Abstract
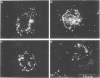
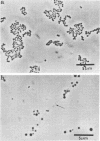
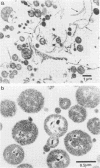
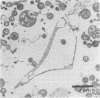
Evidence obtained using nonimmunosuppressed and newborn mice suggests that the immune response of the host plays a role in the rapid removal of a physiologically isotonic L-form of Streptococcus pyogenes, since its inability to persist in vivo was not due to osmotic lysis. With mice immunosuppressed with methylprednisolone sodium succinate, viability and detection of this L-form by fluorescent antibody was prolonged for at least 2 weeks, the approximate duration of immunosuppression in these mice. However, heat-killed L-form cells only persisted for 3 days in such mice. Therefore, persistence of a viable L-form in these treated mice was not simply due to the lack of removal of L-forms by a compromised host. At no time was there any indication of illness in nonimmunosuppressed or immunosuppressed mice after L-form injection, and all internal organs, when examined macroscopically, remained normal. Thus, overt pathogenesis was not a characteristic of this L-form in a suitable host even when its immune response had been compromised. The microscopic morphology of the L-form after isolation from immunosuppressed mice changed drastically. It was typically micrococcal in appearance and exemplified the cellular variability achievable by this organism in vivo. Also, streptolysin S production was increased markedly by passage of the L-form in immunosuppressed mice. However, M protein, as a cellular component, was not detected serologically, nor was any reformation of a rigid cell wall apparent by electron microscopy after isolation of this streptococcal L-form from such mice.
Full text
PDF


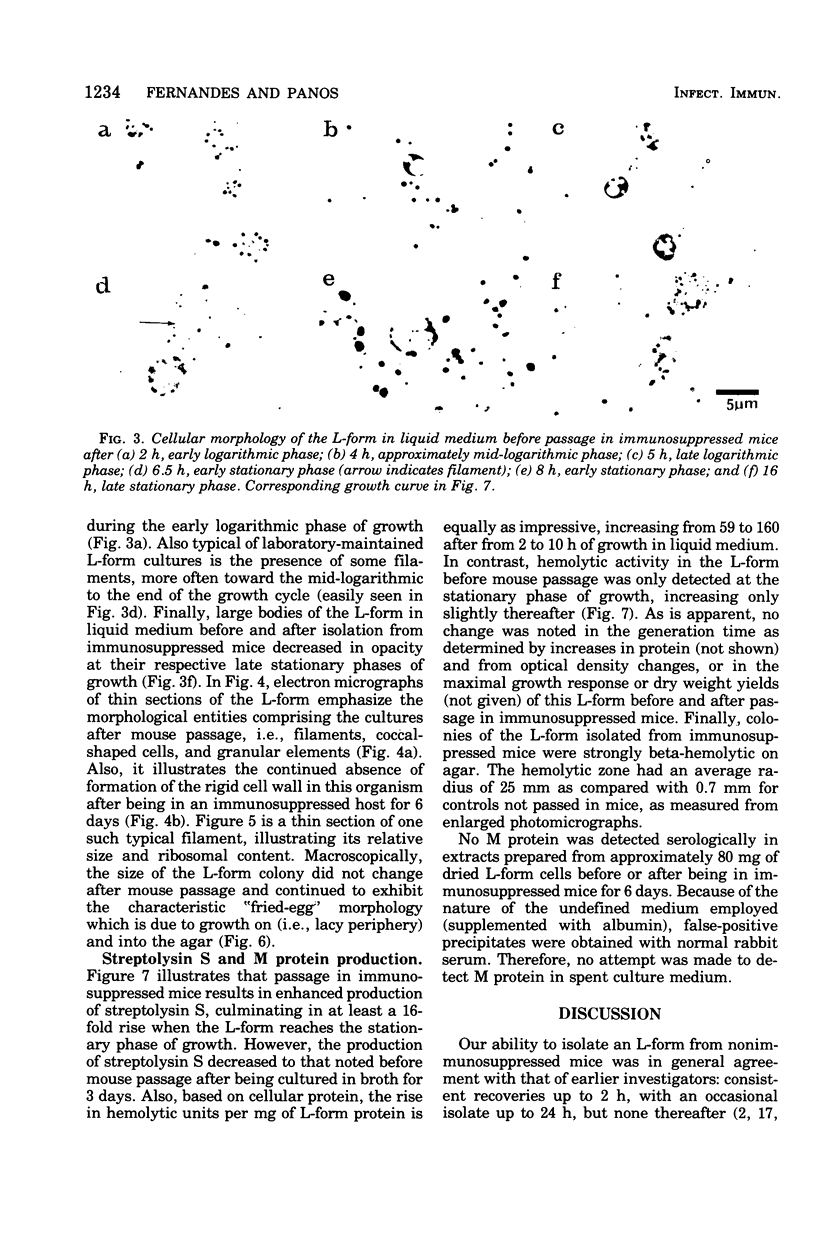
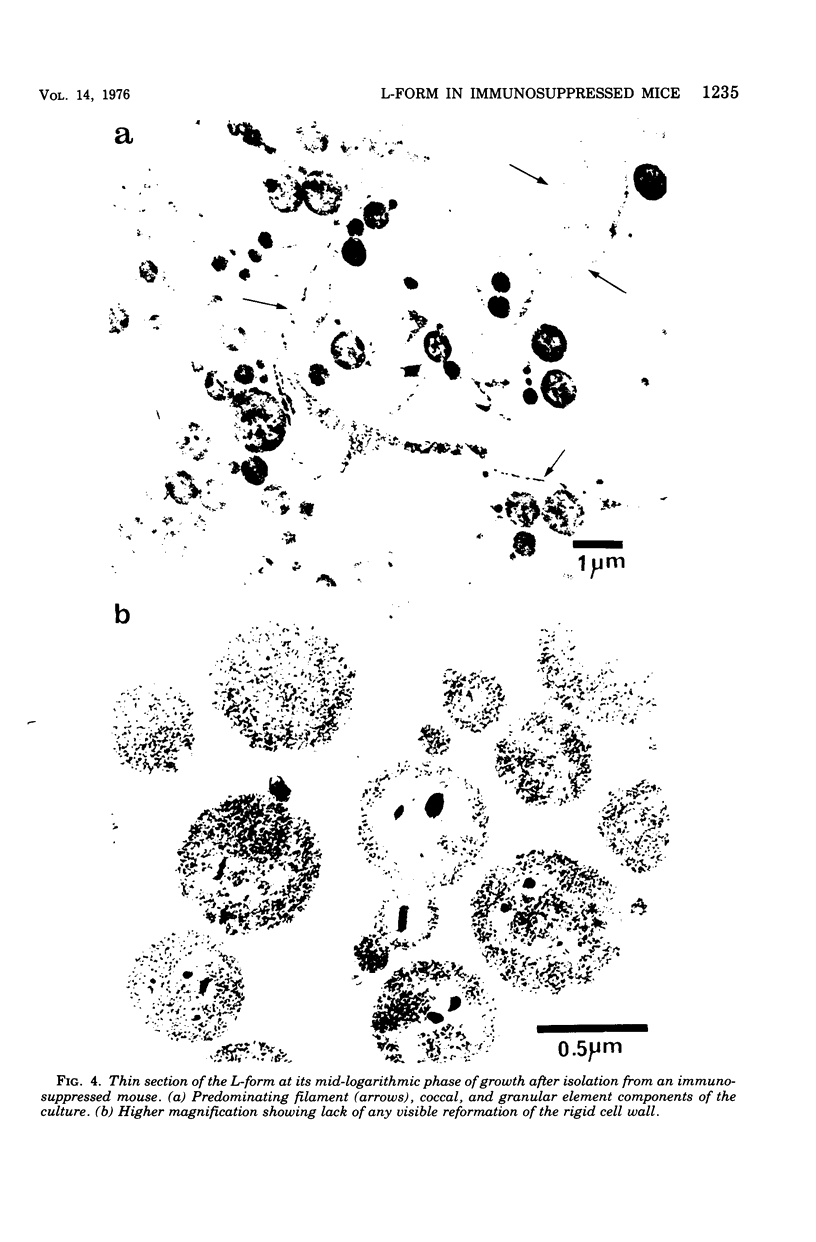

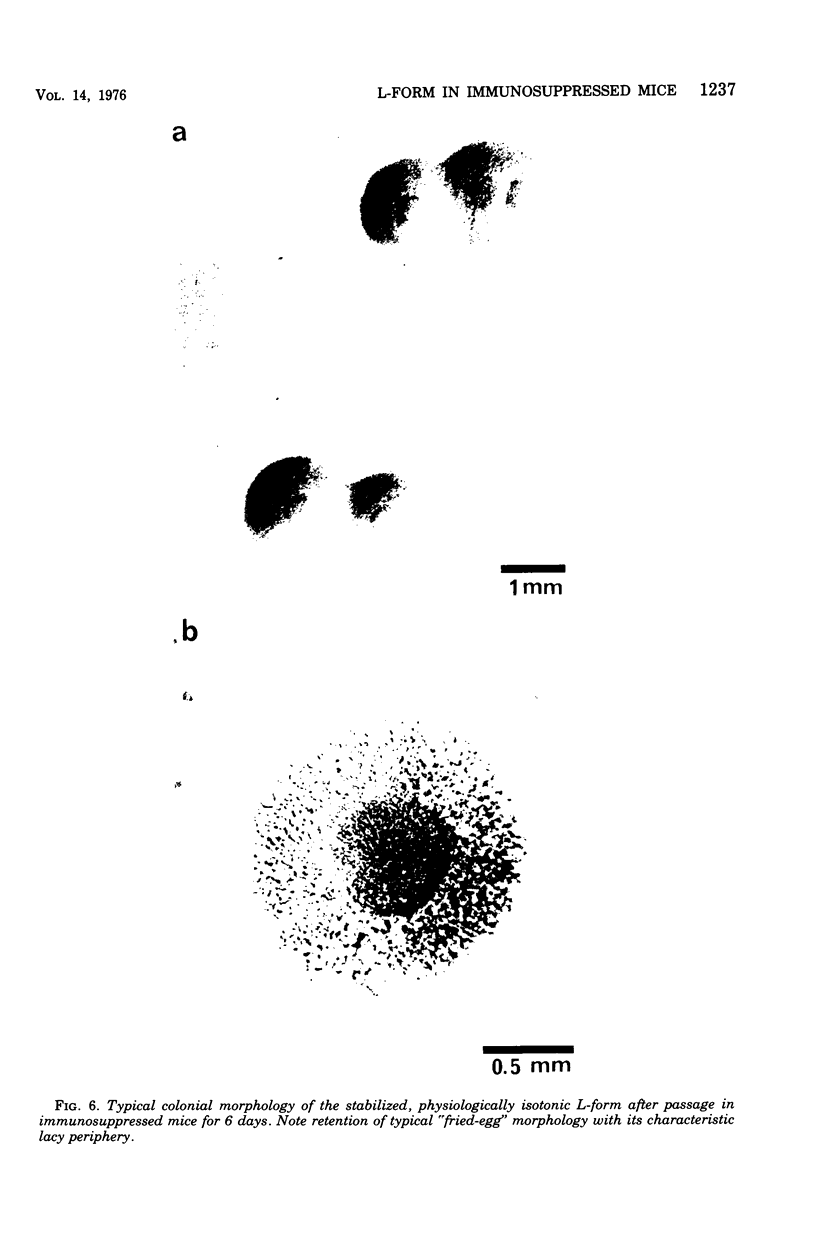
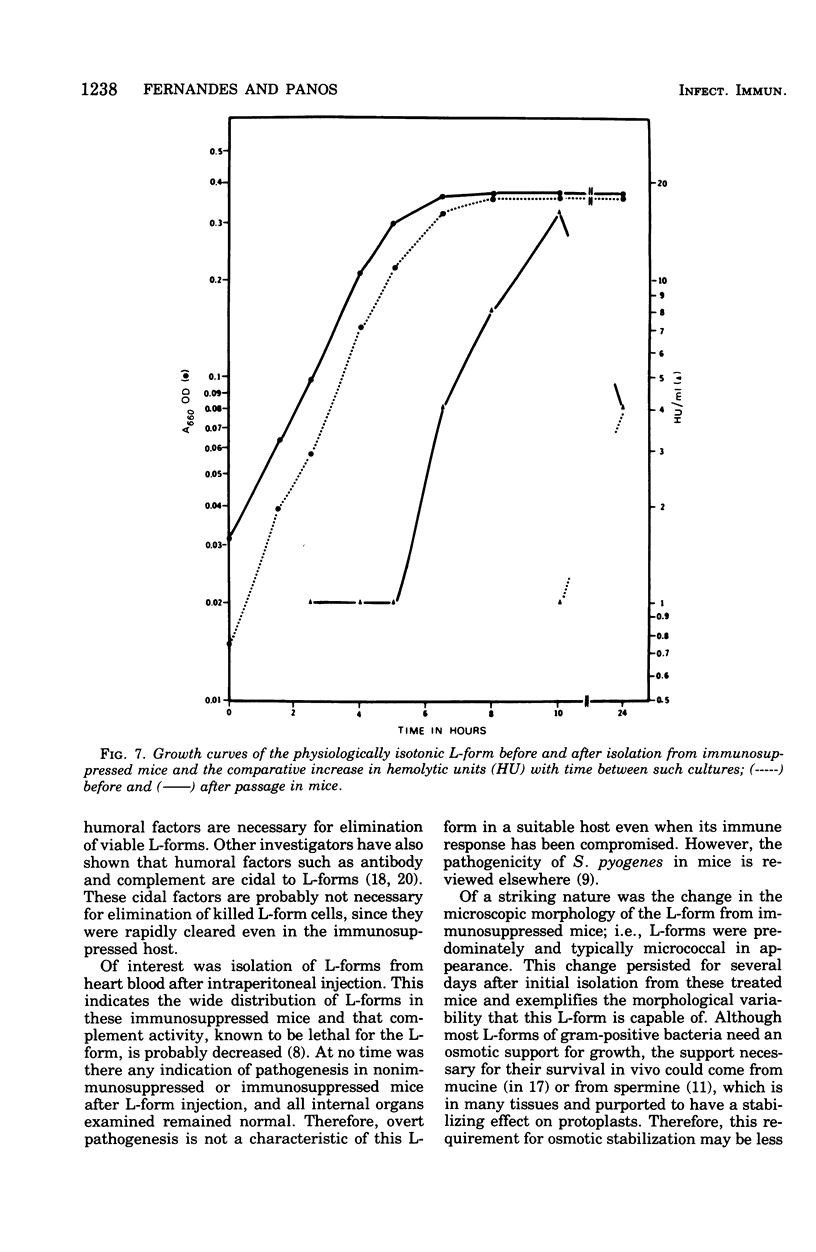


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chevion M., Panos C., Paxton J. Membrane studies of Streptococcus pyogenes and its L-form growing in hypertonic and physiologically isotonic media. An electron spin resonance spectroscopy approach. Biochim Biophys Acta. 1976 Mar 5;426(2):288–301. doi: 10.1016/0005-2736(76)90338-2. [DOI] [PubMed] [Google Scholar]
- Clasener H. A., Ensering H. L., Hijmans W. Persistance in mice of the L-phase of three streptococcal strains adapted to physiological osmotic conditions. J Gen Microbiol. 1970 Aug;62(2):195–202. doi: 10.1099/00221287-62-2-195. [DOI] [PubMed] [Google Scholar]
- Cohen J. O., Pine L. Quantitative aspects of the M protein capillary precipitin test. Appl Microbiol. 1968 Jan;16(1):122–127. doi: 10.1128/am.16.1.122-127.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- DENNY F. W., Jr, THOMAS L. Persistence of group A streptococci in tissues of rabbits after infection. Proc Soc Exp Biol Med. 1955 Feb;88(2):260–263. doi: 10.3181/00379727-88-21557. [DOI] [PubMed] [Google Scholar]
- DIENES L., WEINBERGER H. J. The L forms of bacteria. Bacteriol Rev. 1951 Dec;15(4):245–288. doi: 10.1128/br.15.4.245-288.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- GLASER R. J., BERRY J. W., LOEB L. H., WOOD W. B., Jr The effect of cortisone in streptococcal lymphadenitis and pneumonia. J Lab Clin Med. 1951 Sep;38(3):363–373. [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Sep;126(3):294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. STABILIZATION OF STREPTOCOCCUS FAECALIS PROTOPLASTS BY SPERMINE. J Bacteriol. 1964 Nov;88:1416–1420. doi: 10.1128/jb.88.5.1416-1420.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASS E. H., FINLAND M. Corticosteroids and infections. Adv Intern Med. 1958;9:45–80. [PubMed] [Google Scholar]
- LEEDOM J. M., BARKULIS S. S. Studies on virulence of group A beta-hemolytic streptococci. J Bacteriol. 1959 Nov;78:687–694. doi: 10.1128/jb.78.5.687-694.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leon O., Panos C. Adaptation of an osmotically fragile L-form of Streptococcus pyogenes to physiological osmotic conditions and its ability to destroy human heart cells in tissue culture. Infect Immun. 1976 Jan;13(1):252–262. doi: 10.1128/iai.13.1.252-262.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Ratner H. B., Bryant R. E., Rosenthal A. S., Koenig M. G. An antibody-complement system in human serum lethal to L-phase variants of bacteria. J Infect Dis. 1972 Mar;125(3):231–242. doi: 10.1093/infdis/125.3.231. [DOI] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. The reactivity of serum against protoplasts and spheroplasts. J Immunol. 1966 Jul;97(1):46–51. [PubMed] [Google Scholar]
- NICHOLS R. L., McCOMB D. E. Immunofluorescent studies with trachoma and related antigens. J Immunol. 1962 Oct;89:545–554. [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Rickles N., Zilberstein Z., Kraus S., Arad G., Kaufstein M., Ginsburg I. Persistence of group A streptococci labeled with fluorescein isothiocyanate in inflammatory sites in the heart and muscle of mice and rabbits. Proc Soc Exp Biol Med. 1969 Jun;131(2):525–530. doi: 10.3181/00379727-131-33917. [DOI] [PubMed] [Google Scholar]
- Schmitt-Slomska J., Sacquet E., Caravano R. Group A streptococcal L forms. I. Persistence among inoculated mice. J Bacteriol. 1967 Jan;93(1):451–455. doi: 10.1128/jb.93.1.451-455.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., BECHER B., THOMAS L. STUDIES ON LYSOSOMES. V. THE EFFECTS OF STREPTOLYSINS AND OTHER HEMOLYTIC AGENTS ON ISOLATED LEUCOCYTE GRANULES. J Cell Biol. 1964 Jul;22:115–126. doi: 10.1083/jcb.22.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., KEISER H., BERNHEIMER A. W. STUDIES ON LYSOSOMES. III. THE EFFECTS OF STREPTOLYSINS O AND S ON THE RELEASE OF ACID HYDROLASES FROM A GRANULAR FRACTION OF RABBIT LIVER. J Exp Med. 1963 Aug 1;118:205–222. doi: 10.1084/jem.118.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A. D., Hall G. H. Acute post-streptococcal toxaemic renal failure. Br Med J. 1969 Dec 13;4(5684):665–666. doi: 10.1136/bmj.4.5684.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbauer R. H., Gutman L. T., Turck M., Wedgwood R. J., Petersdorf R. G. The role of penicillin-induced bacterial variants in experimental pyelonephritis. J Exp Med. 1967 Apr 1;125(4):607–618. doi: 10.1084/jem.125.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNATHAN E. S., BARKULIS S. S. Effect of some antimetabolites on the production of streptolysin S'. J Bacteriol. 1957 Aug;74(2):151–158. doi: 10.1128/jb.74.2.151-158.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]