Abstract
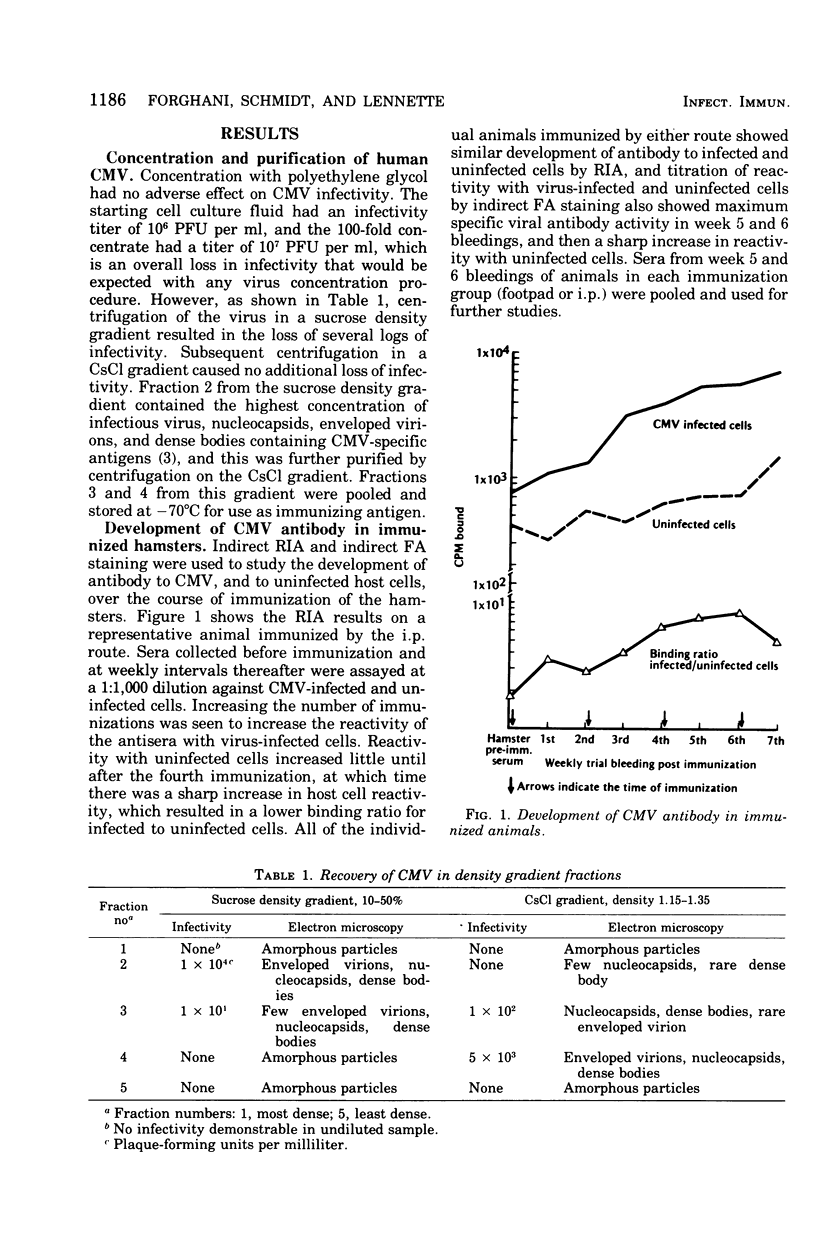
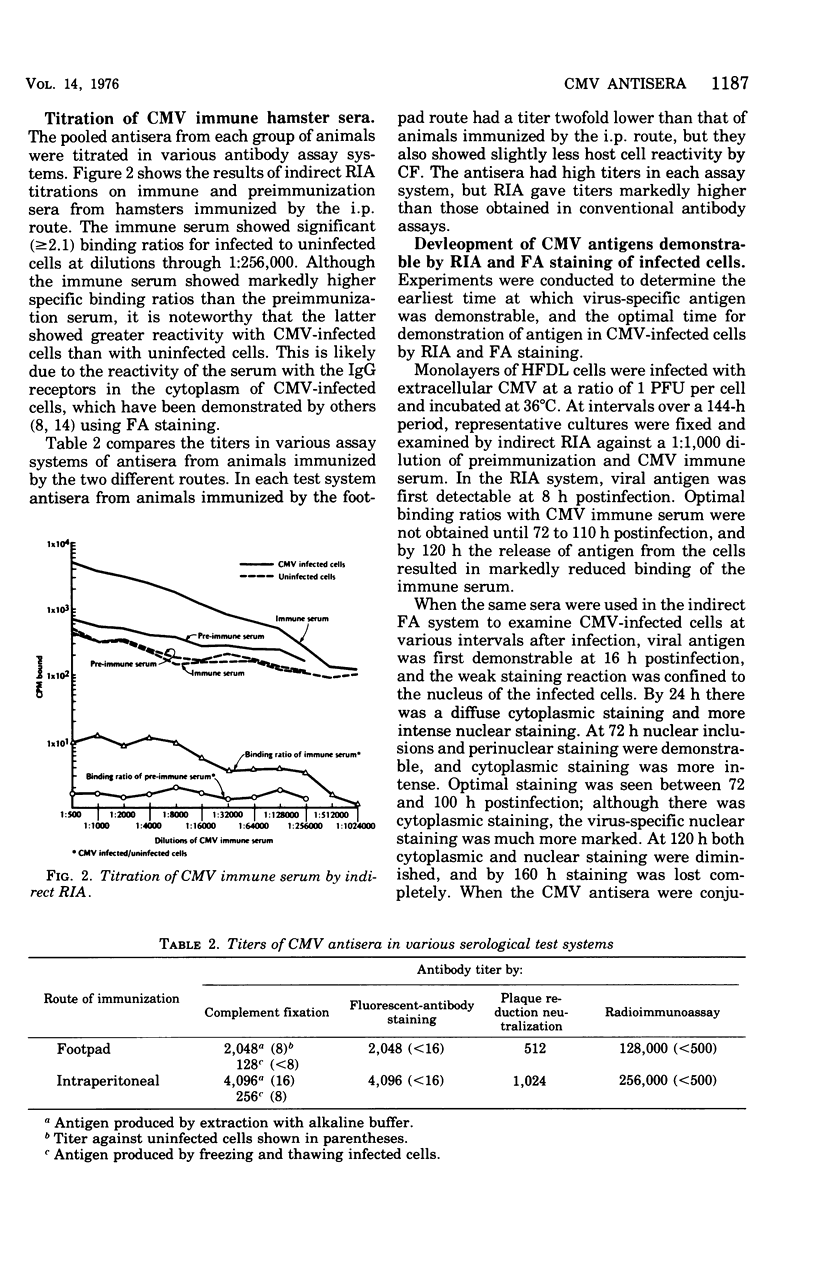
Hamsters immunized with human cytomegalovirus (CMV) concentrated and purified by polyethylene glycol precipitation and density gradient centrifugation produced antisera with high titers of specific viral antibody, and which showed no significant reactivity with human host cell components. The antisera had high titers of CMV antibody in complement fixation, indirect fluorescent-antibody (FA), and neutralization tests, but titers obtained by indirect radioimmunoassay (RIA) were markedly higher. The antisera were used to follow the development of CMV antigen in infected host cells by indirect RIA and indirect FA staining. Virus-specific antigen was first detectable by RIA at 8 h after infection, and by FA staining at 16 h; cells contained optimal amounts of antigen for RIA and FA assays at 72 to 100 h postinfection. Immune globulins from the antisera were labeled with 125I for use in direct RIA. The labeled globulins gave highly specific reactions with CMV-infected cells, including those infected with low-passage isolates, and showed no reactivity with cells infected with other human herpesviruses or certain other human viruses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K. Serologic differentiation of human cytomegalovirus strains using rabbit hyperimmune sera. Brief report. Arch Gesamte Virusforsch. 1971;33(1):187–191. doi: 10.1007/BF01254177. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Rose J. A., Rabson A. S., Bond H. E., Hall W. T. Propagation and purification of high-titer human cytomegalovirus. Appl Microbiol. 1971 Nov;22(5):914–918. doi: 10.1128/am.22.5.914-918.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Kanich R. E., Almeida J. D. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J Virol. 1972 Oct;10(4):766–775. doi: 10.1128/jvi.10.4.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer N. E., Schmidt N. J., Jensen F., Hoffman M., Oshiro L. S., Lennette E. H. Complement-fixing antibody in human sera reactive with viral and soluble antigens of cytomegalovirus. J Clin Microbiol. 1975 Mar;1(3):262–267. doi: 10.1128/jcm.1.3.262-267.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Solid phase radioimmunoassay for identification of Herpesvirus hominis types 1 and 2 from clinical materials. Appl Microbiol. 1974 Oct;28(4):661–667. doi: 10.1128/am.28.4.661-667.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Solid phase radioimmunoassay for typing herpes simplex viral antibodies in human sera. J Clin Microbiol. 1975 Nov;2(5):410–418. doi: 10.1128/jcm.2.5.410-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Hornberger E., Sakuma S., Plotkin S. A. Demonstration of immunoglobulin G receptors induced by human cytomegalovirus. J Clin Microbiol. 1975 Oct;2(4):332–336. doi: 10.1128/jcm.2.4.332-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. J., Minamishima Y., Dresman G. R., Haines H. G., Benyesh-Melnick M. Complement-requiring neutralizing antibodies in hyperimmune sera to human cytomegaloviruses. J Immunol. 1971 Dec;107(6):1618–1630. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haines H. G., Von Essen R., Benyesh-Melnick M., Melnick J. L. Preparation of specific antisera to cytomegaloviruses in goats. Proc Soc Exp Biol Med. 1971 Dec;138(3):846–849. doi: 10.3181/00379727-138-36003. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Huang E. S., Pagano J. S. Antisera to human cytomegaloviruses prepared in the guinea pig: specific immunofluorescence and complement fixation tests. J Immunol. 1974 Feb;112(2):528–532. [PubMed] [Google Scholar]
- Keller R., Peitchel R., Goldman J. N., Goldman M. An IgG-Fc receptor induced in cytomegalovirus-infected human fibroblasts. J Immunol. 1976 Mar;116(3):772–777. [PubMed] [Google Scholar]
- Krech U., Jung M. The development of neutralizing antibodies in guinea pigs following immunization with human cytomegalovirus. Arch Gesamte Virusforsch. 1969;28(2):248–250. doi: 10.1007/BF01249391. [DOI] [PubMed] [Google Scholar]
- Martos L. M., Ablashi D. V., Gilden R. V., Sigüenza R. F., Hampar B. Preparation of immune rabbit sera with neutralizing activity against human cytomegalovirus and varicella-zoster virus. J Gen Virol. 1970;7(2):169–171. doi: 10.1099/0022-1317-7-2-169. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. Preparation of cytomegalovirus immune serum in rabbits with alkaline-extracted virus. Acta Pathol Microbiol Scand. 1968;72(2):345–346. doi: 10.1111/j.1699-0463.1968.tb01348.x. [DOI] [PubMed] [Google Scholar]
- RIGGS J. L., LOH P. C., EVELAND W. C. A simple fractionation method for preparation of fluorescein-labeled gamma globulin. Proc Soc Exp Biol Med. 1960 Dec;105:655–658. doi: 10.3181/00379727-105-26207. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Lennette E. H. Plaque reduction neutralization test for human cytomegalovirus based upon enhanced uptake of neutral red by virus-infected cells. J Clin Microbiol. 1976 Jul;4(1):61–66. doi: 10.1128/jcm.4.1.61-66.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Woodie J. D., Ho H. H. Immunofluorescent staining in the laboratory diagnosis of varicella-zoster virus infections. J Lab Clin Med. 1965 Sep;66(3):403–412. [PubMed] [Google Scholar]
- Waner J. L. Partial characterization of a soluble antigen preparation from cells infected with human cytomegalovirus: properties of antisera prepared to the antigen. J Immunol. 1975 May;114(5):1454–1457. [PubMed] [Google Scholar]



