimoto Y, Franke AA, Cooney RV, Wilkens LR, et al. Non-Hodgkin Lymphoma and Circulating Markers of Inflammation and Adiposity in a Nested Case-Control Study: The Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-12-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purdue MP, Lan Q, Bagni R, Hocking WG, Baris D, Reding DJ, et al. Prediagnostic serum levels of cytokines and other immune markers and risk of non-hodgkin lymphoma. Cancer Res. 2011;71:4898–907. doi: 10.1158/0008-5472.CAN-11-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–6. [PubMed] [Google Scholar]
- 66.Ljungberg B, Grankvist K, Rasmuson T. Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. Eur J Cancer. 1997;33:1794–8. doi: 10.1016/s0959-8049(97)00179-2. [DOI] [PubMed] [Google Scholar]
- 67.Wojciechowska-Lacka A, Adamiak E, Stryczynska G, Lacki JK. Prognostic value of serial serum interleukin-6 level estimation in patients with lung cancer: a preliminary report. Yale J Biol Med. 1997;70:139–48. [PMC free article] [PubMed] [Google Scholar]
- 68.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol. 1997;66:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 69.Fayad L, Cabanillas F, Talpaz M, McLaughlin P, Kurzrock R. High serum interleukin-6 levels correlate with a shorter failure-free survival in indolent lymphoma. Leuk Lymphoma. 1998;30:563–71. doi: 10.3109/10428199809057568. [DOI] [PubMed] [Google Scholar]
- 70.Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, Kurzrock R. Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med. 1997;127:186–94. doi: 10.7326/0003-4819-127-3-199708010-00002. [DOI] [PubMed] [Google Scholar]
- 71.Lai R, O'Brien S, Maushouri T, Rogers A, Kantarjian H, Keating M, et al. Prognostic value of plasma interleukin-6 levels in patients with chronic lymphocytic leukemia. Cancer. 2002;95:1071–5. doi: 10.1002/cncr.10772. [DOI] [PubMed] [Google Scholar]
- 72.De Vita F, Romano C, Orditura M, Galizia G, Martinelli E, Lieto E, et al. Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J Interferon Cytokine Res. 2001;21:45–52. doi: 10.1089/107999001459150. [DOI] [PubMed] [Google Scholar]
- 73.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 74.Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, et al. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2009;18:215–22. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 76.Wu J, Du J, Liu L, Li Q, Rong W, Wang L, et al. Elevated pretherapy serum IL17 in primary hepatocellular carcinoma patients correlate to increased risk of early recurrence after curative hepatectomy. PLoS One. 2012;7:e50035. doi: 10.1371/journal.pone.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Valk P, Herman CJ. Leukocyte functions. Lab Invest. 1987;56:127–37. [PubMed] [Google Scholar]
- 78.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. British journal of cancer. 2006;94:227–30. doi: 10.1038/sj.bjc.6602922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 81.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 82.Holmes EC. Immunology of tumor infiltrating lymphocytes. Ann Surg. 1985;201:158–63. doi: 10.1097/00000658-198502000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 85.Locksley RM. The Roaring Twenties. Immunity. 2008;28:437–9. doi: 10.1016/j.immuni.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 88.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohm I. Quantification of absolute peripheral white blood cells and their subsets in patients with lupus erythematosus: comparison with other inflammatory diseases with and without autoimmune background. Biomed Pharmacother. 2006;60:92–5. doi: 10.1016/j.biopha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 90.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gratama JW, Kraan J, Van den Beemd R, Hooibrink B, Van Bockstaele DR, Hooijkaas H. Analysis of variation in results of flow cytometric lymphocyte immunophenotyping in a multicenter study. Cytometry. 1997;30:166–77. doi: 10.1002/(sici)1097-0320(19970815)30:4<166::aid-cyto2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 92.Parish CR, Glidden MH, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0409s84. Chapter 4:Unit4 9. [DOI] [PubMed] [Google Scholar]
- 93.Gratama JW, Kraan J, Adriaansen H, Hooibrink B, Levering W, Reinders P, et al. Reduction of interlaboratory variability in flow cytometric immunophenotyping by standardization of instrument set-up and calibration, and standard list mode data analysis. Cytometry. 1997;30:10–22. [PubMed] [Google Scholar]
- 94.Maecker HT, McCoy JP, Jr., Amos M, Elliott J, Gaigalas A, Wang L, et al. A model for harmonizing flow cytometry in clinical trials. Nat Immunol. 2010;11:975–8. doi: 10.1038/ni1110-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maecker HT, Rinfret A, D'Souza P, Darden J, Roig E, Landry C, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–8. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loughlin PM, Cooke TG, George WD, Gray AJ, Stott DI, Going JJ. Quantifying tumour-infiltrating lymphocyte subsets: a practical immuno-histochemical method. Journal of immunological methods. 2007;321:32–40. doi: 10.1016/j.jim.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 98.Steinkamp JA, Habbersett RC, Stewart CC. A modular detector for flow cytometric multicolor fluorescence measurements. Cytometry. 1987;8:353–65. doi: 10.1002/cyto.990080403. [DOI] [PubMed] [Google Scholar]
- 99.Aust S, Bachmayr-Heyda A, Pils D, Zhao L, Tong W, Berger A, et al. Determination of tumor-infiltrating CD8+ lymphocytes in human ovarian cancer. Int J Gynecol Pathol. 2013;32:269–76. doi: 10.1097/PGP.0b013e31826a63f8. [DOI] [PubMed] [Google Scholar]
- 100.Yamada Y, Saito H, Ikeguchi M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J Surg Res. 2012;178:685–91. doi: 10.1016/j.jss.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 101.Li H, Bradbury JA, Dackor RT, Edin ML, Graves JP, DeGraff LM, et al. Cyclooxygenase-2 regulates Th17 cell differentiation during allergic lung inflammation. Am J Respir Crit Care Med. 2011;184:37–49. doi: 10.1164/rccm.201010-1637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shete A, Thakar M, Abraham PR, Paranjape R. A review on peripheral blood CD4+ T lymphocyte counts in healthy adult Indians. Indian J Med Res. 2010;132:667–75. [PMC free article] [PubMed] [Google Scholar]
- 103.Margolis KL, Rodabough RJ, Thomson CA, Lopez AM, McTiernan A. Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch Intern Med. 2007;167:1837–44. doi: 10.1001/archinte.167.17.1837. [DOI] [PubMed] [Google Scholar]
- 104.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 105.Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.D'Andrilli A, Natoli G, Scarpino S, Rendina EA. Stage I non-small cell lung cancer: the presence of the lymphocyte-specific protein tyrosin kinase in the tumour infiltrate is associated with a better long-term prognosis. Interact Cardiovasc Thorac Surg. 2012;15:148–51. doi: 10.1093/icvts/ivr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–10. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 108.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 109.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–7. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 111.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58:1107–16. doi: 10.1111/j.1365-2559.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- 113.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, et al. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–6. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 114.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–54. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 116.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185:4063–71. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–9. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–9. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. 1999;17:1019–25. doi: 10.1016/s0736-4679(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 120.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 121.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–44. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 122.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 123.Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 124.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 125.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control. 2007;18:399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 126.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 127.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–8. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McKeown DJ, Brown DJ, Kelly A, Wallace AM, McMillan DC. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. British journal of cancer. 2004;91:1993–5. doi: 10.1038/sj.bjc.6602248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khuseyinova N, Imhof A, Trischler G, Rothenbacher D, Hutchinson WL, Pepys MB, et al. Determination of C-reactive protein: comparison of three high-sensitivity immunoassays. Clin Chem. 2003;49:1691–5. doi: 10.1373/49.10.1691. [DOI] [PubMed] [Google Scholar]
- 130.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–18. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 132.Platz EA, Sutcliffe S, De Marzo AM, Drake CG, Rifai N, Hsing AW, et al. Intra-individual variation in serum C-reactive protein over 4 years: an implication for epidemiologic studies. Cancer Causes Control. 2010;21:847–51. doi: 10.1007/s10552-010-9511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 134.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. International journal of cancer Journal international du cancer. 2008;123:1133–40. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- 135.Chaturvedi AK, Caporaso NE, Katki HA, Wong HL, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–26. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15:381–4. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 137.Zhang SM, Lin J, Cook NR, Lee IM, Manson JE, Buring JE, et al. C-reactive protein and risk of breast cancer. J Natl Cancer Inst. 2007;99:890–4. doi: 10.1093/jnci/djk202. [DOI] [PubMed] [Google Scholar]
- 138.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–24. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 139.McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. C-reactive protein concentrations and subsequent ovarian cancer risk. Obstet Gynecol. 2007;109:933–41. doi: 10.1097/01.AOG.0000257126.68803.03. [DOI] [PubMed] [Google Scholar]
- 140.Gunter MJ, Cross AJ, Huang WY, Stanczyk FZ, Purdue M, Xue X, et al. A prospective evaluation of C-reactive protein levels and colorectal adenoma development. Cancer Epidemiol Biomarkers Prev. 2011;20:537–44. doi: 10.1158/1055-9965.EPI-10-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toriola AT, Cheng TY, Neuhouser ML, Wener MH, Zheng Y, Brown E, et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int J Cancer. 2013;132:2648–58. doi: 10.1002/ijc.27942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou B, Liu J, Wang ZM, Xi T. C-reactive protein, interleukin 6 and lung cancer risk: a meta-analysis. PLoS One. 2012;7:e43075. doi: 10.1371/journal.pone.0043075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee JG, Cho BC, Bae MK, Lee CY, Park IK, Kim DJ, et al. Preoperative C-reactive protein levels are associated with tumor size and lymphovascular invasion in resected non-small cell lung cancer. Lung Cancer. 2009;63:106–10. doi: 10.1016/j.lungcan.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 144.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O'Dowd C, McRae LA, McMillan DC, Kirk A, Milroy R. Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010;5:988–92. doi: 10.1097/JTO.0b013e3181da78f9. [DOI] [PubMed] [Google Scholar]
- 146.Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–82. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 147.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. doi: 10.1016/s0002-9610(01)00684-5. [DOI] [PubMed] [Google Scholar]
- 148.Trautner K, Cooper EH, Haworth S, Ward AM. An evaluation of serum protein profiles in the long-term surveillance of prostatic cancer. Scand J Urol Nephrol. 1980;14:143–9. doi: 10.3109/00365598009179552. [DOI] [PubMed] [Google Scholar]
- 149.Stamatiadis AP, Manouras AJ, Triantos GN, Katergiannakis VA, Apostolidis NS. Combination of serum carcino-embryonic antigen and C-reactive protein--a useful test in preoperative staging of colorectal cancer. Eur J Surg Oncol. 1992;18:41–3. [PubMed] [Google Scholar]
- 150.Wigmore SJ, McMahon AJ, Sturgeon CM, Fearon KC. Acute-phase protein response, survival and tumour recurrence in patients with colorectal cancer. Br J Surg. 2001;88:255–60. doi: 10.1046/j.1365-2168.2001.01669.x. [DOI] [PubMed] [Google Scholar]
- 151.McMillan DC, Wotherspoon HA, Fearon KC, Sturgeon C, Cooke TG, McArdle CS. A prospective study of tumor recurrence and the acute-phase response after apparently curative colorectal cancer surgery. Am J Surg. 1995;170:319–22. doi: 10.1016/s0002-9610(99)80296-7. [DOI] [PubMed] [Google Scholar]
- 152.Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998;176:335–8. doi: 10.1016/s0002-9610(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 153.Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S, et al. Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2011;26:209–15. doi: 10.5301/JBM.2011.8872. [DOI] [PubMed] [Google Scholar]
- 154.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 155.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–36. [PubMed] [Google Scholar]
- 156.Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–8. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 157.Soler L, Gutierrez A, Martinez-Subiela S, Ceron JJ. Fast measurement of serum amyloid A in different specimens from swine by using a new one-step time-resolved fluorescent immunoassay. J Vet Diagn Invest. 2011;23:902–8. doi: 10.1177/1040638711416623. [DOI] [PubMed] [Google Scholar]
- 158.Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, et al. Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis. 2010;31:712–8. doi: 10.1093/carcin/bgq010. [DOI] [PubMed] [Google Scholar]
- 159.Zhang G, Sun X, Lv H, Yang X, Kang X. Serum amyloid A: A new potential serum marker correlated with the stage of breast cancer. Oncology letters. 2012;3:940–4. doi: 10.3892/ol.2012.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–67. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cho WC, Yip TT, Cheng WW, Au JS. Serum amyloid A is elevated in the serum of lung cancer patients with poor prognosis. Br J Cancer. 2010;102:1731–5. doi: 10.1038/sj.bjc.6605700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang JY, Zheng YZ, Yang J, Lin YH, Dai SQ, Zhang G, et al. Elevated levels of serum amyloid A indicate poor prognosis in patients with esophageal squamous cell carcinoma. BMC Cancer. 2012;12:365. doi: 10.1186/1471-2407-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hansen MT, Forst B, Cremers N, Quagliata L, Ambartsumian N, Grum-Schwensen B, et al. A link between inflammation and metastasis: serum amyloid A1 and A3 induce metastasis, and are targets of metastasis-inducing S100A4. Oncogene. 2014 doi: 10.1038/onc.2013.568. [DOI] [PubMed] [Google Scholar]
- 164.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–41. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 165.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic Biol Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 169.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Berquist BR, Wilson DM., 3rd Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012;327:61–72. doi: 10.1016/j.canlet.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992;267:166–72. [PubMed] [Google Scholar]
- 172.Sauvain JJ, Setyan A, Wild P, Tacchini P, Lagger G, Storti F, et al. Biomarkers of oxidative stress and its association with the urinary reducing capacity in bus maintenance workers. J Occup Med Toxicol. 2011;6:18. doi: 10.1186/1745-6673-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cooke MS, Loft S, Olinski R, Evans MD, Bialkowski K, Wagner JR, et al. Recommendations for standardized description of and nomenclature concerning oxidatively damaged nucleobases in DNA. Chemical research in toxicology. 2010;23:705–7. doi: 10.1021/tx1000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 175.el Ghissassi F, Barbin A, Nair J, Bartsch H. Formation of 1,N6-ethenoadenine and 3,N4-ethenocytosine by lipid peroxidation products and nucleic acid bases. Chem Res Toxicol. 1995;8:278–83. doi: 10.1021/tx00044a013. [DOI] [PubMed] [Google Scholar]
- 176.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–20. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 177.Marnett LJ. Inflammation and cancer: chemical approaches to mechanisms, imaging, and treatment. J Org Chem. 2012;77:5224–38. doi: 10.1021/jo300214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:8598–602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat Res. 2005;591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 180.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 181.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–94. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 183.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jablonska E, Kiersnowska-Rogowska B, Ratajczak W, Rogowski F, Sawicka-Powierza J. Reactive oxygen and nitrogen species in the course of B-CLL. Adv Med Sci. 2007;52:154–8. [PubMed] [Google Scholar]
- 185.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 186.Yuen JW, Benzie IF. Hydrogen peroxide in urine as a potential biomarker of whole body oxidative stress. Free Radic Res. 2003;37:1209–13. doi: 10.1080/10715760310001616032. [DOI] [PubMed] [Google Scholar]
- 187.Halliwell B, Long LH, Yee TP, Lim S, Kelly R. Establishing biomarkers of oxidative stress: the measurement of hydrogen peroxide in human urine. Curr Med Chem. 2004;11:1085–92. doi: 10.2174/0929867043365404. [DOI] [PubMed] [Google Scholar]
- 188.Rahman I, Kelly F. Biomarkers in breath condensate: a promising new non-invasive technique in free radical research. Free Radic Res. 2003;37:1253–66. doi: 10.1080/10715760310001623331. [DOI] [PubMed] [Google Scholar]
- 189.Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–7. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- 190.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–26. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 191.Cadet J, Poulsen H. Measurement of oxidatively generated base damage in cellular DNA and urine. Free Radic Biol Med. 2010;48:1457–9. doi: 10.1016/j.freeradbiomed.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 192.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 193.Loft S, Danielsen P, Lohr M, Jantzen K, Hemmingsen JG, Roursgaard M, et al. Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch Biochem Biophys. 2012;518:142–50. doi: 10.1016/j.abb.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 194.Kasai H. Chemistry-based studies on oxidative DNA damage: formation, repair, and mutagenesis. Free Radic Biol Med. 2002;33:450–6. [PubMed] [Google Scholar]
- 195.Irie M, Asami S, Nagata S, Miyata M, Kasai H. Psychological mediation of a type of oxidative DNA damage, 8-hydroxydeoxyguanosine, in peripheral blood leukocytes of non-smoking and non-drinking workers. Psychotherapy and psychosomatics. 2002;71:90–6. doi: 10.1159/000049351. [DOI] [PubMed] [Google Scholar]
- 196.Lee KF, Chung WY, Benzie IF. Urine 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), a specific marker of oxidative stress, using direct, isocratic LC-MS/MS: Method evaluation and application in study of biological variation in healthy adults. Clin Chim Acta. 2010;411:416–22. doi: 10.1016/j.cca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 197.Inaba Y, Koide S, Yokoyama K, Karube I. Development of urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) measurement method combined with SPE. J Chromatogr Sci. 2011;49:303–9. doi: 10.1093/chrsci/49.4.303. [DOI] [PubMed] [Google Scholar]
- 198.Smith KA, Shepherd J, Wakil A, Kilpatrick ES. A comparison of methods for the measurement of 8-isoPGF(2alpha): a marker of oxidative stress. Ann Clin Biochem. 2011;48:147–54. doi: 10.1258/acb.2010.010151. [DOI] [PubMed] [Google Scholar]
- 199.Cooke MS, Olinski R, Loft S. Measurement and meaning of oxidatively modified DNA lesions in urine. Cancer Epidemiol Biomarkers Prev. 2008;17:3–14. doi: 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- 200.Barregard L, Moller P, Henriksen T, Mistry V, Koppen G, Rossner P, Jr., et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine. Antioxid Redox Signal. 2013;18:2377–91. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bartsch H, Nair J. Ultrasensitive and specific detection methods for exocylic DNA adducts: markers for lipid peroxidation and oxidative stress. Toxicology. 2000;153:105–14. doi: 10.1016/s0300-483x(00)00307-3. [DOI] [PubMed] [Google Scholar]
- 202.Breusing N, Grune T, Andrisic L, Atalay M, Bartosz G, Biasi F, et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic Res. 2010;44:1203–15. doi: 10.3109/10715762.2010.499907. [DOI] [PubMed] [Google Scholar]
- 203.Lang J, Celotto C, Esterbauer H. Quantitative determination of the lipid peroxidation product 4-hydroxynonenal by high-performance liquid chromatography. Anal Biochem. 1985;150:369–78. doi: 10.1016/0003-2697(85)90525-1. [DOI] [PubMed] [Google Scholar]
- 204.Borovic S, Tirzitis G, Tirzite D, Cipak A, Khoschsorur GA, Waeg G, et al. Bioactive 1,4-dihydroisonicotinic acid derivatives prevent oxidative damage of liver cells. Eur J Pharmacol. 2006;537:12–9. doi: 10.1016/j.ejphar.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 205.Hanaoka T, Nair J, Takahashi Y, Sasaki S, Bartsch H, Tsugane S. Urinary level of 1,N(6) -ethenodeoxyadenosine, a marker of oxidative stress, is associated with salt excretion and omega 6-polyunsaturated fatty acid intake in postmenopausal Japanese women. Int J Cancer. 2002;100:71–5. doi: 10.1002/ijc.10437. [DOI] [PubMed] [Google Scholar]
- 206.Sun X, Karlsson A, Bartsch H, Nair J. New ultrasensitive 32P-postlabelling method for the analysis of 3,N4-etheno-2'-deoxycytidine in human urine. Biomarkers. 2006;11:329–40. doi: 10.1080/13547500600709606. [DOI] [PubMed] [Google Scholar]
- 207.Taghizadeh K, McFaline JL, Pang B, Sullivan M, Dong M, Plummer E, et al. Quantification of DNA damage products resulting from deamination, oxidation and reaction with products of lipid peroxidation by liquid chromatography isotope dilution tandem mass spectrometry. Nat Protoc. 2008;3:1287–98. doi: 10.1038/nprot.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nair J, Barbin A, Guichard Y, Bartsch H. 1,N6-ethenodeoxyadenosine and 3,N4-ethenodeoxycytine in liver DNA from humans and untreated rodents detected by immunoaffinity/32P-postlabeling. Carcinogenesis. 1995;16:613–7. doi: 10.1093/carcin/16.3.613. [DOI] [PubMed] [Google Scholar]
- 209.Sun X, Nair J, Linseisen J, Owen RW, Bartsch H. Lipid peroxidation and DNA adduct formation in lymphocytes of premenopausal women: Role of estrogen metabolites and fatty acid intake. Int J Cancer. 2012;131:1983–90. doi: 10.1002/ijc.27479. [DOI] [PubMed] [Google Scholar]
- 210.Tsikas D. Measurement of nitrotyrosine in plasma by immunoassays is fraught with danger: commercial availability is no guarantee of analytical reliability. Clin Chem Lab Med. 2010;48:141–3. doi: 10.1515/CCLM.2010.015. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- 211.Utsumi H, Yamada K. In vivo electron spin resonance-computed tomography/nitroxyl probe technique for non-invasive analysis of oxidative injuries. Arch Biochem Biophys. 2003;416:1–8. doi: 10.1016/s0003-9861(03)00285-6. [DOI] [PubMed] [Google Scholar]
- 212.Frost MT, Halliwell B, Moore KP. Analysis of free and protein-bound nitrotyrosine in human plasma by a gas chromatography/mass spectrometry method that avoids nitration artifacts. Biochem J. 2000;345(Pt 3):453–8. [PMC free article] [PubMed] [Google Scholar]
- 213.Tsikas D, Mitschke A, Gutzki FM. Measurement of 3-nitro-tyrosine in human plasma and urine by gas chromatography-tandem mass spectrometry. Methods Mol Biol. 2012;828:255–70. doi: 10.1007/978-1-61779-445-2_20. [DOI] [PubMed] [Google Scholar]
- 214.Chuma M, Hige S, Nakanishi M, Ogawa K, Natsuizaka M, Yamamoto Y, et al. 8-Hydroxy-2'-deoxy-guanosine is a risk factor for development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2008;23:1431–6. doi: 10.1111/j.1440-1746.2008.05502.x. [DOI] [PubMed] [Google Scholar]
- 215.Kumar A, Pant MC, Singh HS, Khandelwal S. Assessment of the redox profile and oxidative DNA damage (8-OHdG) in squamous cell carcinoma of head and neck. J Cancer Res Ther. 2012;8:254–9. doi: 10.4103/0973-1482.98980. [DOI] [PubMed] [Google Scholar]
- 216.Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer. 2007;109:54–9. doi: 10.1002/cncr.22386. [DOI] [PubMed] [Google Scholar]
- 217.Kuo HW, Chou SY, Hu TW, Wu FY, Chen DJ. Urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) and genetic polymorphisms in breast cancer patients. Mutat Res. 2007;631:62–8. doi: 10.1016/j.mrgentox.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 218.Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209–14. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 219.Chang D, Wang F, Zhao YS, Pan HZ. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci. 2008;21:286–9. doi: 10.1016/S0895-3988(08)60043-4. [DOI] [PubMed] [Google Scholar]
- 220.Khadem-Ansari MH, Shahsavari Z, Rasmi Y, Mahmoodlo R. Elevated levels of urinary 8-hydroxy-2'-deoxyguanosine and 8-isoprostane in esophageal squamous cell carcinoma. J Carcinog. 2011;10:14. doi: 10.4103/1477-3163.79683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Diakowska D, Lewandowski A, Kopec W, Diakowski W, Chrzanowska T. Oxidative DNA damage and total antioxidant status in serum of patients with esophageal squamous cell carcinoma. Hepatogastroenterology. 2007;54:1701–4. [PubMed] [Google Scholar]
- 222.Rasanen JV, Sihvo EI, Ahotupa MO, Farkkila MA, Salo JA. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumours. Eur J Surg Oncol. 2007;33:1164–8. doi: 10.1016/j.ejso.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 223.Loft S, Olsen A, Moller P, Poulsen HE, Tjonneland A. Association between 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiol Biomarkers Prev. 2013;22:1289–96. doi: 10.1158/1055-9965.EPI-13-0229. [DOI] [PubMed] [Google Scholar]
- 224.Loft S, Svoboda P, Kawai K, Kasai H, Sorensen M, Tjonneland A, et al. Association between 8-oxo-7,8-dihydroguanine excretion and risk of lung cancer in a prospective study. Free Radic Biol Med. 2012;52:167–72. doi: 10.1016/j.freeradbiomed.2011.10.439. [DOI] [PubMed] [Google Scholar]
- 225.Rossner P, Jr., Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:639–44. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 226.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women's Health Study. J Clin Oncol. 2009;27:2482–8. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cai F, Dupertuis YM, Pichard C. Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr Opin Clin Nutr Metab Care. 2012;15:99–106. doi: 10.1097/MCO.0b013e32834feab4. [DOI] [PubMed] [Google Scholar]
- 228.Cobanoglu U, Demir H, Cebi A, Sayir F, Alp HH, Akan Z, et al. Lipid peroxidation, DNA damage and coenzyme Q10 in lung cancer patients--markers for risk assessment? Asian Pac J Cancer Prev. 2011;12:1399–403. [PubMed] [Google Scholar]
- 229.Peddireddy V, Siva Prasad B, Gundimeda SD, Penagaluru PR, Mundluru HP. Assessment of 8-oxo-7, 8-dihydro-2'-deoxyguanosine and malondialdehyde levels as oxidative stress markers and antioxidant status in non-small cell lung cancer. Biomarkers. 2012;17:261–8. doi: 10.3109/1354750X.2012.664169. [DOI] [PubMed] [Google Scholar]
- 230.Leufkens AM, van Duijnhoven FJ, Woudt SH, Siersema PD, Jenab M, Jansen EH, et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: a cohort-nested case-control study in the European Prospective Investigation Into Cancer and Nutrition. Am J Epidemiol. 2012;175:653–63. doi: 10.1093/aje/kwr418. [DOI] [PubMed] [Google Scholar]
- 231.Beevi SS, Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol. 2004;34:379–85. doi: 10.1093/jjco/hyh058. [DOI] [PubMed] [Google Scholar]
- 232.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, et al. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6:4768–75. [PubMed] [Google Scholar]
- 233.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 234.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 235.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, et al. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–51. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 236.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tsikas D. Application of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry to assess in vivo synthesis of prostaglandins, thromboxane, leukotrienes, isoprostanes and related compounds in humans. J Chromatogr B Biomed Sci Appl. 1998;717:201–45. doi: 10.1016/s0378-4347(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 238.Il'yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol. 2004;14:793–7. doi: 10.1016/j.annepidem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 239.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Analytical biochemistry. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 240.Cao H, Xiao L, Park G, Wang X, Azim AC, Christman JW, et al. An improved LC-MS/MS method for the quantification of prostaglandins E(2) and D(2) production in biological fluids. Analytical biochemistry. 2008;372:41–51. doi: 10.1016/j.ab.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim S, Taylor JA, Milne GL, Sandler DP. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev Res (Phila) 2013;6:511–8. doi: 10.1158/1940-6207.CAPR-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dong LM, Shu XO, Gao YT, Milne G, Ji BT, Yang G, et al. Urinary prostaglandin E2 metabolite and gastric cancer risk in the Shanghai women's health study. Cancer Epidemiol Biomarkers Prev. 2009;18:3075–8. doi: 10.1158/1055-9965.EPI-09-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Peng L, Zhou Y, Wang Y, Mou H, Zhao Q. Prognostic significance of COX-2 immunohistochemical expression in colorectal cancer: a meta-analysis of the literature. PLoS One. 2013;8:e58891. doi: 10.1371/journal.pone.0058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis G, Cohen C. COX-2 expression in invasive breast cancer: correlation with prognostic parameters and outcome. Appl Immunohistochem Mol Morphol. 2007;15:255–9. doi: 10.1097/01.pai.0000213130.63417.b3. [DOI] [PubMed] [Google Scholar]
- 245.Ferrandina G, Lauriola L, Zannoni GF, Distefano MG, Legge F, Salutari V, et al. Expression of cyclooxygenase-2 (COX-2) in tumour and stroma compartments in cervical cancer: clinical implications. Br J Cancer. 2002;87:1145–52. doi: 10.1038/sj.bjc.6600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ferrandina G, Lauriola L, Zannoni GF, Fagotti A, Fanfani F, Legge F, et al. Increased cyclooxygenase-2 (COX-2) expression is associated with chemotherapy resistance and outcome in ovarian cancer patients. Ann Oncol. 2002;13:1205–11. doi: 10.1093/annonc/mdf207. [DOI] [PubMed] [Google Scholar]
- 247.Ferrandina G, Ranelletti FO, Lauriola L, Fanfani F, Legge F, Mottolese M, et al. Cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), and Her-2/neu expression in ovarian cancer. Gynecol Oncol. 2002;85:305–10. doi: 10.1006/gyno.2002.6620. [DOI] [PubMed] [Google Scholar]
- 248.Czachorowski MJ, Amaral AF, Montes-Moreno S, Lloreta J, Carrato A, Tardon A, et al. Cyclooxygenase-2 expression in bladder cancer and patient prognosis: results from a large clinical cohort and meta-analysis. PLoS One. 2012;7:e45025. doi: 10.1371/journal.pone.0045025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 250.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 251.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 252.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 254.Park GY, Christman JW. Nuclear factor kappa B is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets. 2006;7:661–8. doi: 10.2174/138945006777435317. [DOI] [PubMed] [Google Scholar]
- 255.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 256.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–31. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bottero V, Imbert V, Frelin C, Formento JL, Peyron JF. Monitoring NF-kappa B transactivation potential via real-time PCR quantification of I kappa B-alpha gene expression. Mol Diagn. 2003;7:187–94. doi: 10.1007/BF03260037. [DOI] [PubMed] [Google Scholar]
- 261.Blaecke A, Delneste Y, Herbault N, Jeannin P, Bonnefoy JY, Beck A, et al. Measurement of nuclear factor-kappa B translocation on lipopolysaccharide-activated human dendritic cells by confocal microscopy and flow cytometry. Cytometry. 2002;48:71–9. doi: 10.1002/cyto.10115. [DOI] [PubMed] [Google Scholar]
- 262.White KL, Vierkant RA, Phelan CM, Fridley BL, Anderson S, Knutson KL, et al. Polymorphisms in NF-kappaB inhibitors and risk of epithelial ovarian cancer. BMC Cancer. 2009;9:170. doi: 10.1186/1471-2407-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Andersen V, Christensen J, Overvad K, Tjonneland A, Vogel U. Polymorphisms in NFkB, PXR, LXR and risk of colorectal cancer in a prospective study of Danes. BMC Cancer. 2010;10:484. doi: 10.1186/1471-2407-10-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Seufert BL, Poole EM, Whitton J, Xiao L, Makar KW, Campbell PT, et al. IkappaBKbeta and NFkappaB1, NSAID use and risk of colorectal cancer in the Colon Cancer Family Registry. Carcinogenesis. 2013;34:79–85. doi: 10.1093/carcin/bgs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat Commun. 2013;4:2299. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
- 266.Scartozzi M, Bearzi I, Pierantoni C, Mandolesi A, Loupakis F, Zaniboni A, et al. Nuclear factor-kB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J Clin Oncol. 2007;25:3930–5. doi: 10.1200/JCO.2007.11.5022. [DOI] [PubMed] [Google Scholar]
- 267.Coghill AE, Newcomb PA, Poole EM, Hutter CM, Makar KW, Duggan D, et al. Genetic variation in inflammatory pathways is related to colorectal cancer survival. Clin Cancer Res. 2011;17:7139–47. doi: 10.1158/1078-0432.CCR-11-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Piperdi B, Ling YH, Liebes L, Muggia F, Perez-Soler R. Bortezomib: understanding the mechanism of action. Mol Cancer Ther. 2011;10:2029–30. doi: 10.1158/1535-7163.MCT-11-0745. [DOI] [PubMed] [Google Scholar]
- 269.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–52. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.McCormack VA, Hung RJ, Brenner DR, Bickeboller H, Rosenberger A, Muscat JE, et al. Aspirin and NSAID use and lung cancer risk: a pooled analysis in the International Lung Cancer Consortium (ILCCO). Cancer Causes Control. 2011;22:1709–20. doi: 10.1007/s10552-011-9847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–50. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 272.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature reviews Cancer. 2006;6:130–40. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 273.Baandrup L, Faber MT, Christensen J, Jensen A, Andersen KK, Friis S, et al. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: systematic review and meta-analysis of observational studies. Acta Obstet Gynecol Scand. 2013;92:245–55. doi: 10.1111/aogs.12069. [DOI] [PubMed] [Google Scholar]
- 274.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–89. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 275.Ladurner P, Pfister D, Seifarth C, Scharer L, Mahlknecht M, Salvenmoser W, et al. Production and characterisation of cell- and tissue-specific monoclonal antibodies for the flatworm Macrostomum sp. Histochem Cell Biol. 2005;123:89–104. doi: 10.1007/s00418-004-0722-9. [DOI] [PubMed] [Google Scholar]
- 276.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 277.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 278.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 279.Vanoirbeek E, Krishnan A, Eelen G, Verlinden L, Bouillon R, Feldman D, et al. The anti-cancer and anti-inflammatory actions of 1,25(OH)(2)D(3). Best Pract Res Clin Endocrinol Metab. 2011;25:593–604. doi: 10.1016/j.beem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–36. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 281.Shimizu M, Tanaka T, Moriwaki H. Obesity and hepatocellular carcinoma: targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin Immunopathol. 2013;35:191–202. doi: 10.1007/s00281-012-0336-6. [DOI] [PubMed] [Google Scholar]
- 282.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]