Abstract
Depressive severity has been associated with attenuated neocortical frontal midline theta (Fm-θ) power/evoked activity. Mindfulness-Based Cognitive Therapy (MBCT) has shown to be a successful novel intervention for Major Depressive Disorder (MDD), albeit precise working mechanisms remain elusive. We examined the hypothesis that MBCT would have modulating effects upon evoked Fm-θ power, in addition to investigating possible mediation of induced event-related de/synchronisation (ERD/ERS) dynamics. Fifty one patients with a primary diagnosis of MDD (26 exposed to MBCT vs. 25 wait-list/WL controls) undertook a Go/NoGo task consisting of positive, negative and neutral words, further stratified into abstract versus trait adjective matrices. Depressive symptom severity and rumination were also examined. A pattern of enhanced induced Fm-θ synchronisation during the latter 400–800 ms temporal-window pre-to-post MBCT was observed; the contrary in the WL. Modulated ERD/ERS dynamics correlated to amelioration in depressive and rumination symptoms in the MBCT group. We propose the primary action pathway alluded to a neural disengagement mechanism enacting upon tonic neuronal assemblies implicated in emotional and self-related processing. Due to the complexity and presently undiscovered complete unified scientific understanding of neuro-oscillatory-dynamics, and associated clinical interplays; we hypothesise that the electro-cortical and connected clinical working pathways of MBCT in depression are multi-levelled constituting nonlinear and interdependent mechanisms, represented by mediated EEG synchronisation dynamics.
Keywords: Fm-theta, Major Depressive Disorder (MDD), Mindfulness-Based Cognitive Therapy (MBCT), Event-related (de-)synchronisation (ERD/ERS), Oscillatory EEG, Rumination
Introduction
Major Depressive Disorder is highly debilitating and often non-responsive to pharmacological and psychological interventions (Guilloux et al. 2012). This is further exacerbated where absence of full remission following a first depressive episode increases the probability of recurrent episodes, ensuing significant burden upon patients’, their immediate support, and societal and economic resources in general (McIntyre and O’Donovan 2004). Thus, developing alternative interventions for patients who appear to be medication and/or psychotherapy treatment-resistant is pertinent.
A conjectured EEG biomarker of MDD is Fm-θ (4–8 Hz bandwidth) activity, primarily generated by frontal lobe-ACC circuits (Ekstrom et al. 2005). Enhanced Fm-θ purportedly indicates greater mental effort and sustained attention (Mitchell et al. 2008), where lower levels may correlate to greater depression and anxiety severity (Gold et al. 2013). Corroborating research indicates medication-related increase in EEG θ-power alongside successful paroxetine (Knott et al. 2002), and nortriptyline (Pizzagalli et al. 2001) antidepressant treatment, and increased frontal cortical MEG θ-activity following efficacious ECT (Heikman et al. 2001). Increased resting θ-activity within the rACC has been reported in responders to citalopram and reboxetine medications (Mulert et al. 2007), whereby elevated levels theoretically improve the probability of antidepressant success via increased synchrony between the DMN and TPN, associated with cognitive control (Pizzagalli 2011). Moreover, elevated pre-treatment θ current density within rACC and mOFC regions correlate with symptom improvement in responders to venlafaxine and fluoxetine (Korb et al. 2009). Collectively, these findings implicate heightened θ-power in symptom alleviation and better treatment outcome for MDD.
The development of innovative treatments aside pharmacological interventions for depression have given rise to the emergence of Mindfulness-Based Cognitive Therapy (MBCT), i.e. the combination of CBT and structured attention training encompassing formal meditative techniques (Segal et al. 2012). Originally conceived for relapse prevention in recurrent depression (Teasdale et al. 2000; Ma and Teasdale 2004), MBCT has also been successful for treatment-resistant (Kenny and Williams 2007), and current depression (van Aalderen et al. 2012), suicide vulnerability (Williams et al. 2006), Bipolar Disorder (Williams et al. 2008), and anxiety (Evans et al. 2008). However, despite accumulating clinical success, precise working mechanisms of MBCT remain relatively under-researched/undefined.
Here, we utilised EEG with the aim to decipher potential neural working mechanisms of MBCT in the treatment of recurrent depression. Electroencephalography provides a multi-dimensional index of brain function, where linear decompositions of EEG signals, such as evoked oscillatory activity and event-related potentials (ERPs), provide phase-locked cortical information associated with populations of neuronal transients directly manifested via the stimulus/event. Such uni-dimensional brain measures can mask underlying and potentially more complex neural dynamics, such as non-phase-locked EEG, which purportedly reflect oscillations generated by diverging higher-order synchronous neuronal assemblies not evoked directly by stimuli, but rather are induced via nonlinear and likely discrete mechanisms, representing multi-levelled tonic cortical activity in time–frequency space. We employed non-linear signal analysis to measure ERD/ERS in the extraction of both evoked phase-locked and induced non-phase-locked components. Non-phase-locked ERD/ERS defines cortical synchrony in terms of: (1) ERD; whereby integrant neuronal assemblies underlying neuronal circuitry function in a relatively independent way, representing an ‘activated’ system with maximal readiness and potential for cortical processing, i.e. ‘cortical excitation’, (2) ERS; reflecting state deactivation/’inhibition’, or the decreased excitability of cortical neurons and more stable/coherent synchrony between neuronal sub-populations, independent from neuronal firing rates which can be higher compared to ERD (Harris and Thiele 2011).
Hypotheses
(1) MBCT would increase Fm-θ evoked power, in line with extant anti-depressant studies, based on the proposed mechanisms of MBCT upon attention and emotion regulation (Hölzel et al. 2011); (2) MBCT would mediate underlying non-phase-locked neuronal θ-synchrony. Due to its novelty, alongside the fact that induced and evoked activity are not necessarily linearly entangled, no point of reference/rationale was available to predict a direction in results, constituting an exploratory element of the study; (3) We reasoned that reduced Fm-θ in depression, where increased levels correlate with improved symptom severity and/or treatment response, plausibly underpin sub-optimal cognitive and attention processing implicated in rumination (Demeyer et al. 2012). Interestingly, Bipolar Disorder patients show increased θ-power (4–7 Hz) and θ/β ratio activity during a sustained attention task following treatment, suggesting MBCT improves comparatively low levels of attentional readiness and frontal cortical control (Howells et al. 2012). In this light, we hypothesised that increased θ-activity pre-to-post MBCT would correlate with ameliorated depressive severity and rumination.
Methods
Recruitment and sample
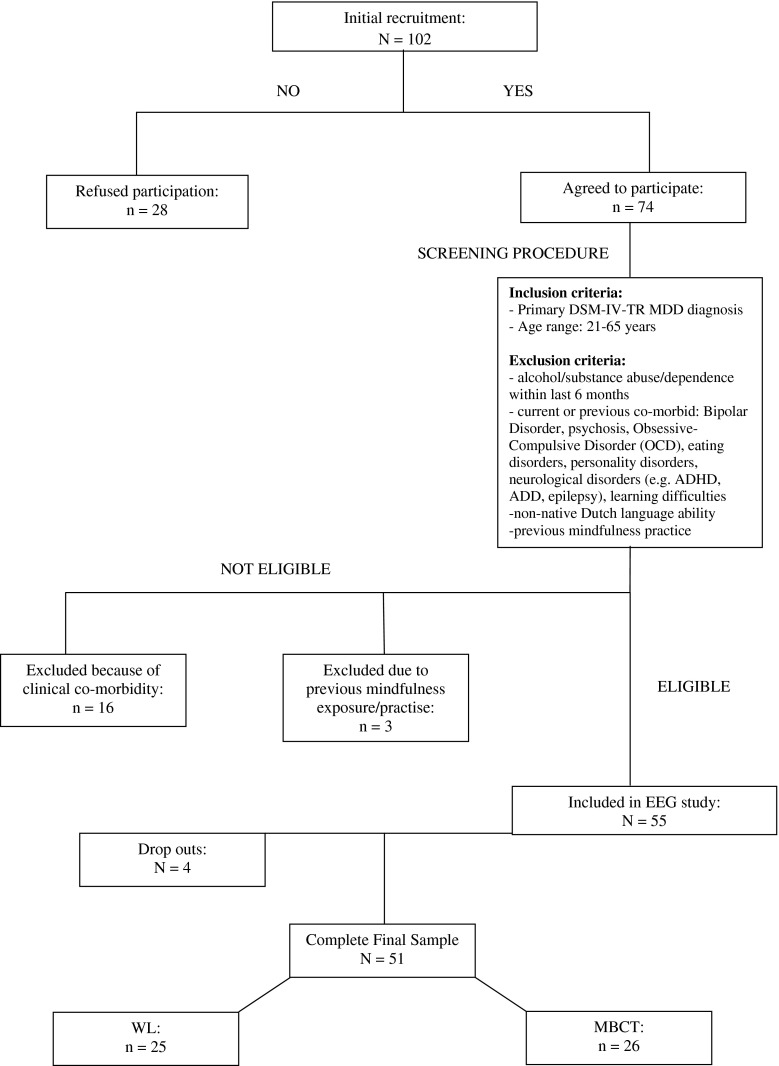
Figure 1 outlines the recruitment procedure. We aimed to recruit approximately n = 25 per group (MBCT vs. WL), based on A-priori power calculation whereby a minimum of n = 21 per group was required to achieve 0.80 power to detect significant difference (α = .05), with an effect size d = 0.8. Sample demographics, clinical, and task performance/behavioural measures, are reported in Table 1. Included patients had suffered between 1 and 3 previous depressive episodes. Patients were recruited via the Radboud University Medical Centre Nijmegen (UMCN) psychiatry outpatient clinic and associated UMCN Mindfulness Centre via specialist referrals. Primary diagnosis of MDD was ascertained according to the DSM-IV-TR by a consultant psychiatrist. Patients were allocated to experimental group depending upon when they had applied for their MBCT course, i.e. patients accepted onto an MBCT course with 8-weeks + until its onset were allocated to the WL group.
Fig. 1.
Recruitment procedure
Table 1.
Demographic, clinical, and task performance/behavioural, data
| Demographics | MBCT | WL | Between-group comparison |
|---|---|---|---|
| Sex | Female = 20 [76.9 %], Male = 6 [23.1 %] | Female = 12 [48 %], Male = 13 [52 %] | p = .05* |
| Age/years | 47.8 (12.1) | 51.2 (8.5) | p = .27 |
| Medicated | Medicated = 19 [73.1 %], non-med = 7 [26.9 %] | Medicated = 17 [68 %], non-med = 8 [32 %] | p = .76 |
| Primary diagnosis | CD = 11 [42.3 %], RD = 15 [57.7 %] | CD = 7 [28 %], RD = 18 [72 %] | p = .30 |
| Pre | Post | Within-group comparison | Pre | Post | Within-group comparison | Baseline: T1/Pre | |
|---|---|---|---|---|---|---|---|
| Clinical variables | |||||||
| IDS | 27.3 (9.4) | 19.3 (9.3) | p = .02* | 25.1 (12.3) | 25.3 (13.2) | p = .89 | p = .55 |
| RRS | 60.6 (11.9) | 55.1 (11.0) | p = .03* | 60.2 (13.2) | 59.7 (13.8) | p = .81 | p = .93 |
| FFMQ global | 114.9 (22.8) | 131.3 (17.4) | p = .02* | 119.7 (14.9) | 119.8 (15.7) | p = .96 | p = .43 |
| FFMQ observe | 24.4 (5.9) | 28.6 (4.7) | p = .005** | 25.1 (4.9) | 24.8 (5.2) | p = .51 | p = .70 |
| FFMQ describe | 27.7 (4.5) | 28.6 (4.7) | p = .64 | 28.7 (6.0) | 28.6 (6.1) | p = .77 | p = .56 |
| FFMQ Non-judge | 22.3 (8.0) | 26.7 (5.3) | p = .03* | 24.1 (6.8) | 24.2 (6.6) | p = .94 | p = .45 |
| FFMQ Non-reaction | 19.0 (5.2) | 22.9 (4.3) | p = .01* | 18.0 (4.7) | 18.0 (4.5) | p = .87 | p = .49 |
| FFMQ awareness | 21.5 (5.8) | 24.7 (6.1) | p = .09 | 23.9 (5.2) | 24.3 (5.5) | p = .55 | p = .20 |
| ZC global | 83.7 (25.8) | 107.0 (26.5) | p < .0001** | 81.7 (22.8) | 84.0 (23.1) | p = .45 | p = .81 |
| STAI T | 47.0 (3.4) | 46.0 (5.0) | p = .32 | 46.9 (4.6) | 46.4 (4.7) | p = .55 | p = .93 |
| STAI S | 43.8 (5.7) | 43.6 (3.9) | p = .81 | 41.6 (3.8) | 41.3 (3.1) | p = .76 | p = .15 |
| CDS G | 41.4 (26.0) | 36.9 (29.3) | p = .37 | 39.1 (45.8) | 42.5 (43.7) | p = .29 | p = .83 |
| CDS EN | 11.1 (8.2) | 8.4 (7.9) | p = .11 | 11.8 (14.5) | 12.6 (13.1) | p = .55 | p = .82 |
| Behavioural measures | |||||||
| Correct-NoGo ALL | 13.6 (3.0) | 13.9 (2.6) | p = .59 | 13.6 (2.9) | 13.9 (3.3) | p = .39 | p = .94 |
| Correct-Go ALL | 70.0 (8.4) | 71.9 (4.3) | p = .12 | 71.8 (4.6) | 72.3 (4.8) | p = .77 | p = .16 |
| Correct-NoGo POS | 14.2 (3.6) | 14.6 (3.0) | p = .59 | 13.9 (3.1) | 14.3 (3.3) | p = .33 | p = .81 |
| Correct-Go POS | 69.2 (9.8) | 72.2 (4.4) | p = .23 | 72.1 (4.6) | 71.9 (4.9) | p = .60 | p = .20 |
| Correct-NoGo NEG | 13.9 (3.4) | 14.0 (2.8) | p = .84 | 14.0 (3.2) | 14.1 (3.4) | p = .82 | p = .88 |
| Correct-Go NEG | 69.0 (10.0) | 73.0 (6.3) | p = .10 | 73.1 (5.3) | 73.1 (6.6) | p = .94 | p = .08 |
| Correct-NoGo NEU | 12.7 (2.8) | 13.0 (2.8) | p = .56 | 12.9 (3.0) | 13.4 (3.5) | p = .27 | p = .74 |
| Correct-Go NEU | 68.7 (8.1) | 71.0 (5.8) | p = .19 | 70.3 (6.7) | 72.0 (6.3) | p = .27 | p = .48 |
| Correct Hits (Go) RT | |||||||
| ALL | 579.7 (75.5) | 587.6 (88.5) | p = .50 | 565.6 (68.4) | 553.8 (60.0) | p = .37 | p = .51 |
| POS | 582.5 (83.2) | 589.9 (84.3) | p = .66 | 562.1 (64.6) | 559.7 (61.5) | p = .79 | p = .36 |
| NEG | 592.1 (85.8) | 601.9 (103.8) | p = .51 | 571.1 (69.1) | 560.8 (54.9) | p = .55 | p = .36 |
| NEU | 564.5 (67.2) | 570.9 (83.4) | p = .52 | 563.5 (79.1) | 541.0 (70.7) | p = .15 | p = .96 |
Bold font indicates statistical significance
Procedure
MBCT consisted of eight weekly sessions of 2.5 h, one full silent training day, and daily homework lasting 0.75 h (for exercise details, see Segal et al. 2012). Training was administered by health care professionals with longstanding clinical and mindfulness experience, meeting the teaching criteria of the Dutch Association of Mindfulness Trainers (www.vmbn.nl). Informed written consent to participate in an ethically approved (CMO, Arnhem-Nijmegen) research study was obtained.
Patients undertook an affective Go/No-Go task concomitant to EEG recording. The experimental task was structured in 12 × 100 stimuli blocks [N = 1,200 stimuli], with rest intervals between each block. Stimuli consisted of Positive, Negative, and Neutral Dutch words, assimilated from two standardised word databases (Arnold et al. 2011, Hermans and De Houwer 1994). Each block consisted of 80×Go and 20×NoGo stimuli (20 % inhibition rate), constituting 6 possible “block types” [i.e.: 1. Positive (Go)-Negative (NoGo); 2. Positive (Go)-Neutral (NoGo); 3. Negative (Go)-Positive (NoGo); 4. Negative (Go)-Neutral (NoGo); 5. Neutral (Go)-Positive (NoGo); 6. Neutral (Go)-Negative (NoGo)]. Between each block standardised instructions were given onscreen, verified verbally by the experimenter, indicating which word valence to press/not press for, in addition to: “There are no right or wrong responses. Please press when the word is *positive/negative/neutral* to you”.
Positive and Negative words were further divided into abstract (e.g. freedom, cruelty) and trait (e.g. ambitious, unhappy) words. We anticipated MDD patients would be particularly self-referential concerning negative trait words compared to negative abstract words for example. Block presentation and word stimuli within each block were randomised. Within the overall experiment, 600 different word stimuli aimed to reduce stimuli habituation/familiarity, alongside random stimulus presentation duration (500–1,500 ms), and random ISI (800–1,750 ms).
Clinical scales
The following instruments were administered at T1 and T2; (a) IDS (Rush et al. 1996), gauging depressive symptom severity; (b) RRS (Nolen-Hoeksema and Morrow 1991) gauging response patterns towards feelings of sadness/depression; (c) FFMQ (Baer et al. 2008); (d) SCS (Neff 2003); (e) STAI-S/T (Spielberger et al. 1983); (f) CDS (Sierra and Berrios 2000). Due to the high co-morbidity of depersonalisation in depression (20–30 %), we included this scale to examine potential interplays between the ‘emotional numbing’ (EN) sub-category and the MBCT process, i.e. attending in the present to emotional experience.
Electrophysiological recording
EEG data were acquired using Brain Vision Recorder 1.03 and QuikAmps 72 hardware (www.BrainProducts.com), recorded from 30 Ag/AgCl active electrode sensors with integrated noise subtraction circuits (actiCAP: Brain Products) located in accordance with the 10–10 electrode system (sites: Fp1, Fp2, AFz, F7, F3, Fz, F4, F8, FC5, FC1, FCz, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, O1, Oz, O2). Average online reference was used (AFz), and referenced to the right mastoid offline. Ground electrode was placed on the forehead. Vertical and horizontal ocular activity were calculated by bipolar derivations of electro-oculogram signals recorded using Ag/AgCl cup electrodes above and below the left eye, and 1 cm to the outer canthi of each eye, respectively. Impedance was maintained ≤10 KΩ. Electrical signal was continuously sampled at a digitization rate of 500 Hz, with a band-pass filter of 0.1–100 Hz.
Event-related (de-)synchronisation (ERD/ERS) analysis
Oscillatory EEG analysis was conducted using Brain Vision Analyzer 2.0.2. Occular artefacts were removed using ICA (Graimann and Pfurthscheller 2006), and data were segmented into 1,400 ms epochs [length = −500 to 900 ms relative to stimulus onset]: (1) stimulus-locked correct NoGo trials (NoGo-T), (2) stimulus-locked correct Go trials (Go-T). NoGo-T and Go-T data included the following sub-segmentations: (1) Valence (Positive, Negative, Neutral), and (2) Object versus Self (abstract words, trait words). Artifact rejection removed electromyographic activity and/or amplifier saturation, where voltages exceeding ±50 µV were discarded. Data were band-pass filtered (Butterworth zero-phase, 24 dB/octave) into θ-frequency range activity (4–8 Hz).
A series of ERD/ERS analyses were then applied to extract induced and evoked oscillatory activity. Point-to-point ‘intertrial variance method’ (formulaic calculations, see Kalcher and Pfurtscheller 1995) was employed to calculate event-related induced activity by averaging all epochs and subtracting the average from each individual epoch constituting the average, thus extracting phase-locked activity. ERD/ERS was quantified as percentage change in average inter-trial variance (A) during the time windows (TW): 0–400 ms (early time-window [E-TW]), and 400–800 ms (late time-window [L-TW]), in the nth channel, compared to the average inter-trial variance of the baseline reference (R) = −500–0 ms, relative to stimulus-onset, defined as follows:
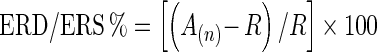 |
To ascertain whether underlying neuronal states were predominantly synchronised or desynchronised within the defined temporal windows of interest following stimulus presentation, mean induced ERD/ERS % during the E-TW (0–400 ms) and L-TW (400–800 ms) were subsequently calculated and used in statistical analyses.
For phase-locked evoked activity; following bandpass filtering, epoch voltages were squared and subsequently averaged, and expressed as instantaneous power (µV2). Mean power for the E-TW (0–400 ms) and L-TW (400–800 ms) were then calculated.
Statistical analyses
Separate repeated-measures (rm-) ANCOVA (co-variates: sex, medication status) were conducted for: (1) behavioural accuracy data, (2) behavioural reaction time (RT) data, (3) EEG evoked power data, and (4) EEG induced ERD/ERS dynamics. Rm-ANCOVA examined Time (2 level: pre/T1, post/T2) × Condition (2 level: Go, NoGo) × Valence (3 level: Positive, Negative, Neutral) x Group (2 level: MBCT, WL), for accuracy, and RT behavioural data. Rm-ANCOVA examined Time (2 level: T1, T2) × Time-Window/TW (2 level: E: 0–400 ms, L: 400–800 ms) × Condition (2 level: Go, NoGo) × Valence (3 level: Positive, Negative, Neutral) × Group (2 level: MBCT, WL) matrices for mean induced ERD/ERS % dynamics, and evoked power, measured at the FCz electrode. Additionally, ERD/ERS and evoked power were examined (separately) via Time (2 level: T1, T2) × TW (2 level: E, L), × Condition (2 levels: Go, NoGo) × Self/Abstract (2 levels: self/trait words, abstract/objective words) x Group (2 level: MBCT, WL) rm-ANCOVAs. N.B. Analyses were conducted in this way so to avoid Type I errors, due to too small numbers per cell if stratifying the data by Valence and Self/Abstract substrata within the same rm-ANCOVA. Greenhouse Geisser corrections were used when assumptions of sphericity were violated. Where significant effects/interactions were revealed from the main rm-ANCOVA/s, follow-up one-way ANOVAs were applied to increment change from T1-to-T2 (T2–T1), i.e. cortical change relative to individual baseline, so to disentangle main effects/interactions.
Results
Due to technical failure, 22 WL and 20 MBCT behavioural datasets were available for statistical task performance analysis. Due to practical restraints (e.g. time, patient fatigue, refusal, partially completed questionnaires), 38 full questionnaire datasets were collected at both T1 and T2 in the EEG testing sessions. For ERD/ERS data, we dropped the dataset for the Neutral condition of one patient who had too few correct NoGo trials.
Behavioural data
Regarding accuracy scores; as expected main effect of Condition (F(1, 38) = 1,558.493, p < .0001), was found. There were no main effects of Group (p = .09), Medication (p = .44), or Group interactions, indicating no behavioural task-performance changes related to the MBCT. Regarding RT; main effect of Condition (F(1, 38) = 27.830, p < .0001), and Condition*Group (F(1, 38) = 6.436, p = .02), and Condition*Valence*Group (F(2, 76) = 3.586, p = .04) interactions were apparent. Although, follow up tests indicated any differences between and within groups were not significant.
Baseline EEG
A series of one-way ANOVAs were applied to baseline (pre/T1) data for each EEG variable, with Group (MBCT, WL) as Factor. Significant differences between MBCT vs. WL at baseline pertained to induced-θ only: for (1) Negative NoGo during the E-TW (F(1, 49) = 12.806, p = .001: [MBCT = 9.69 % ERS vs. WL = -9.99 % ERD]), (2) Self NoGo during the E-TW (F(1, 49) = 4.956, p = .03: [MBCT = .81 % ERS vs. WL = −8.43 % ERD]), and (3) Positive NoGo during the L-TW (F(1, 49) = 5.067, p = .03: [MBCT = 44.03 % ERS vs. WL = 87.07 % ERS]).
Induced ERD/ERS (%)
Valence
Main effect of TW (F(1, 46) = 12.790, p = .001), showed θ-ERD for the E-TW (0–400 ms), and greater ERS during the L-TW (400–800 ms), in both groups. Main effect of Condition (F(1, 46) = 9.519, p = .003), Valence (F(2, 92) = 3.228, p = .05), and TW*Condition (F(1, 46) = 5.615, p = .022), TW*Valence (F(2, 92) = 7.956, p = .001), Time*Valence*Group (F(2, 92) = 4.487, p = .01), and Time*Condition*Valence*Group (F(2, 92) = 5.459, p = .006), interactions were found. However, no main effects of Group (F(1, 46) = .645, p = .43), Sex (F(1, 46) = .997, p = .32), or Sex interactions were evident, suggesting the uneven sex distribution had no effect on ERD/ERS activity.
To better disentangle these findings in light of any baseline differences, posthoc one-way ANOVAs were applied to incremental changes from T1-to-T2 (T2–T1) for each variable, i.e. cortical change relative to individual baseline. No significant posthoc findings were evident for Go-T. However, an overall pattern showed increased cortical inhibition for Positive and Neutral stimuli opposed cortical activation for Negative stimuli during the E-TW from T1-to-T2, in the MBCT group. The WL group showed a contrary pattern albeit with smaller degrees of incremental change. Marked difference from T1-to-T2 was evident between groups during the L-TW, whereby mean ERS/cortical inhibition was apparent across valence conditions in the MBCT group, compared to mean ERD/cortical activation in the WL.
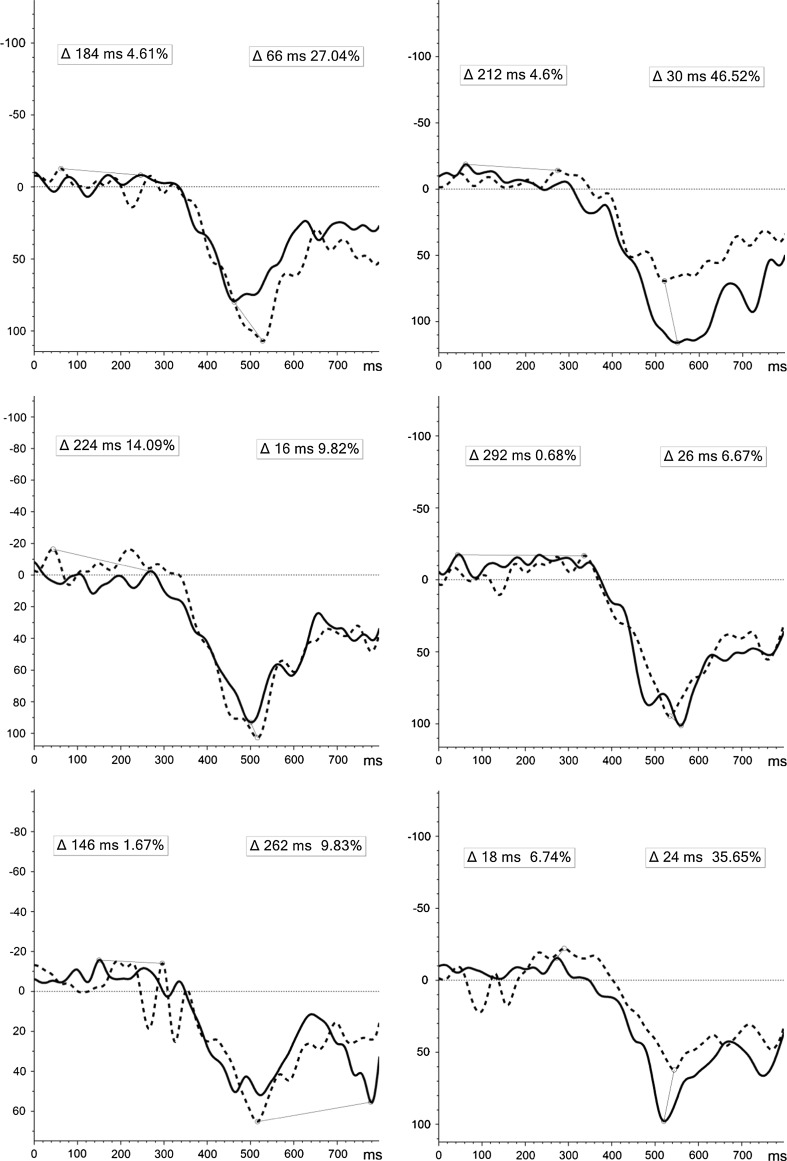
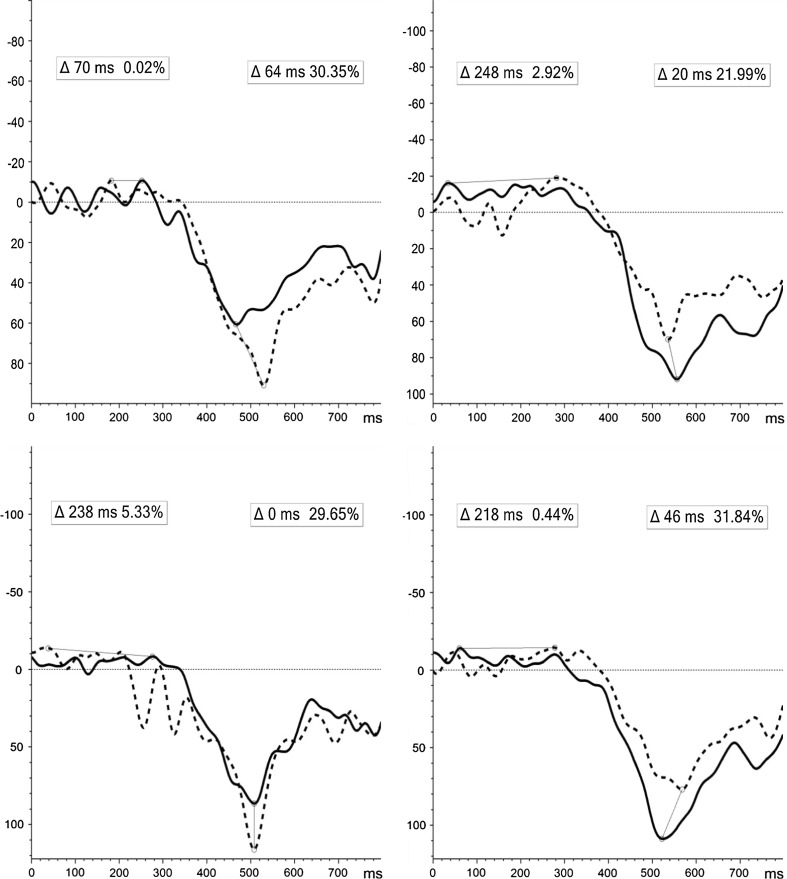
Significant incremental changes were exclusive to induced θ-activity for NoGo-T (Fig. 2). Despite variability in (1) Positive stimuli during the L-TW and (2) Negative stimuli during E-TW between groups at T1, a significant incremental change (controlling for individual baseline) was evident for both: (1) (F(1, 49) = 10.933, p = .002), showing increased ERS pre-to-post-MBCT [44.03 to 55.81 %], compared to decreased ERS in the WL [87.07 to 51.26 %]; and 2) (F(1, 49) = 5.099, p = .03), indicating a shift from synchronised to desynchronised cortical state pre-to-post [9.69 % to −1.83 %] MBCT, compared to attenuated ERD in the WL [−9.99 % to −3.99 %] (Fig. 3).
Fig. 2.
ERD/ERS induced activity for NoGo-T positive (above), negative (middle), and neutral (below) stimuli at T1 (line) and T2 (dot) for MBCT (left) and WL (right) groups [x-axis = full 800 ms TW; y-axis = desynchronisation (−), synchronisation (+)]. Δ = latency (ms) and peak (%) of difference in induced activity from T1 to T2 for E-TW: 0–400 ms (left) and L-TW: 400–800 ms (right)
Fig. 3.
Induced fronto-central ERD/ERS incremental change (T2–T1) for NoGo-T during E:0–400 ms (above) and L:400–800 ms (below) epochs
Medication effects (valence)
No main effect of Medication (F(1, 46) = 1.158, p = .288) was found, although a Time*TW*Condition*Valence*Medication (F(2, 92) = 3.392, p = .04) interaction was evident, where posthoc one-way ANOVAs showed significant increment change (pre-to-post) for NoGo trials in the late TW (400–800 ms) to Positive stimuli (F(1, 35) = 6.350, p = .02) in the medicated group only. Follow-up paired-tests, with a Group*Medication dataset split, showed these pre-to-post differences were not significant; medicated patients receiving MBCT showed increased ERS pre-to-post (t(18) = −.892, p = .38 − [45.8 % to 52.4 %]), as did non-medicated patients exposed to MBCT (t(6) = −1.407, p = .21 − [39.3 % to 65.1 %]). The opposite of decreased ERS from T1-to-T2 was apparent in the WL group, regardless of medicated (t(16) = 2.252, p = .04 - [99.1 % to 57.8 %]), or non-medicated (t(7) = 4.353, p =.003 - [61.6 % to 37.3 %]), status. These analyses must be approached with caution due to the low numbers per cell when stratified by Group*Medication (i.e. medicated MBCT n = 19, WL n = 17 vs. non-medicated MBCT n = 7, WL n = 8).
Self/trait versus object/abstract
Condition (F(1, 47) = 8.199, p = .006), TW (F(1, 47) = 13.071, p = .001), and TW*Condition (F(1, 47) = 7.114, p = .01), Time*Group (F(1, 47) = 4.037, p = .05), and Time*TW*Group (F(1, 47) = 5.481, p = .02) interactions, were evident. No main effects of Group (p = .52), Medication (p = .23), or Sex (p = .33).
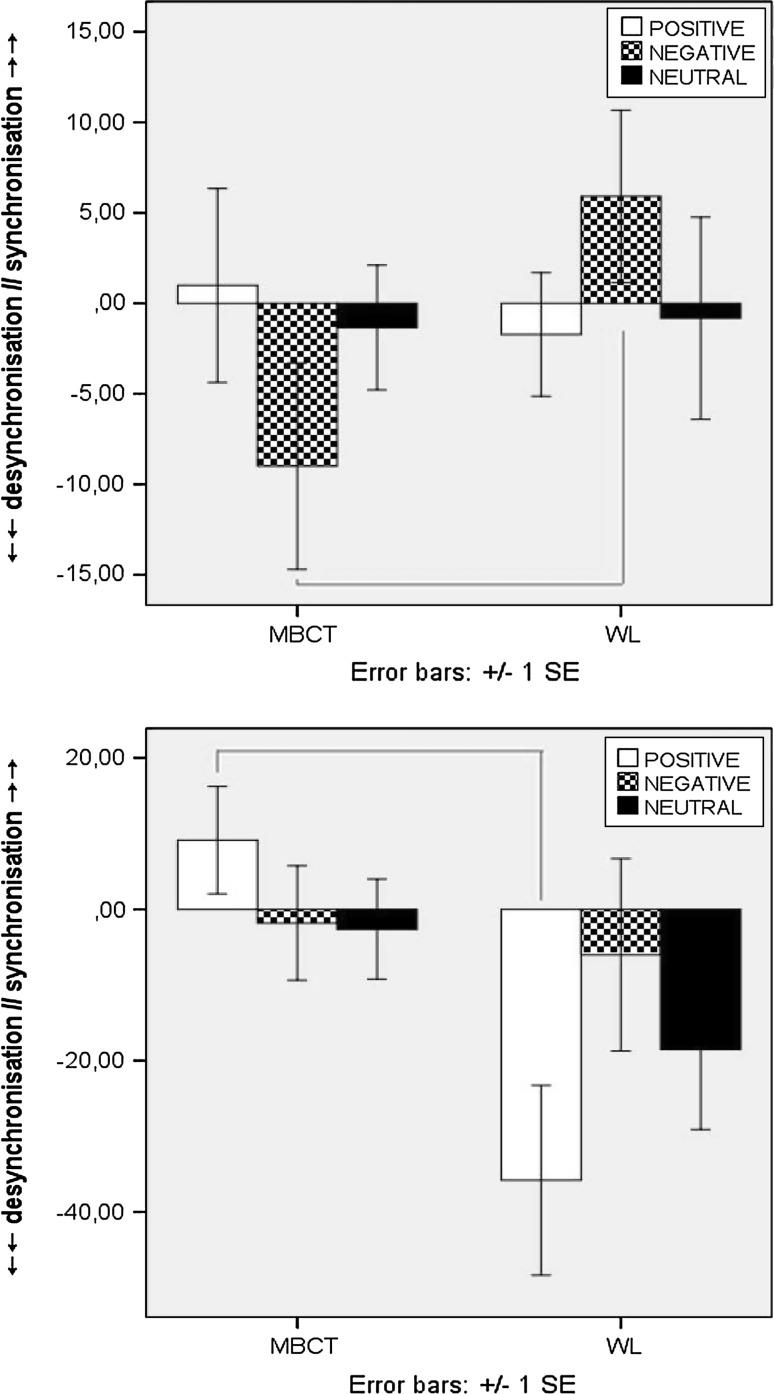
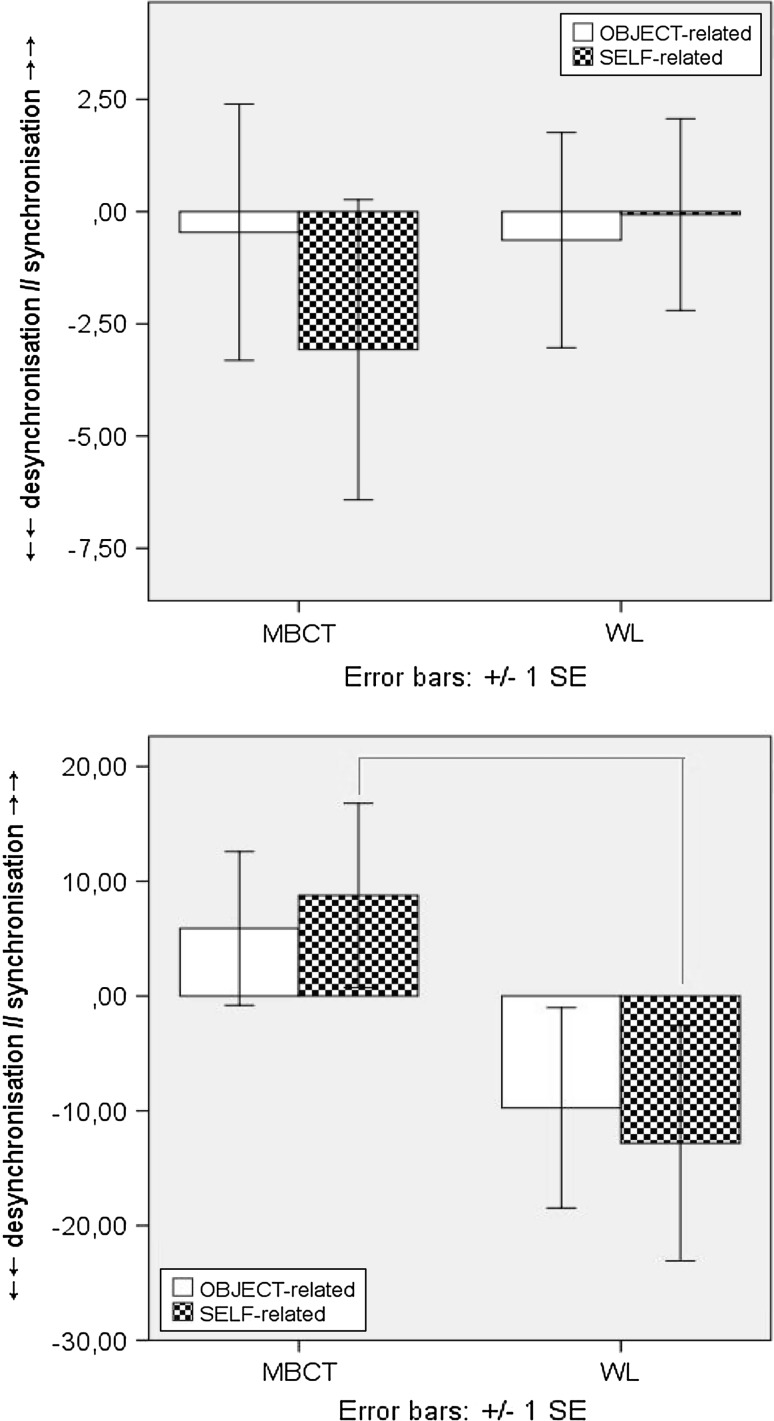
Follow up analyses examining incremental change, to account for any baseline differences, showed both groups increased cortical activation between T1-to-T2 for Go-T, whereby incremental change was greater for Self-related/trait stimuli in the MBCT group. However, during the L-TW, oscillatory dynamics in the MBCT group shifted to greater cortical inhibition, which was not apparent in the WL (F(1, 49) = 4.647, p = .036), (Fig. 4a, b).
Fig. 4.
a ERD/ERS induced activity for Go-T Self-related (above), and object-related (below), stimuli at T1 (line) and T2 (dot) for MBCT (left) and WL (right) groups. b Induced fronto-central ERD/ERS incremental change (T2–T1) for Go-T during E: 0–400 ms (above) and L: 400–800 ms (below) epochs [x-axis = full 800 ms TW; y-axis = desynchronisation (−), synchronisation (+)]. Δ = latency (ms) and peak (%) of difference in induced activity from T1 to T2 for E-TW: 0–400 ms (left) and L-TW: 400–800 ms (right)
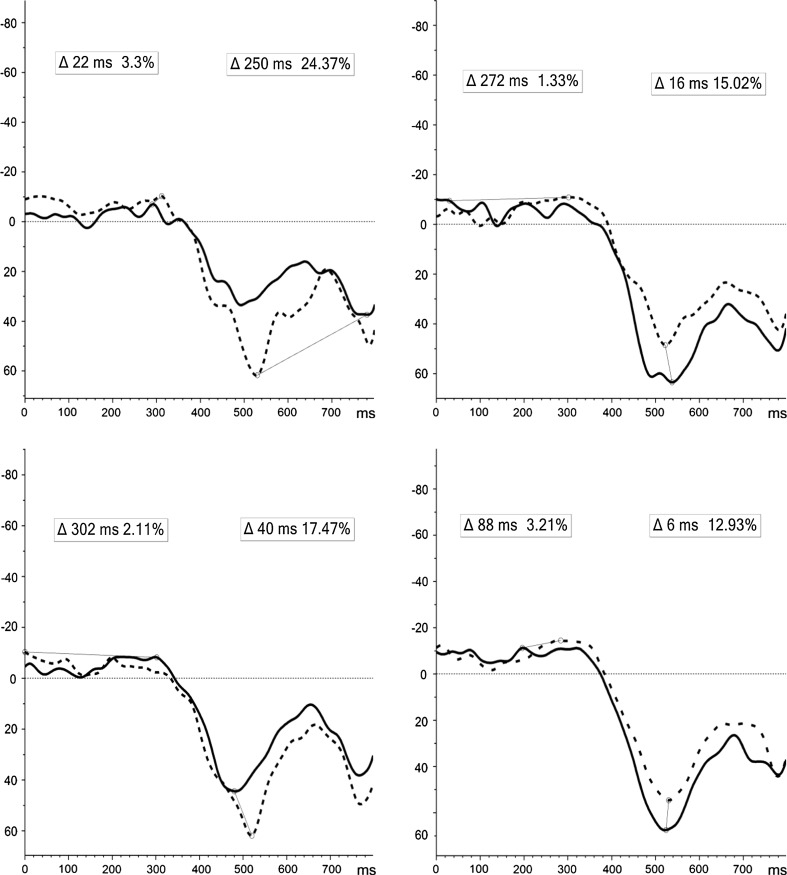
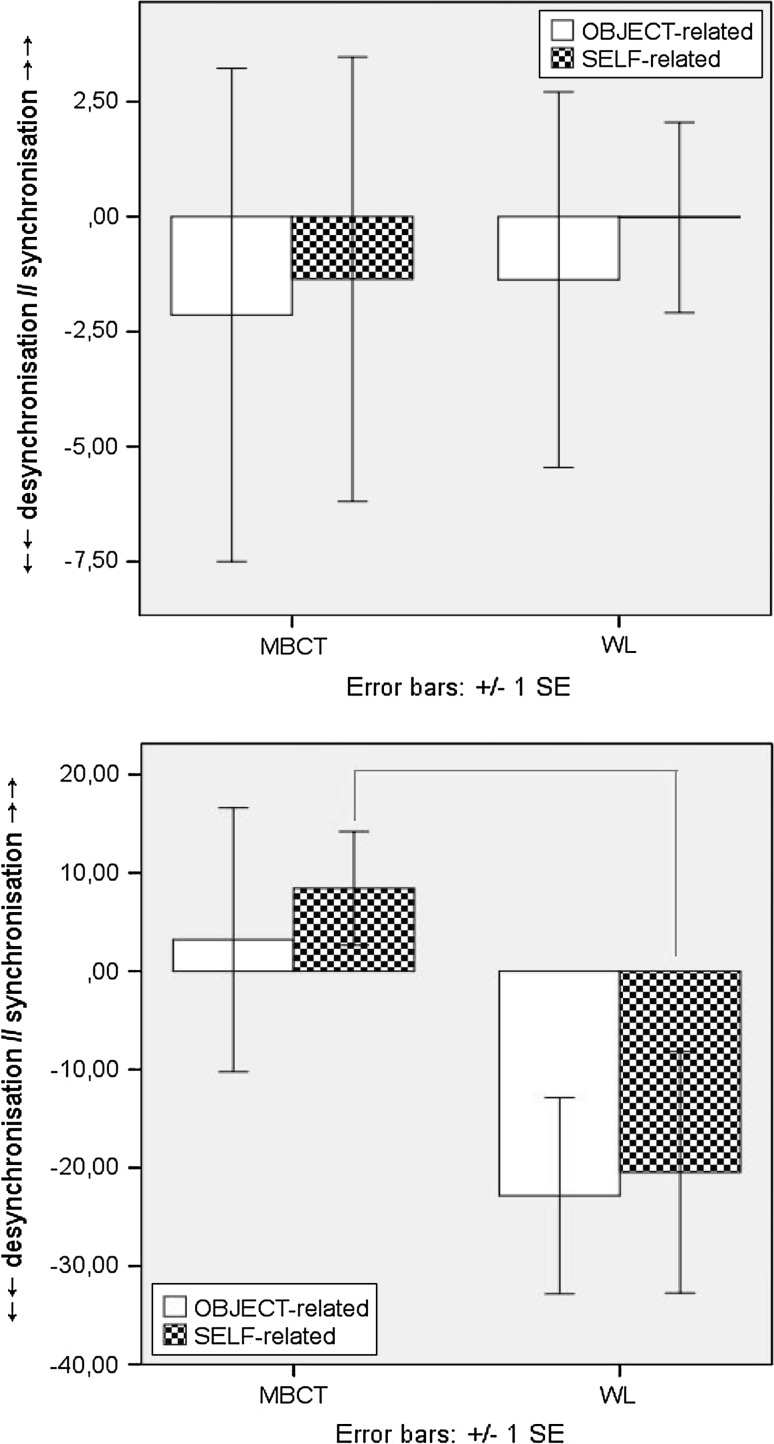
For NoGo-T incremental changes; during the E-TW, both groups showed a tendency for overall mean ERD/cortical activation from T1-to-T2 for both conditions, and to a larger degree for Self-related stimuli in the MBCT group. Significantly increased ERS/cortical inhibition for Self stimuli during the L-TW (F(1, 49) = 4.647, p = .036) was apparent pre-to-post MBCT, compared to attenuated ERS in the WL (Fig. 5a, b).
Fig. 5.
a ERD/ERS induced activity for NoGo-T self-related (above), and object-related (below), stimuli at T1 (line) and T2 (dot) for MBCT (left) and WL (right) groups. b Induced fronto-central ERD/ERS incremental change (T2–T1) for NoGo-T during E:0–400 ms (above) and L:400–800 ms (below) epochs [x-axis = full 800 ms TW; y-axis = desynchronisation (−), synchronisation (+)]. Δ = latency (ms) and peak (%) of difference in induced activity from T1 to T2 for E-TW: 0–400 ms (left) and L-TW: 400–800 ms (right)
Evoked power (µ2)
Valence
Main effect of TW (F(1, 46) = 4.446, p = .04), and TW*Valence (F(1, 46) = 7.505, p = .002), Time*TW*Condition (F(1, 46) = 4.115, p = .05), Time*Condition*Valence (F(2, 92) = 4.683, p = .02), interactions were found. Moreover, a Time*Condition*Valence*Medication (F(2, 92) = 5.169, p = 01) interaction was found, although follow-up posthoc tests did not reveal significant differences. Furthermore, no main effect of Group (F(1, 46) = .229, p = .64), Medication (p = .633), Sex (p = .97), or Group/Sex interactions were apparent, demonstrating that MBCT exposure did not affect evoked θ-activity.
Self/trait versus object/abstract
There were no significant findings, specifically main effects of Group (p = .88), Medication (p = 72), Sex (p = .68), or Group interactions.
Correlations: clinical data + θ activity
Clinical variables are outlined in Table 1. A Time*Group (F(1, 34) = 6.329, p = .02) interaction for IDS scores was evident. Significant correlations between reduced IDS scores pre-to-post, and increased ERD induced theta activity were evident in the MBCT group only. Specifically, decreased IDS scores correlated with increased ERD [−4.61 % to −8.49 %] for false alarms (FAs) to NoGo Neutral stimuli during the E-TW (r(15) = −.543, p = .04); and increased ERD [1.06 % to −1.08 %] during the E-TW for FAs to Object stimuli (r(15) = −.583, p = .022).
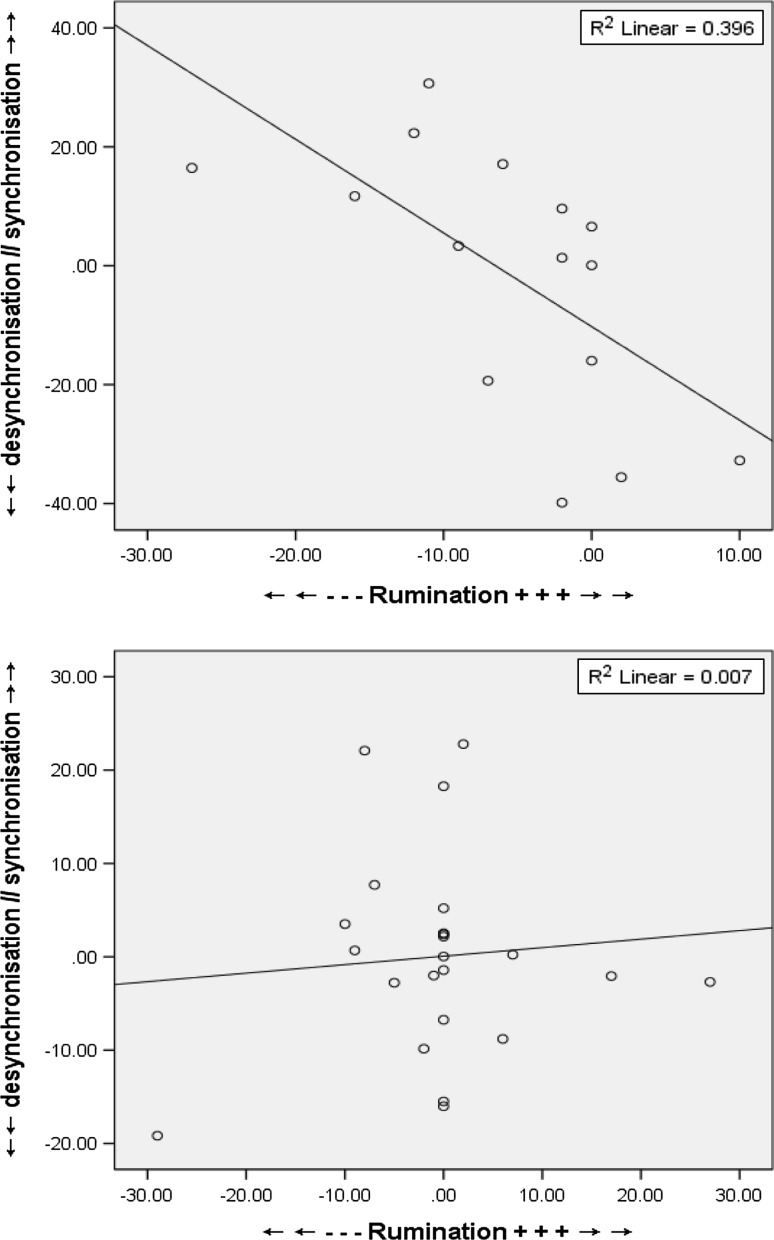
There was no main effect of Group (p = .45), or Time*Group (p = .32) interaction for rumination scores. There was no main effect of medication (p = .95), however, a Time*Medication interaction (F(1, 34) = 5.340, p = .03) showed medication elicited a general decrease in RRS scores pre-to-post compared to non-medicated patients. Reduced RRS scores pre-to-post MBCT exposure were associated with a mean shift from ERS to ERD [.81 % to −.55 %] for FAs to Self stimuli during the E-TW (r(15) = −.629, p = .01), no correlations were evident for the WL (Fig. 6). Albeit, due to the baseline differences between groups for this specific measure, additional to the incomplete available clinical data, it would be wise to approach such correlation with caution.
Fig. 6.
Scatterplots outlining correlations between rumination (RRS scores) increment change and ERD/ERS activity for FAs to NoGo Self trials during the E-TW for MBCT (above) versus WL (below)
Discussion
To our knowledge, this is the first examination of Fm-θ ERD/ERS cortical dynamics relative to MBCT exposure in MDD patients. Contrary to expectations, we did not see enhanced phase-locked evoked oscillatory activity, in line with absent behavioural performance change. As hypothesised, significant findings pertained to non-phase-locked induced neural synchrony, for valence-related and object/self-related matrices during the 400–800 ms post-stimulus onset temporal-window, suggesting mediation of nonlinear (task-independent) synchrony related to underlying θ-oscillations. Specifically, MBCT and WL groups showed diverging directions of induced synchrony; (1) during the early 0–400 ms temporal-window post-stimulus, whereby MBCT patients yielded increased pre-to-post desynchronisation for negative material, more evident in NoGo-T trials compared to Go-T, contrary to an opposite pattern in the WL group; and (2) the late 400–800 ms temporal-window was characterised by a large shift in synchronisation, where T1-to-T2 incremental change controlling for individual baseline variability, revealed synchronisation significantly increased in the MBCT group suggesting overall cortical inhibition, with the opposite direction in the WL group. Modulation in non-linear oscillatory dynamics also correlated with amelioration in depressive and ruminative symptoms. These findings reflect complex and nonlinear (non-phase-locked) mechanisms of action, whose potential exploration and discussion will ensue.
Attention allocation shifting
Non-clinical studies have connected enhanced, particularly frontal, θ-ERS with increased executive functioning, such as optimised working memory and the allocation of attention to task-specific demands (Doppelmayr et al. 2000; McEvoy et al. 2001; Missonnier et al. 2006; Deiber et al. 2007). Moreover, increases in frontal midline theta (Fm-θ) power (increased ERS = increased power), distinct from the regionally ubiquitous θ rhythm associated with low-level alertness or hypnagogic states (Schachter 1977), have been reported in high-order cortical allocation during error processing (Luu et al. 2004; Trujillo and Allen 2007), language processing (Bastiaansen and Hagoort 2003), working memory (Bastiaansen and Hagoort 2003; Gevins et al. 1997; Jensen and Tesche 2002), and increased attentional control via meditation (Aftanas and Golocheikine 2001; Travis and Shear 2010). Related to the latter point, enhanced Fm-θ appears specific to open monitoring meditation, a distinct meditative technique aiming to increase reflective, non-reactive, and non-judgemental moment-to-moment awareness of internal and external experience (Travis and Shear 2010).
Taken together, we can logically premise that the increase in event-related θ-synchrony (ERS) for positive trials during the task-processing and execution epoch (0–400 ms) exclusive to the MBCT, reflected the optimised allocation of attentional resources and higher order cognition specific to positively valenced material. This mechanistic interpretation has empirical and clinical value for future hypotheses and inquiry pertaining to mood disorders underpinned by the over-allocation, or attentional bias, of negative versus hypo-allocation towards positive, stimuli.
Neural disengagement mechanism
Interconnecting to the postulation above, the late 400–800 ms temporal-window was characterised by a large shift in significantly increased synchronisation in the MBCT group suggesting overall cortical inhibition, with the opposite direction of decreased synchronisation in the WL group. Decreased θ-synchrony, ergo inhibition of multi-levelled neuronal assemblies, during the latter 400–800 ms in the MBCT group, presents an attentional disengagement mechanism. We may infer neural disengagement did not occur in the WL patients as mean synchrony dynamics shifted in the opposite direction from T1-to-T2 representing greater cortical excitation, which was highest for negative stimuli. Accordingly, attenuated attention towards negative stimuli alongside reduced inhibition of attention to positive stimuli in patients with a history of depression has been reported post-MBCT (De Raedt et al. 2012), which is in line with our neural disengagement and attention allocation shift (section above) findings and hypothesis here.
The plausibility of a cognitive disengagement mechanism is an important priority in terms of a therapeutic working pathway of MBCT for MDD considering the evidence suggesting attentional biases, or inability to disinhibit attention, towards negative material (Foland-Ross et al. 2013) appear key to the disorder’s aetiology and maintenance. From our findings, we may surmise that MCBT facilitated the top-down resynchronisation of underlying cortical circuitry, i.e. ‘resetting’ of neural synchrony giving rise to a recalibration of cortical state, in turn, promoting optimal readiness for newly incoming task demands and processing whilst adaptively disengaging from previously processed material. Furthermore, the mediatory effects of MBCT on tonic non-phase-locked Fm-θ plausibly connects to DMN dynamics in MDD. That is, frontal-θ serves as an electrocortical index of DMN activity (Scheeringa et al. 2008), and increased DMN activity dominance over the TPN has been linked to higher levels of maladaptive rumination in depression (Hamilton et al. 2011). In this vein, our results showed a shift in non-phase-locked Fm-θ from ERD to ERS for FAs to Self-related material correlated with ameliorated rumination.
Induced Fm-θ and emotion regulation
Functionally, Fm-θ has links to emotion processing, where its neuroanatomical generation has been sourced to the ACC (Pizzagalli et al. 2003), a key structure of the limbic system, alongside interplays with other limbic areas and the PFC in the top-down control of emotion regulation circuitry (Etkin et al. 2011). For example, increased Fm-θ correlates with auditory perception of consonant (harmonic and stable, i.e. likely perceived as beautiful), opposed to dissonant (disharmonic and unstable, i.e. likely perceived as chaotic and unsettling) music perception (Sammler et al. 2007), and during musical mood induction eliciting joyful and pleasurable emotional states (Lin et al. 2010). Enhanced frontal and parietal absolute θ have been associated with mystical states in long-term Carmelite nuns, i.e. during experiences of an all-encompassing union with the ‘divine’ or ‘God’ according to the Carmelite order (Beauregard and Paquette 2008).
Here, the significant shift in θ-ERS (corresponding to increased θ-power) during the latter time window (400–800 ms) pre-to-post MBCT, thus may have also been connected with the ensuing amelioration in depressive symptom severity. For example, an extant study reported enhanced θ-synchronisation during heightened positive emotional states of ‘bliss’ and increased internalised awareness in meditators (Aftanas and Golocheikine, 2001), indicating a modulatory role on positive mood. Interestingly, an RCT found increased appraisal and momentary experience of positive emotions post-intervention in patients with a history of depression and current residual symptoms (Geschwind et al. 2011). Further related evidence pertains to low-anxiety participants exhibiting higher θ-ERS to pleasant compared to threatening images, with the converse observed in high-anxiety traits (Aftanas et al. 2003). Whilst depression and anxiety often share co-morbidity, we stringently screened out anxiety-disorders from our patient sample, so to ‘purely’ evaluate the neural mechanisms of MBCT in MDD. Within this context, enhancement of MBCT-related θ-ERS and its likely implications in mood regulation of our depressed sample appears empirically and clinically apt.
Overall, significantly altered induced synchrony suggests that the mediating disengagement mechanism of the MBCT was via higher-order synchrony of neuronal assemblies associated with non-linear cortical dynamics associated with non-phase-locked (or ‘background’) rhythmic oscillations. In this light, θ-band activity in general may reflect cortical binding properties in the gating of information processing flow in limbic brain regions via the facilitation of transmission between differing structures of the limbic system (Pizzagalli et al. 2003; Vinogradova 1995), pertinent in emotion processing.
Self-related processing
Non-phase-locked induced neural oscillations also appear to have pivotal multi-levelled involvement in self-related processing. For example, gamma (35–44 Hz) band desynchronisation during states of dissolution and reconstitution of a sense of ‘self’ reported during meditation (Lehmann et al. 2001), midline frontal and left hemispheric beta (12–24 Hz) synchronisation associated with intentional inhibition related to self-control and volition (Walsh et al. 2010), medial frontal θ phase resetting correlating to face-specific visual self-representations (Miyakoshi et al. 2010), and greater induced θ-ERS 700–800 ms post stimulus during the memory encoding of trait judgements related to self, compared to a significant other (Mu and Han 2010).
In the latter study (Mu and Han 2010), wavelet analyses disentangled multiple time–frequency dynamics related to self-processing. Greater Fm-θ ERS significantly correlated with a self-reference effect during memory retrieval, suggesting that non-phase-locked cortical synchrony is engaged during self-reflexive cognition, and that differing oscillatory bandwidths are involved at discrete stages of self-processing, whereby θ-band specifically engages synchronous binding properties enabling the integration of cognitive and affective factions during self-related processing. A related study found enhanced θ-power emitted across the entire scalp when participants were asked to ruminate over an issue of self-related/personal meaning, compared to nominal subject matter (Andersen et al. 2009). We found a correlation between shifting ERS to ERD (or declining ERS) during the 0–400 ms window and decreased rumination post-MBCT. Furthermore, increased ERS (corresponding to increased θ-power) was also evident for FAs to self-related stimuli during the post-processing phase of 400–800 ms. This pattern potentially implies MBCT facilitated self-related task-processing whilst simultaneously having a regulatory effect particularly to salient errors/false-alarms, serving to disengage prolonged maladaptive self-related processing. In line with our earlier purported hypothesis; that increased synchrony at later processing stages may represent a neural disengagement mechanism; plausibly constitutes the proposed regulatory system so that self-related processing remained adaptive.
Monoamine synthesis and θ-dynamics
From an overarching neurophysiological perspective, neural θ binding properties admissibly have an intrinsic relationship with neurotransmission interplays. For example, norepinephrine reuptake inhibitor antidepressant medications have shown to increase induced θ-synchronisation of the septo-hippocampal system enacting a mediatory role on various functions pertaining to this limbic circuitry (Hajós et al. 2003), not replicated with serotonin reuptake inhibitor pharmacology, suggesting that the efficacy of both medications have differing treatment pathways. We may extend that a viable therapeutic mechanism of MBCT targets the norepinephrine system, whereby norepinephrine neurons arise from cell bodies in the brain stem, the locus coeruleus, and project to the frontal cortical and limbic structures, enacting key regulatory roles on primary depressive symptoms (Morey and Briley 2011). Furthermore, as glutaminergic neurons activate the locus coeruleus, it is postulated that noradrenergic deficits in MDD are epiphenomenal to chronic elevated levels of glutaminergic input into the locus coeruleus associated with norepinephrine depletion (Paul and Skolnick 2003), contributing to the manifestation of depressive symptomatology. Antidepressant pharmacology reducing glutaminergic activity show mood stabilising effects in patients with depression (Krystal et al. 2002). Cortical glutamine concentrations also have a functional relationship with neocortical θ-activity, where glutaminergic cells modulate θ-oscillations and subsequent coherent processing and plasticity across distributed neural networks during cognitive and behavioural processing (Gallinat et al. 2006). A pertinent limitation of this study is the lack of an active control to directly measure neuroamine changes. Hence, we can only present inferential conjectures regarding possible modulatory effects of MBCT on underlying neurotransmission systems from Fm-θ activity changes. Based on the inherent functional interconnection between neurotransmission and EEG oscillations, this hypothesis provides the impetus for future investigations into multi-levelled treatment pathways of MBCT, which may impact psychopathologies differently based on their distinct neuromodulatory signatures. Further important limitations pertain to the lack of a fully randomised procedure, and importantly, the incomplete clinical and behavioural data, enforces us to interpret the presented significant correlations with considerable caution.
Summary
MBCT modulated synchrony and stability of underlying cortical circuitry related to tonic non-phase-locked Fm-θ oscillations, inferring a non-linear multi-dimensional action pathway. Specifically, enhanced Fm-θ synchronisation during the latter 400–800 ms epoch plausibly suggests MBCT facilitated a disengagement mechanism related to cognitive and emotional processing, serving to functionally recalibrate underlying neuronal assemblies towards optimal readiness for new discrete processing devoid of interference from previously processed, particularly negative, material.
Acknowledgments
This work was supported by the BrainGain SmartMix Programme for the Netherlands Ministry of Economic Affairs and Netherlands Ministry of Education, Culture and Science, funded by the Organisation for Scientific Research (NWO); and the Netherlands Institute for Advanced Study in the Humanities and Social Sciences. Much appreciation and gratitude to all those who participated in the experiments. We thank Addy de Graaf for assistance with research co-ordination; Magdalena Kowalczuk and Katrin Scheibe for help with data collection; Sietske Heusinveld, Joyce Besselink, Danique Smeijers, Irma Veliscek-van Maren, and the Radboud University Medical Centre for Mindfulness team for their generous assistance with patient recruitment.
Conflict of interest
None.
Abbreviations
- ACC
Anterior cingulate cortex
- Ag/AgCl
Silver/silver-chloride
- CBT
Cognitive Behavioural Therapy
- CD
Currently depressed
- CDS
Cambridge Depersonalization Scale
- CMO
Commissie Mensgebonden Onderzoek
- DMN
Default-mode network
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Health Disorders (version IV-TR)
- ECT
Electroconvulsive therapy
- EEG
Electroencephalography/electroencephalographic
- EN
Emotional numbing
- ERD/ERS
Event-related (de-)synchronization
- ERD
Event-related desynchronization
- ERP
Event-related potential
- ERS
Event-related synchronization
- FA
False alarm (i.e. response to NoGo stimuli)
- FFMQ
Five Facet Mindfulness Questionnaire
- Fm-θ
Front midline theta
- ICA
Independent Component Analysis
- IDS
Inventory of Depressive Symptomatology
- ISI
Inter-stimulus-interval
- MBCT
Mindfulness-Based Cognitive Therapy
- MDD
Major Depressive Disorder
- MEG
Magnetoencephalography
- mOFC
Medial orbitofrontal cortex
- rACC
Rostral ACC
- PFC
Prefrontal cortex
- RCT
Randomized controlled trial
- RD
Remitted depressed
- Rm-ANOVA
Repeated measures-Analysis of variance
- RRS
Ruminative Response Scale
- SCS
Self-Compassion Scale
- STAI—S/T
State-trait anxiety inventory (state/trait)
- TPN
Task-positive network
- TW
Time window: E/L = Early [0–400 ms]/Late [400–800 ms]
- WL
Wait-list
Units of measurement
- dB
Decibel
- Hz
Hertz
- KΩ
Kilo-ohm
- ms
Millisecond
- µV
Microvolt
References
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high resolution EEG investigation of meditation. Neurosci Lett. 2001;310:57–60. doi: 10.1016/S0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Aftanas LI, Pavlov SV, Reva NV, Varlamov AA. Trait anxiety impact on the EEG theta band power changes during appraisal of threatening and pleasant visual stimuli. Int J Psychophysiol. 2003;50:205–212. doi: 10.1016/S0167-8760(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Andersen SB, Moore RA, Venables L, Corr PJ. Electrophysiological correlates of anxious rumination. Int J Psychophysiol. 2009;71:156–169. doi: 10.1016/j.ijpsycho.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Arnold JF, Fitzgerald DA, Fernández G, Rijpkema M, Rinck M, Eling PA, Becker ES, Speckens A, Tendolkar I. Rose or black-coloured glasses? Altered neural processing of positive events during memory formation is a trait marker of depression. J Affect Disord. 2011;131:214–223. doi: 10.1016/j.jad.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Lykins E, Button D, Krietemeyer J, Sauer S, Walsh E, Duggan D, Williams MG. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15:329–342. doi: 10.1177/1073191107313003. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window of the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/S0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Paquette V. EEG activity in Carmelite nuns during a mystical experience. Neurosci Lett. 2008;444:1–4. doi: 10.1016/j.neulet.2008.08.028. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Baert S, Demeyer I, Goeleven E, Raes A, Visser A, Wysmans M, Jansen E, Schacht R, Van Aalderen JR, Speckens A. Changes in attentional processing of emotional information following mindfulness-based cognitive therapy in people with a history of depression: towards an open attention for all emotional experiences. Cogn Ther Res. 2012;36:612–620. doi: 10.1007/s10608-011-9411-x. [DOI] [Google Scholar]
- Deiber MP, Missonnier P, Bertrand O, Gold G, Fazio-Costa L, Ibanez V. Distinction between perceptual and attentional processing in working memory tasks: a study of phase-locked and induced oscillatory brain dynamics. J Cogn Neurosci. 2007;19:158–172. doi: 10.1162/jocn.2007.19.1.158. [DOI] [PubMed] [Google Scholar]
- Demeyer I, De Lissnyder E, Koster EHW, De Raedt R. Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: a prospective study in remitted depressed adults. Behav Res Ther. 2012;50:292–297. doi: 10.1016/j.brat.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Stadler W, Rohm D. The time locked theta response reflects interindividual differences in human memory performance. Neurosci Lett. 2000;278:141–144. doi: 10.1016/S0304-3940(99)00925-8. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2008;22:716–721. doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Hamilton PJ, Joorman J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in Major Depression. Psychol Sci. 2013;24:334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Kunz D, Senkowski D, Kienast T, Seifert F, Schubert F, Heinz A. Hipocampal glutamine concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology. 2006;187:103–111. doi: 10.1007/s00213-006-0397-0. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Drukker M, van Os J, Wichers M. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: a randomised controlled trial. J Consult Clin Psychol. 2011;79:618–628. doi: 10.1037/a0024595. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practise. Cereb Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gold C, Fachner J, Erkkilä J. Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scand J Psychol. 2013;54:118–126. doi: 10.1111/sjop.12022. [DOI] [PubMed] [Google Scholar]
- Graimann B, Pfurthscheller G. Quantification and visualisation of event-related changes in oscillatory brain activity in the time-frequency domain. In: Neuper C, Klimesch W, editors. Event-related dynamics of brain oscillations. Amsterdam: Elsevier; 2006. pp. 79–97. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, David DJ, Samuels BA, David I, Gardier AM, Guiard BP (2012) Non-response to initial antidepressant therapy. In: Rossi GP (ed) Psychology—selected papers technical. doi:10.5772/37783
- Hajós M, Hoffmann WE, Robinson DD, Yu JH, Hajós-Korcsok E. Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology. 2003;28:857–864. doi: 10.1038/sj.npp.1300116. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nat Rev. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikman P, Salmelin R, Mäkelä JP, Hari R, Katila H, Kuoppasalmi K. Relation between frontal 3-7 Hz MEG activity and the efficacy of ECT in Major depression. J ECT. 2001;17:136–140. doi: 10.1097/00124509-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Hermans D, De Houwer J. Affective and subjective familiarity ratings of 740 Dutch words. Psychol Belg. 1994;34:115–139. [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Howells FM, Ives-Deliperi VL, Horn NR, Stein DJ. Mindfulness based cognitive therapy improves frontal control in bipolar disorder: a pilot EEG study. BMC Psychiatry. 2012;12:15. doi: 10.1186/1471-244X-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kalcher J, Pfurtscheller G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalogr Clin. 1995;94:381–384. doi: 10.1016/0013-4694(95)00040-6. [DOI] [PubMed] [Google Scholar]
- Kenny M, Williams JMG. Treatment resistant depressed patients show a good response to mindfulness-based cognitive therapy. Behav Res Ther. 2007;45:617–625. doi: 10.1016/j.brat.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V, Mahoney C, Kennedy S, Evans K. EEG correlates of acute and chronic paroxetine treatment in depression. J Affect Disord. 2002;69:241–249. doi: 10.1016/S0165-0327(01)00308-1. [DOI] [PubMed] [Google Scholar]
- Korb AS, Hunter AM, Cook IA, Leuchter AF. Rostral anterior cingulate cortex theta current density and response to antidepressants and placebo in major depression. Clin Neurophysiol. 2009;120:1313–1319. doi: 10.1016/j.clinph.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamine and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Faber PL, Achermann P, Jeanmonod D, Gianotti LRR, Pizzagalli D. Brain sources of EEG gamma frequency during volitionally meditation-induced, altered states of consciousness, and experience of the self. Psychiatry Res Neuroimaging. 2001;108:111–121. doi: 10.1016/S0925-4927(01)00116-0. [DOI] [PubMed] [Google Scholar]
- Lin YP, Duann JR, Chen JH, Jung TP. Electroencephalographic dynamics of musical emotion perception revealed by independent spectral components. NeuroReport. 2010;21:410–415. doi: 10.1097/WNR.0b013e32833774de. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Pellouchoud E, Smith ME, Gevins A. Neurophysiological signals of working memory in normal aging. Brain Res Cogn Brain Res. 2001;11:363–376. doi: 10.1016/S0926-6410(01)00009-X. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, O’Donovan C. The human cost of not achieving full remission in depression. Can J Psychiatry. 2004;49:10S–16S. [PubMed] [Google Scholar]
- Missonnier P, Deiber MP, Gold G, Millet P, Gex-Fabry Pun M, Fazio-Costa L. Frontal theta event-related synchronization: comparison of directed attention and working memory load effects. J Neural Transm. 2006;113:1477–1486. doi: 10.1007/s00702-005-0443-9. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Miyakoshi M, Kanayama N, Iidaka T, Ohira H. EEG evidence of face-specific visual self-representation. Neuroimage. 2010;50:1666–1675. doi: 10.1016/j.neuroimage.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Morey C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7:9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Han S. Neural oscillations involved in self-referential processing. Neuroimage. 2010;53:757–768. doi: 10.1016/j.neuroimage.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Mӧller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Neff KD. Development and validation of a scale to measure self-compassion. Self Ident. 2003;2:223–250. doi: 10.1080/15298860309027. [DOI] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J Pers Soc Psychol. 1991;61:115–121. doi: 10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann NY Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes T, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/S0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sammler D, Grigutsch M, Fritz T, Koelsch S. Music and emotion: electrophysiological correlates of the processing of pleasant and unpleasant music. Psychophysiology. 2007;44:293–304. doi: 10.1111/j.1469-8986.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- Schachter DL. EEG theta waves and psychological phenomena: a review and analysis. Biol Psychol. 1977;5:47–82. doi: 10.1016/0301-0511(77)90028-X. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MCM, Petersen KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression, 2nd edn. New York: The Guildford Press; 2012. [Google Scholar]
- Sierra M, Berrios G. The Cambridge depersonalisation scale: a new instrument for the measurement of depersonalisation. Psychiatry Res. 2000;93:153–164. doi: 10.1016/S0165-1781(00)00100-1. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Teasdale JD, Segal ZW, Williams JMG, Ridgeway VA, Souslby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037/0022-006X.68.4.615. [DOI] [PubMed] [Google Scholar]
- Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19:1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- van Aalderen JR, Donders ART, Giommi F, Spinhoven P, Barendregt HP, Speckens AEM. The efficacy of mindfulness-based cognitive therapy in recurrent depressed patients with and without a current depressive episode: a randomized controlled trial. Psychol Med. 2012;42:989–1001. doi: 10.1017/S0033291711002054. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Expression, control, and probable functional significance of the neuronal theta-rhythm. Prog Neurobiol. 1995;45:523–583. doi: 10.1016/0301-0082(94)00051-I. [DOI] [PubMed] [Google Scholar]
- Walsh E, Kühn S, Brass M, Wenke D, Haggard P. EEG activations during intentional inhibition of voluntary action: an electrophysiological correlate of self-control? Neuropsychologia. 2010;48:619–626. doi: 10.1016/j.neuropsychologia.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Duggan DS, Crane C, Fennell MJV. Mindfulness-based cognitive therapy for prevention of recurrence of suicidal behaviour. J Clin Psychol. 2006;62:201–210. doi: 10.1002/jclp.20223. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Alatiq Y, Crane C, Barnhofer T, Fennell MJV, Duggan DS, Hepburn S, Goodwin GM. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J Affect Disord. 2008;107:275–279. doi: 10.1016/j.jad.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]