ABSTRACT
Matrix metalloproteinases (MMPs) 2 and 9 are zinc-dependent endopeptidases that contribute to the control of breakdown and reconstitution of extracellular matrix under both normal and pathological conditions. The main objective of this study was to identify the presence of MMP-2 and -9 in the mucosa of the small and large intestines of clinically healthy beagle dogs using gelatin zymography technique. Intestinal mucosa samples from four different parts of the intestine (duodenum, jejunum, ileum and colon) were taken from 12 healthy laboratory beagle dogs and examined histologically. Based on WSAVA histology standards, recorded findings of all samples were considered insignificant. Pro-MMP-2 and -9 activities were found in 17/48 (35%) and 25/48 (52%) of the samples, respectively. Among four different parts of the intestine of 12 dogs, the ileum had the highest positivity rates of 7/12 (58.3%) and 8/12 (66.7%) for pro-MMP-2 and -9 activities, respectively. However, statistical analysis showed no significant difference of pro-MMP-2 and -9 activities between the separate parts of the intestine (P>0.05). None of the intestinal samples showed gelatinolytic activity corresponding to the control bands of active MMP-2 and MMP-9. This study showed that pro-MMP-2 and -9 could be detected in the intestinal mucosa of healthy dogs using zymography, which seems to be a useful tool to evaluate the role of MMP-2 and -9 in the pathogenesis of canine chronic enteropathies, including inflammatory bowel diseases.
Keywords: Scanine, intestine, matrix metalloproteinase, zymography
Matrix metalloproteinases (MMPs) are a group of zinc-dependent endopeptidases that contribute to the control of breakdown and reconstitution of the extracellular matrix (ECM) under both normal and pathological conditions [24, 31, 39]. They have been divided into subtypes based on substrate specificity and structural homology including the collagenases, stromelysins, gelatinases, elastases, membrane-type MMPs and others (e.g. matrilysin, enamelysin) [39]. The activity of MMPs is controlled by several types of inhibitors, of which the tissue inhibitors of metalloproteinases (TIMPs) and alpha-macroglobulins are the most important [41]. Among the MMPs, MMP-2 and MMP-9 are also referred to as gelatinases A (72 kDa) and B (92 kDa), respectively. Both MMP-2 and -9 degrade similar substrates, such as gelatin, collagen types IV and V, elastin, laminin, fibronectin and proteoglycans [11, 12, 40]. MMP-2 is most commonly expressed in normal adult tissue and is primarily produced by stromal cells, including fibroblasts, myofibroblasts and endothelial cells [10, 16]. MMP-9 is mainly synthesized by inflammatory cells, particularly polymorphonuclear leukocytes (PMNL, mainly neutrophils and eosinophils) and is found to a lesser extent in monocytes, macrophages, lymphocytes and epithelial cells [10, 12, 14, 15, 23]. In immunohistochemical studies of normal human colon, MMP-2 staining has been observed mainly in stromal cells [10, 16]. Positive staining for MMP-9 was observed in PMNL, epithelial cells and the stroma of normal colon [10, 16, 23, 24]. Under normal conditions, MMPs are present in intestinal tissues at low levels, usually in the pro-form where they are responsible for normal physiological intestinal epithelial-cell turnover [39].
In normal human colon mucosa, pro-MMP-2 and -9 were mostly detected using gelatin zymography assay [2, 10]. Zymography is one of the most common techniques for evaluating samples for gelatinolytic MMPs. It is a gel-based enzymatic assay for detection and semi-quantitative assessment of gelatinolytic enzymes’ activities [3, 21, 35]. An important advantage of zymography is the possibility of identifying both the pro-enzyme and active forms of MMPs in the same assay on the basis of their molecular weight [3, 8, 41].
Recent research in human medicine has focused on the presence and role of MMPs in the intestinal tissue of healthy subjects and patients suffering from inflammatory bowel diseases (IBD) [10, 16, 24]. IBD is a common gastrointestinal disorder in humans and companion animals, such as dogs, and is characterized in both by chronic periods of exacerbation and remission, even when the histologic background of human and canine IBD differs [5, 34]. In humans, MMP-2 and -9 play an important role in the pathogenesis of IBD, and their activities have been determined in both normal and IBD colons [2, 10, 16, 24]. In intestinal IBD tissues, protein levels of MMP-2 and -9 are reported to be increased when determined by either zymography, enzyme-linked immunosorbent assay (ELISA) or western blotting [2, 10, 16].
In dogs, there have been only few studies on MMPs. In healthy dogs, MMP-2 and -9 have so far been determined in cerebrospinal fluid (CSF), aqueous humor and iridocorneal drainage angle tissue [3, 43]. MMP studies in serum and/or tissues of canine patients included several disorders, such as rheumatoid arthritis [6], Alzheimer’s-like disease [20], dilated cardiomyopathy [13], pulmonary eosinophilia [35], myxomatous mitral valve disease [21], mammary tumors [1], osteosarcomas [18], mast cell tumors [19] and other tumors [22]. In dogs, zymography technique was used in all these studies [1, 3, 6, 13, 18,19,20,21,22, 35, 43] to determine MMP-2 and -9 activities. However, immunohistochemistry [1, 22], ELISA [3, 6] and western blotting [35] were also used in some studies. To our knowledge, thus far, there have been no reports about MMP determination in intestinal samples from healthy dogs or dogs suffering from IBD.
Using zymography for MMP detection, the aim of this study was to follow the hypothesis that MMP-2 and -9 are present in the mucosa of the small and large intestines of clinically healthy beagle dogs.
MATERIALS AND METHODS
Samples collection and processing: Tissue samples were taken during post mortem examinations from the intestine of 12 healthy laboratory beagle dogs (8 males/4 females, age 10–13 years), after finishing another non-related study which was approved by the Finnish National Animal Experiment Board (study license No. ESLH-2007-09833/Ym-23). The dogs participated in dietary studies and were kept under the same environmental and nutritional conditions before they needed to be euthanized. They were housed according to European Union guidelines in groups in indoor pens with possibilities to use outdoor runs. The indoor environmental temperature was maintained at a range of 15–24°C. The dogs were exposed to both natural and artificial light (from 07:00 to 16:00). They were fed with a standard commercial diet and were determined to be healthy based on history, physical examination, complete blood count, serum biochemistry and fecal examination. Immediately after euthanasia, the intestine was opened longitudinally and flushed with cold saline. Full-thickness tissue samples were taken from four different parts of the intestine (duodenum, jejunum, ileum and colon) and were snap frozen in liquid nitrogen and stored at −80°C until histological and zymographic analyses. For histologic evaluation, parts of the frozen intestinal tissue samples were later slowly thawed and fixed in 4% formaldehyde solution in phosphate buffered saline (PBS) at 8°C under permanent automatic rotation of the sample tube. Then, the samples were trimmed and paraffin wax embedded. Sections (3–5 µm) were prepared and stained with hematoxylin and eosin for histological examination.
Assessment of intestinal health: All dogs were clinically healthy without any signs of gastrointestinal diseases based on history, physical examination and laboratory examination of feces and blood. Histological assessment of the intestinal samples was performed using the guide lines of the World Small Animal Veterinary Association (WSAVA) Gastrointestinal Standardization Group [7]. In all small bowel samples, 5 morphological features (villous stunting, epithelial injury, crypt distention, lacteal dilation and mucosal fibrosis) and 3 types of infiltrated leukocytes (intraepithelial lymphocytes, lamina propria lymphocytes and lamina propria neutrophils) were selected and scored from 0 to 3 according to the WSAVA standardization guidelines. In the colonic samples, crypt hyperplasia, dilatation and distortion were additionally evaluated. The total scores were classified as insignificant (scores 0–4), mild (scores 5–9), moderate (scores 10–14), severe (scores 15–19) or very severe (scores ≥20).
Sample preparation for zymography: Mucosa samples (50 mg) were taken from the snap frozen intestinal tissues and homogenized for 2 × 50 sec with 5,000 × g in ice-cold extraction buffer (20 µl/mg tissue) containing 50 mM Tris Base, 150 mM NaCl, 10 mM CaCl2, 0.2 mM NaN3 and 0.01% Triton X-100 (pH 7.6) in the presence of EDTA-free protease cocktail tablets (Complete EDTA-free tablets, Roche, Basel, Switzerland) using Precellys 24 ceramic beads (Bertin technologies, Paris, France) [4]. After homogenization, samples were centrifuged at 13,000 × g at 4°C for 10 min, and the supernatants were collected and stored at –80°C for measurement of MMP-2 and MMP-9 [31]. Protein concentrations of the supernatants were measured with bicinchoninic acid protein assay reagents (Pierce, Rockford, IL, U.S.A.).
Zymography: Gelatinolytic activities of MMP-2 and MMP-9 in supernatant were measured by gelatin zymography in mini-gels as previously described in detail [21]. Supernatants were separated by electrophoresis in 11% polyacrylamide gel impregnated with 0.7 mg/ml of gelatin as a substrate (porcine skin gelatin, G-8150, Sigma, St. Louis, MO, U.S.A.) under non-reducing conditions. Each lane of an 11% SDS-polyacrylamide gel was loaded with 20 µl of supernatants containing either 10 µg or 25 µg of total protein mixed with a 10 µl aliquot of loading buffer. All samples were made in duplicate and averaged. Loading buffer consisted of 0.04 g/l bromophenol blue (Art. 8122) (BDH), 20% glycerol and 6% sodium dodecyl sulphate (SDS, Prod. 44244) (BDH) at pH 6.8. Electrophoresis was performed by using a mini-PROTEAN Tetra Cell electrophoresis system (Bio- Rad Laboratories, Hercules, CA, U.S.A.) under a constant current of 60 MA for 10 min and then 30 MA until the bromophenol blue reaches the bottom of the gel.
After electrophoresis, the gels were washed in distilled water and then soaked (2 × 30 min) in renaturing buffer (2.5% Triton X-100) with gentle shaking at room temperature in order to remove the sodium dodecyl sulfate. Then, the gels were soaked in zymogram developing buffer (50 mM Tris Base, pH 7.5 containing 200 mM NaCl, 5 mM CaCl2·2H2O and 0.02% Brilj-35) for 30 min at room temperature, then replaced with fresh developing buffer and incubated for another 18 hr at 37°C. After washing the gels with distilled water 3 times for 10 min, they were stained with PageBlue™ Protein Staining Solution (Fermentas) and stained with gentle agitation for 5 hr. The areas of proteinase activity were visualized as clear bands by washing the gels with distilled water.
As a control, each gel was loaded with a diluted (1:600) recombinant human MMP-2 (9 µl) and -9 (2 µl) (R&D Systems, Minneapolis, MN, U.S.A.), respectively, to determine whether MMPs in canine intestinal tissue are equivalent in molecular size and activity to pro-MMP-2 (72 kDa gelatinase) and pro-MMP-9 (92 kDa gelatinase). For quantification of gelatin degradation, gels were scanned and were assessed by densitometer analysis method creating an arbitrary unit (AU) for each band by calculating the integrated area under each peak (Alpha-imager densitometer, Alpha Innotech, San Leandro, CA, U.S.A.). The activity levels of pro- and active MMP-2 and -9 for each sample were expressed in AU related to the level of pro-MMP-2 of the positive-control standard loaded on each gel. Each band’s activity was reported as the mean of two different measurements of the same sample.
Statistical analysis: The differences between the separate parts of the intestine (duodenum, jejunum, ileum and colon) in their proportions of samples with pro-MMP-2 and -9 activities were analyzed with exact McNemar’s test. Bonferroni adjustment was used to control for multiplicity issues. Data are presented as number (%) or median (range) as appropriate. P values <0.05 were considered statistically significant. All statistical analyses were performed using the SAS 9.3 statistical software (SAS Institute Inc., Cary, NC, U.S.A.).
RESULTS
Histological examination: Based on the histological examination of the intestine, the median total WSAVA score of all samples was 0 (range 0–3) classifying all findings as insignificant. There were no histological abnormalities in the submucosa, the muscularis externa and the serosa in any of the samples.
Zymography: Zymography was performed with 48 samples from four different intestinal areas of 12 healthy dogs. When positive, it showed gelatinolytic activity at the same level as the positive control bands of pro-MMP-2 and -9 and was therefore considered to represent canine pro-MMP-2 and pro-MMP-9 (Fig. 1). Since the majority of samples containing 10 µg of total protein showed no activity (pro-MMP-2: 40/48, 83%; pro-MMP-9: 33/48, 69%), only the results for samples containing 25 µg of total protein are presented in this paper. Pro-MMP-2 and -9 activities were found in 17/48 (35%) and 25/48 (52%) of the samples, respectively. None of the intestinal samples showed gelatinolytic activity corresponding to the control bands of active MMP-2 and MMP-9.
Fig.1.
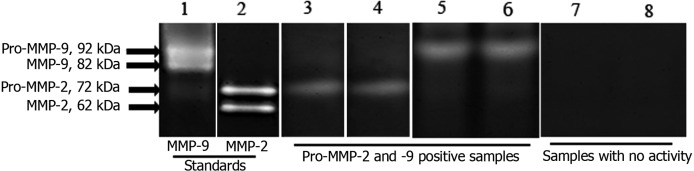
Representative zymogram of protein (25 µg) from intestinal mucosa samples {Pro-MMP-2 and -9 positive samples (lanes 3–6) and samples with no activity (lanes 7 and 8)} and recombinant human pro-MMP-2 (lane 2) and -9 (lane 1).
Results of the McNemar test for statistical analysis showed no significant difference of pro-MMP-2 and -9 activities between the four separate parts of the intestine (duodenum, jejunum, ileum and colon) (P>0.05). However, for pro-MMP-2, the number (and percentage) of positive samples in four different parts of the intestine of 12 dogs were from the highest to the lowest as follows: ileum, 7/12 (58.3%); jejunum, 5/12 (41.7%); duodenum, 3/12 (25%); and colon, 2/12 (16.7%). For pro-MMP-9, ileum had the highest positivity rate (8/12; 66.7%), followed by jejunum (6/12; 50%), duodenum (6/12; 50%) and colon (5/12; 41.7%). From all 48 canine intestinal mucosa samples examined with zymography, no pro-MMP-2 and -9 activity was found in 31/48 (65%) and 23/48 (48%), respectively.
The enzyme activities ranged for pro-MMP-2 between 0.015 and 6.449 AU and for pro-MMP-9 between 0.018 and 5.680 AU. In the examined four different parts of the intestine, the median (range) pro-MMP-2 activity was from the highest to the lowest: ileum, 0.175 (0–3.337) AU; jejunum, 0 (0–6.449) AU; duodenum, 0 (0–4.301) AU; and colon, 0 (0–0.146) AU. The highest pro-MMP-9 activity (median and range) was found in the ileum (0.086 (0–1.443) AU), followed by colon (0.018 (0–1.652) AU), jejunum (0.011 (0–2.810) AU) and duodenum (0 (0–5.680) AU). In the majority of positive samples (36/42; 86%), either pro-MMP-2 or -9 activities were lower than 2 AU throughout the whole intestine (Table 1). Comparably high activities (AU ≥2) of pro-MMP-2 were recorded in two mucosa samples from duodenum and jejunum and one ileum. Two duodenal mucosa samples and one jejunum sample had the highest activities of pro-MMP-9 (Table 1).
Table 1. Distribution of pro-MMP-2 and -9 gelatinolytic activities in mucosa samples from duodenum, jejunum, ileum and colon of 12 healthy dogs AU: arbitrary units, Pro: pro-enzyme.
| Intestinal part | Variable | Dog’s No |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Duodenum | Pro-MMP-2 (AU) | 0 | 0 | 0 | 0 | 4.30 | 0 | 0 | 0 | 0.51 | 1.46 | 0 | 0 |
| Pro-MMP-9 (AU) | 0.23 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 | 5.68 | 5.42 | 0.62 | 0 | |
| Jejunum | Pro-MMP-2 | 0 | 0 | 0 | 0 | 6.45 | 0.58 | 1.53 | 0.23 | 0.23 | 0 | 0 | 0 |
| Pro-MMP-9 | 0.02 | 0 | 0 | 0 | 0 | 0 | 1.09 | 0.18 | 2.81 | 0.02 | 0 | 0.73 | |
| Ileum | Pro- MMP-2 | 0.15 | 0.29 | 0 | 0 | 0 | 0.56 | 0 | 3.34 | 0.20 | 0.50 | 0 | 1.88 |
| Pro-MMP-9 | 0.59 | 0 | 1.44 | 0.06 | 0 | 0.10 | 0 | 0.62 | 0.18 | 0.08 | 0.09 | 0 | |
| Colon | Pro-MMP-2 | 0.15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 |
| Pro-MMP-9 | 1.65 | 0 | 0.14 | 0.09 | 0 | 0 | 0 | 0 | 0.04 | 0.05 | 0 | 0.04 | |
DISCUSSION
This study aimed to detect the presence of MMP-2 and -9 in four different parts of the intestinal mucosa of clinically healthy beagle dogs by using gelatin zymography. The method has been used earlier to study MMP-2 and -9 in intestinal samples from humans [2, 10, 17], but to the best of our knowledge, not yet in samples from canine intestinal mucosa.
In the current study, we used intestinal mucosa samples from 12 clinically healthy beagle dogs. To avoid the unnecessary use of laboratory animals for experimental studies, we sampled intestinal mucosa during necropsy from all 12 dogs after they were euthanized when finishing another non-related study. The collected samples were then stored at –80°C for later research. This approach complies with the principles of replacement, reduction and refinement of animal experiments [9]. It seems unlikely that the previous studies could have interfered with our results since all dogs had the same living and nutritional conditions and were clinically healthy at the time of euthanasia. In addition, the histological examination of the intestinal mucosa was a part of our study to investigate the possibility of subclinical intestinal inflammation which might have influence on the interpretation of the study results.
MMP-2 and -9 were extracted from canine intestinal mucosa according to Castaneda et al. [4]. However, in the current study, Precellys device (Bertin technologies) with tubes containing ceramic beads was used for grinding the mucosa samples. To prevent temperature rising during the lysis process and to protect sensitive molecules from degradation, cold air (–50°C) was sprayed by Cryolys device (Bertin technologies) beside the tubes so that temperature during homogenization remained at approximately 4°C.
Gelatin zymography is a highly sensitive technique and is still widely used in the determination of MMP-2 and -9 in tissue samples. This technique can detect even small amounts of MMP-2 and -9 from tissue-homogenization supernatant [41]. The gelatin zymography used here was described by Rajamäki et al. selecting both, 10 and 25 µg of total protein in the extracted samples [35]. The amount of 10 µg protein is often used in human IBD research for determination of MMPs by gelatin zymography. However, the majority of our samples containing 10 µg of total protein showed no activity. It is likely that the levels of MMP-2 and -9 activities in healthy dogs are too low to be detected in 10 µg protein samples in comparison to 25 µg protein samples. Therefore, only the results for samples containing 25 µg of total protein are presented in this paper for the characterization of MMP-2 and -9 in the intestinal mucosa of healthy dogs. To assess whether higher protein amounts lead to MMP detection in negative samples, we used also larger protein amounts of 100 µg leading also to negative results.
In our canine samples, the zymography method produced distinct bands on the gel which corresponded to the standards with human pro-MMP-2 and -9. We did not detect any substantial difference between the migration of these enzymes from human standards and intestinal mucosa samples obtained from healthy dogs, which is in concordance with MMP-2 and -9 enzymes being highly conserved among species [6].
Gelatin zymography appears to be a useful method for the evaluation of intestinal mucosa samples, because it requires only a small piece of intestinal tissue sample (5 mg mucosa) which is the weight of about 1–2 endoscopically taken biopsies.
In the present study, 65% and 48% of all samples showed no activity for pro-MMP-2 and -9, respectively, and the colon showed the lowest activity. One possible reason for the high amount of undetectable activity might be that the level of gelatinolytic activity of both MMPs was less than the detection limit of the gelatin zymography assay. In addition, the extraction of MMPs from tissues has some limitations for the following reasons: (1) MMPs, such as MMP-1, MMP-2 and MMP-9, are tightly bound to the ECM; (2) it is difficult to ascertain whether all MMPs are extracted; (3) specific MMPs localized in a relatively small part of the tissue may not be detected due to dilution in the entire tissue extract [41, 44]. Therefore, difficulties in the extraction of MMPs from tissues might also be another reason for samples without MMP-2 and -9 activities. Similar results were reported for normal human colon mucosa samples. In the study by Baugh et al., pro-MMP-2 and -9 were not detected in 20% and 56% of their samples, respectively, using gelatin zymography when examining colon mucosa from nine healthy human subjects [2]. In our study, none of the intestinal samples showed gelatinolytic activity corresponded to the control bands of active MMP-2 and MMP-9. Similar to our results, Baugh et al. did not detect active forms of MMP-2 and -9 in any of the colonic mucosal samples in healthy human subjects [2].
Gao et al. reported the mean (± SEM) activities of pro-MMP-2 and pro-MMP-9 in normal colonic mucosa of humans to be <2 AU with 0.64 ± 0.1 and 1.18 ± 0.19 AU, respectively [10]. Similar to findings of Gao et al. [10], the majority of our samples (86%) had pro-MMP-2 and -9 activities <2 AU. However, in the present study, some histologically unremarkable samples had comparably high pro-MMP-2 and -9 activities>2 AU for which we have not found any explanation so far.
Thirty-five percent of the samples were positive for pro-MMP-2 activity in the present study. In healthy dogs, pro-MMP-2 activity was mostly seen in the small intestine. This might be due to high levels of epithelial cell turnover in the small intestine [3]. MMP-2 is commonly expressed in normal intestinal tissues and is believed to participate in the maintenance of collagen homeostasis and the intestinal tissue remodeling [10, 39].
In the current study, pro-MMP-9 activity was detected within 25/48 (52%) of the samples. Under normal conditions, MMPs including MMP-9 are expressed at low levels, usually in the pro-form, and when activated, they play a role in normal tissue ECM turnover, including intestinal tissue [30].
In humans, MMP-2 and MMP-9 seem to be actively involved in the inflammatory and remodeling processes in IBD [2, 10, 16]. In a mouse model of IBD, mucosa-derived MMP-2 serves to protect against tissue damage possibly by regulation of epithelial barrier function [11, 12, 37]. In human IBD, it participates in the ECM remodeling and degradation of the basal membrane type IV collagen leading to intestinal ulceration, epithelial damage and/or fistula formation [10, 28, 29, 42]. The exact role of MMP-2 in mucosal injury of IBD remains to be fully clarified, however. MMP-9 has a crucial role in the induction of intestinal tissue inflammation through defective re-epithelialization, increased paracellular permeability and reduction in adhesion complex integrity, resulting in impaired wound healing, especially in the acute phase [11, 12, 37].
In human IBD, several studies have investigated the role of MMPs and their inhibitors in the differentiating subtypes of IBD [25,26,27, 32, 33, 36, 38, 42]. Based on the study by Mäkitalo et al., MMP-7 has been suggested to aid in differentiation between Crohn’s disease and ulcerative colitis, while MMP-10 and TIMP-3 may be markers of IBD etiology in inflammatory processes of the gut [24]. MMP-profiles in operated inflammatory disease (IBD) patients have also been looked at to evaluate the etiology of inflammatory flares - IBD or other - to properly treat them [25, 42]. To our knowledge, MMP profiles are not yet used for therapeutic purposes, but efforts to characterize IBD and to examine how treatment affects MMP expression have been made [26, 27, 32, 33]. MMP expression may also predict precancerous potential [36] and long-term remission [38]. While treatment for IBD subtypes share similarities, the most notable difference is found in surgical treatment, being more conservative in Crohn’s disease. MMP expression in the gut or elsewhere in the body, e.g. serum, may in the future help in properly differentiating the disease subtypes and severity and to enable tailored treatment choices for individuals.
Whether MMP-2 and -9 are also involved in the pathogenesis of canine IBD remains unknown and requires further investigation. In future studies, however, immunohistochemistry should be included to also assess the localization of MMPs in canine intestinal mucosa and their correlation with intestinal pathologies in dogs.
The activity of MMP-2 and MMP-9 in different parts of the intestinal mucosa of healthy dogs has been determined here, laying the groundwork for future studies in canine chronic intestinal diseases, including IBD. Pro-MMP-2 and -9 activities were found in the intestinal mucosa of the small and large intestines in healthy dogs, mostly in the ileum. Active forms of MMP-2 and -9 were not detected in the intestinal mucosa of healthy dogs. Understanding the role of MMP-2 and -9 in the pathogenesis of chronic intestinal inflammation in the future and using them as biomarkers for differentiating canine IBD subtypes might offer a possibility to improve the current treatment approach.
The sample processing used for the study led to histological slides of sufficient quality for assessment. A limitation of the study might be that in some occasional samples, it was not possible to interpret epithelial changes due to processing artifact. However, since we used several samples per location for histology, there was enough epithelial layer on the majority of samples from the same intestinal part of every dog to allow a final interpretation. The submucosa, muscularis and serosa were not affected by artifacts.
In conclusion, based on the results of the current study and reports from studies in human IBD [2, 10, 41], zymography presents as an appropriate method to evaluate MMP-2- and -9 profiles in canine intestinal mucosa. The results of this study may provide valuable information for future studies evaluating the role of MMPs in the pathogenesis of canine chronic enteropathies including IBD.
Acknowledgments
The authors would like to express sincere thanks to Professor Anja Kipar, Veterinary Pathology, Faculty of Veterinary Medicine, University of Helsinki, for histological cross examination and introductory training sessions; to Jouni Junnila, 4Pharma, Finland, for performing statistical analysis of the data; to Dr Jennifer Rowland, Sciences Editor, University of Helsinki Language Services, for language revision; and to Laura Parikka, for her technical assistance. The authors also thank the Finnish Centre for Mobility Exchange (CIMO), Finnish Veterinary Foundation, Finnish Foundation of Veterinary Research, and the Doctoral Programme in Clinical Veterinary Medicine (former ANIWEL) for financial support.
REFERENCES
- 1.Aresu L., Giantin M., Morello E., Vascellari M., Castagnaro M., Lopparelli R., Zancanella V., Granato A., Garbisa S., Arico A., Bradaschia A., Mutinelli F., Dacasto M.2011. Matrix metalloproteinases and their inhibitors in canine mammary tumors. BMC Vet. Res. 7: 33. doi: 10.1186/1746-6148-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baugh M. D., Perry M. J., Hollander A. P., Davies D. R., Cross S. S., Lobo A. J., Taylor C. J., Evans G. S.1999. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 117: 814–822. doi: 10.1016/S0016-5085(99)70339-2 [DOI] [PubMed] [Google Scholar]
- 3.Bergman R. L., Inzana K. D., Inzana T. J.2002. Characterization of matrix metalloproteinase-2 and -9 in cerebrospinal fluid of clinically normal dogs. Am. J. Vet. Res. 63: 1359–1362. doi: 10.2460/ajvr.2002.63.1359 [DOI] [PubMed] [Google Scholar]
- 4.Castaneda F. E., Walia B., Vijay-Kumar M., Patel N. R., Roser S., Kolachala V. L., Rojas M., Wang L., Oprea G., Garg P., Gewirtz A. T., Roman J., Merlin D., Sitaraman S. V.2005. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129: 1991–2008. doi: 10.1053/j.gastro.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 5.Cerquetella M., Spaterna A., Laus F., Tesei B., Rossi G., Antonelli E., Villanacci V., Bassotti G.2010. Inflammatory bowel disease in the dog: differences and similarities with humans. World J. Gastroenterol. 16: 1050–1056. doi: 10.3748/wjg.v16.i9.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlan A. R., Robertson D. H., Bennett D., May C., Beynon R. J., Carter S. D.1998. Matrix metalloproteinases 2 and 9 in canine rheumatoid arthritis. Vet. Rec. 143: 219–223. doi: 10.1136/vr.143.8.219 [DOI] [PubMed] [Google Scholar]
- 7.Day M. J., Bilzer T., Mansell J., Wilcock B., Hall E. J., Jergens A., Minami T., Willard M., Washabau R.2008. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 138 Suppl. 1: S1–S43. doi: 10.1016/j.jcpa.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Elkington P. T., Green J. A., Friedland J. S.2009. Analysis of matrix metalloproteinase secretion by macrophages. pp. 253–265. In: Macrophages and Dendritic Cells (Reiner, N. E. ed.), Humana Press, Totowa. [DOI] [PubMed] [Google Scholar]
- 9.Flecknell P.2002. Replacement, reduction and refinement. ALTEX 19: 73–78 [PubMed] [Google Scholar]
- 10.Gao Q., Meijer M. J. W., Kubben F., Sier C. F. M., Kruidenier L., van Duijn W., van den Berg M., van Hogezand R. A., Lamers C., Verspaget H. W.2005. Expression of matrix metalloproteinases-2 and-9 in intestinal tissue of patients with inflammatory bowel diseases. Dig. Liver Dis. 37: 584–592. doi: 10.1016/j.dld.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 11.Garg P., Rojas M., Ravi A., Bockbrader K., Epstein S., Vijay-Kumar M., Gewirtz A. T., Merlin D., Sitaraman S. V.2006. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J. Immunol. 177: 4103–4112. doi: 10.4049/jimmunol.177.6.4103 [DOI] [PubMed] [Google Scholar]
- 12.Garg P., Vijay-Kumar M., Wang L. X., Gewirtz A. T., Merlin D., Sitaraman S. V.2009. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296: G175–G184. doi: 10.1152/ajpgi.90454.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert S. J., Wotton P. R., Tarlton J. F., Duance V. C., Bailey A. J.1997. Increased expression of promatrix metalloproteinase-9 and neutrophil elastase in canine dilated cardiomyopathy. Cardiovasc. Res. 34: 377–383. doi: 10.1016/S0008-6363(97)00011-4 [DOI] [PubMed] [Google Scholar]
- 14.Hogan S. P.2009. Functional role of eosinophils in gastrointestinal inflammation. Immunol. Allergy Clin. North Am. 29: 129–140 xi.doi: 10.1016/j.iac.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J. H., Lee S. Y., Bak S. M., Suh I. B., Shin C., Shim J. J., In K. H., Kang K. H., Yoo S. H.2004. Effects of matrix metalloproteinase inhibitor on LPS-induced goblet cell metaplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 287: L127–L133. doi: 10.1152/ajplung.00047.2003 [DOI] [PubMed] [Google Scholar]
- 16.Kirkegaard T., Pedersen G., Saermark T., Brynskov J.2004. Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin. Exp. Immunol. 135: 146–153. doi: 10.1111/j.1365-2249.2004.02348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleiner D. E., Stetler-Stevenson W. G.1994. Quantitative zymography: detection of picogram quantities of gelatinases. Anal. Biochem. 218: 325–329. doi: 10.1006/abio.1994.1186 [DOI] [PubMed] [Google Scholar]
- 18.Lana S. E., Ogilvie G. K., Hansen R. A., Powers B. E., Dernell W. S., Withrow S. J.2000. Identification of matrix metalloproteinases in canine neoplastic tissue. Am. J. Vet. Res. 61: 111–114. doi: 10.2460/ajvr.2000.61.111 [DOI] [PubMed] [Google Scholar]
- 19.Leibman N. F., Lana S. E., Hansen R. A., Powers B. E., Fettman M. J., Withrow S. J., Ogilvie G. K.2000. Identification of matrix metalloproteinases in canine cutaneous mast cell tumors. J. Vet. Intern. Med. 14: 583–586. doi: 10.1111/j.1939-1676.2000.tb02280.x [DOI] [PubMed] [Google Scholar]
- 20.Lim G. P., Russell M. J., Cullen M. J., Tokes Z. A.1997. Matrix metalloproteinases in dog brains exhibiting Alzheimer-like characteristics. J. Neurochem. 68: 1606–1611. doi: 10.1046/j.1471-4159.1997.68041606.x [DOI] [PubMed] [Google Scholar]
- 21.Ljungvall I., Rajamaki M. M., Crosara S., Olsen L. H., Kvart C., Borgarelli M., Hoglund K., Haggstrom J.2011. Evaluation of plasma activity of matrix metalloproteinase-2 and -9 in dogs with myxomatous mitral valve disease. Am. J. Vet. Res. 72: 1022–1028. doi: 10.2460/ajvr.72.8.1022 [DOI] [PubMed] [Google Scholar]
- 22.Loukopoulos P., Mungall B. A., Straw R. C., Thornton J. R., Robinson W. F.2003. Matrix metalloproteinase-2 and -9 involvement in canine tumors. Vet. Pathol. 40: 382–394. doi: 10.1354/vp.40-4-382 [DOI] [PubMed] [Google Scholar]
- 23.Lubbe W. J., Zhou Z. Y., Fu W., Zuzga D., Schulz S., Fridman R., Muschel R. J., Waldman S. A., Pitari G. M.2006. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin. Cancer Res. 12: 1876–1882. doi: 10.1158/1078-0432.CCR-05-2686 [DOI] [PubMed] [Google Scholar]
- 24.Mäkitalo L., Kolho K. L., Karikoski R., Anthoni H., Saarialho-Kere U.2010. Expression profiles of matrix metalloproteinases and their inhibitors in colonic inflammation related to pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 45: 862–871. doi: 10.3109/00365520903583863 [DOI] [PubMed] [Google Scholar]
- 25.Mäkitalo L., Piekkala M., Ashorn M., Pakarinen M., Koivusalo A., Karikoski R., Natunen J., Saarialho-Kere U., Rintala R., Kolho K. L.2012. Matrix metalloproteinases in the restorative proctocolectomy pouch of pediatric ulcerative colitis. World J. Gastroenterol. 18: 4028–4036. doi: 10.3748/wjg.v18.i30.4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mäkitalo L., Rintamaki H., Tervahartiala T., Sorsa T., Kolho K. L.2012. Serum MMPs 7–9 and their inhibitors during glucocorticoid and anti-TNF-alpha therapy in pediatric inflammatory bowel disease. Scand. J. Gastroenterol. 47: 785–794. doi: 10.3109/00365521.2012.677954 [DOI] [PubMed] [Google Scholar]
- 27.Mäkitalo L., Sipponen T., Kärkkäinen P., Kolho K. L., Saarialho-Kere U.2009. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn’s disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int. J. Colorectal Dis. 24: 1157–1167. doi: 10.1007/s00384-009-0756-5 [DOI] [PubMed] [Google Scholar]
- 28.Matsuno K., Adachi Y., Yamamoto H., Goto A., Arimura Y., Endo T., Itoh F., Imai K.2003. The expression of matrix metalloproteinase matrilysin indicates the degree of inflammation in ulcerative colitis. J. Gastroenterol. 38: 348–354. doi: 10.1007/s005350300062 [DOI] [PubMed] [Google Scholar]
- 29.McKaig B. C., McWilliams D., Watson S. A., Mahida Y. R.2003. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am. J. Pathol. 162: 1355–1360. doi: 10.1016/S0002-9440(10)63931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina C., Radomski M. W.2006. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 318: 933–938. doi: 10.1124/jpet.106.103465 [DOI] [PubMed] [Google Scholar]
- 31.Medina C., Santana A., Paz M. C., Diaz-Gonzalez F., Farre E., Salas A., Radomski M. W., Quintero E.2006. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J. Leukoc. Biol. 79: 954–962. doi: 10.1189/jlb.1005544 [DOI] [PubMed] [Google Scholar]
- 32.Meijer M. J., Mieremet-Ooms M. A., van Duijn W., van der Zon A. M., Hanemaaijer R., Verheijen J. H., van Hogezand R. A., Lamers C. B., Verspaget H. W.2007. Effect of the anti-tumor necrosis factor-alpha antibody infliximab on the ex vivo mucosal matrix metalloproteinase-proteolytic phenotype in inflammatory bowel disease. Inflamm. Bowel Dis. 13: 200–210. doi: 10.1002/ibd.20051 [DOI] [PubMed] [Google Scholar]
- 33.Meijer M. J., Mieremet-Ooms M. A., van Hogezand R. A., Lamers C. B., Hommes D. W., Verspaget H. W.2007. Role of matrix metalloproteinase, tissue inhibitor of metalloproteinase and tumor necrosis factor-alpha single nucleotide gene polymorphisms in inflammatory bowel disease. World J. Gastroenterol. 13: 2960–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuman M. G.2007. Immune dysfunction in inflammatory bowel disease. Transl. Res. 149: 173–186. doi: 10.1016/j.trsl.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 35.Rajamäki M. M., Jarvinen A. K., Sorsa T., Maisi P.2002. Clinical findings, bronchoalveolar lavage fluid cytology and matrix metalloproteinase-2 and -9 in canine pulmonary eosinophilia. Vet. J. 163: 168–181. doi: 10.1053/tvjl.2001.0631 [DOI] [PubMed] [Google Scholar]
- 36.Rath T., Roderfeld M., Graf J., Wagner S., Vehr A. K., Dietrich C., Geier A., Roeb E.2006. Enhanced expression of MMP-7 and MMP-13 in inflammatory bowel disease: a precancerous potential? Inflamm. Bowel Dis. 12: 1025–1035. doi: 10.1097/01.mib.0000234133.97594.04 [DOI] [PubMed] [Google Scholar]
- 37.Ravi A., Garg P., Sitaraman S. V.2007. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm. Bowel Dis. 13: 97–107. doi: 10.1002/ibd.20011 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt C., Giese T., Hermann E., Zeuzem S., Meuer S. C., Stallmach A.2007. Predictive value of mucosal TNF-alpha transcripts in steroid-refractory Crohn’s disease patients receiving intensive immunosuppressive therapy. Inflamm. Bowel Dis. 13: 65–70. doi: 10.1002/ibd.20012 [DOI] [PubMed] [Google Scholar]
- 39.Sengupta N., MacDonald T. T.2007. The role of matrix metalloproteinases in stromal/epithelial interactions in the gut. Physiology (Bethesda) 22: 401–409. doi: 10.1152/physiol.00027.2007 [DOI] [PubMed] [Google Scholar]
- 40.Shimokawa Ki K., Katayama M., Matsuda Y., Takahashi H., Hara I., Sato H., Kaneko S.2002. Matrix metalloproteinase (MMP)-2 and MMP-9 activities in human seminal plasma. Mol. Hum. Reprod. 8: 32–36. doi: 10.1093/molehr/8.1.32 [DOI] [PubMed] [Google Scholar]
- 41.Snoek-van Beurden P. A., Von den Hoff J. W.2005. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 38: 73–83. doi: 10.2144/05381RV01 [DOI] [PubMed] [Google Scholar]
- 42.Stallmach A., Chan C. C., Ecker K. W., Feifel G., Herbst H., Schuppan D., Zeitz M.2000. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut 47: 415–422. doi: 10.1136/gut.47.3.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinstein W. L., Dietrich U. M., Sapienza J. S., Carmichael K. P., Moore P. A., Krunkosky T. M.2007. Identification of ocular matrix metalloproteinases present within the aqueous humor and iridocorneal drainage angle tissue of normal and glaucomatous canine eyes. Vet. Ophthalmol. 10 Suppl.1: 108–116. doi: 10.1111/j.1463-5224.2007.00586.x [DOI] [PubMed] [Google Scholar]
- 44.Yan S. J., Blomme E. A.2003. In situ zymography: a molecular pathology technique to localize endogenous protease activity in tissue sections. Vet. Pathol. 40: 227–236. doi: 10.1354/vp.40-3-227 [DOI] [PubMed] [Google Scholar]


