ABSTRACT
We investigated the phenotypic level of albumin in peripheral blood mononuclear cells (PBMC) of streptozotocin (STZ)-induced diabetic rats. A specific reduction of albumin was identified by 2-dimensional electrophoresis and mass spectrometry. Decreased albumin content was also confirmed by immunoblotting and quantitative real-time PCR. Since albumin is a major and predominant antioxidant in plasma, the PBMC albumin may also contribute to their antioxidant activity. By measuring the amount of H2O2, lipid peroxidation and the redox form of glutathione, it was found that the production of the oxidative stress was elevated in STZ-diabetic rats compared to that of normal control. We suggest, therefore, that decreased albumin content may lead to the decreased antioxidant activity in the PBMC of type 1 diabetic rats.
Keywords: albumin, diabetes, expression, oxidative stress, streptozotocin
Oxidative stress has been suggested to play an important role in the etiology and pathogenesis of the chronic complications associated with diabetes [3, 25]. Streptozotocin (STZ) has been used to generate type 1 diabetes mellitus (DM) in animal models. In STZ treated rats, it has been suggested that hyperglycemia, auto-oxidation of glycated proteins and increased production of reactive oxygen species (ROS) lead to increased oxidative stress that is accompanied by pancreatic β-cell damage [6, 9]. Moreover, STZ has been shown to deplete the antioxidant pool in cells, making them more susceptible to oxidative damage [12].
Albumin, the most abundant circulating protein in plasma accounting for approximately 60% of total plasma protein, has several important physiological and pharmacological functions. It transports metals, fatty acids, cholesterol, bile pigments and drugs. In general, albumin is also a major predominant antioxidant in plasma, a body compartment known to be exposed to continuous oxidative stress [21]. Previous studies have shown that more than 70% of the free radical-trapping activity of serum was due to serum albumin [4]. The implication of this function is that hypoalbuminemic patients have reduced potential for scavenging of oxygen radical [14].
Peripheral blood mononuclear cells (PBMC) are the population of blood cells having a round nucleus, such as lymphocytes, monocytes and macrophages. These cells have a major responsibility for carrying out immunological functions. Both B and T lymphocytes especially have membrane-bound albumin as a ‘hidden’ component due to the fact that the functional significance of the membrane albumin is unknown and that the protein is radiolabeled within the plasma membrane but not from the cell exterior [22]. Since the protein serves as a carrier for a variety of small molecules, one possible role of membrane albumin in the lymphocytes is that it may be taken up by endocytosis in order to deliver albumin-bound nutrients into cells [22]. In spite of these studies, however, further intensive works should be undertaken to reveal the exact localization of albumin in lymphocytes as well as its cellular function (s).
We have suggested that the expression level of cytochrome P450 2E1 involving in the etiology and pathology of many diseases with a concomitant increase in ROS production was increased in several tissues of STZ-induced diabetic rats [1]. Here, we expanded the previous study and investigated differential protein levels of PBMC between diabetic and normal rats using two-dimensional polyacrylamide gel-electrophoresis (2D-PAGE) combined with mass spectroscopy (MALDI-TOF) and immunoblotting. Changes in oxidative stresses were also examined in the diabetic PBMC, and their possible relation with phenotypic changes of protein was suggested.
MATERIALS AND METHODS
Male Sprague Dawley rats, weighing 200–250 g (6 weeks old), were obtained from Samtako Bio Korea (Osan, South Korea) and used for inducing type I DM after one week of quarantine and acclimation. The PlusOne silver staining kit was obtained from Amersham Biosciences (Piscataway, NJ, U.S.A.). The Amplex® Red assay kit containing horseradish peroxidase and standard H2O2 solution was acquired from Invitrogen (Carlsbad, CA, U.S.A.). Monoclonal antibody against albumin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). All equipments and buffer solutions for 2D-PAGE were purchased from GE Healthcare Biosciences (Piscataway, NJ, U.S.A.).
Experimental animals: The rats were housed individually in stainless steel cages in a room with a controlled temperature of 23 ± 3°C and lighting (alternating 12 hr period of light and dark). They were fed a commercial rodent chow (Samyang Feed, Wonju, South Korea). The experimental design was approved by the committee for the care and use of laboratory animals at the Chonnam National University.
Induction of DM: Diabetes was induced by a single intraperitoneal injection of STZ (100 mg/kg body weight in 0.1 M citrate buffer). Rats of non-diabetic group were injected with the same volume of 0.1 M citrate buffer only. The severity of the induced diabetic state was assessed by daily monitoring of blood glucose levels using reagent strips (ACCUTREND, Roche Diagnostics GmbH, Mannheim, Germany). Rats with blood glucose levels exceeding 300 mg/dl were classified as diabetic rats. For a span of 2 weeks after STZ-injection, 2 groups of rats (n=10 for each group) were sacrificed. Each rat’s blood was collected from the caudal vena cava.
Blood biochemistry: Blood samples from the caudal vena cava were centrifuged to obtain hemolysis-free clear serum. The activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) and the levels of blood urea nitrogen (BUN), creatinine (CRE), glucose (GLU), total cholesterol (T-CHO), total bilirubin (T-BIL), total protein (TP), albumin (ALB) and triglyceride (TG) were assayed using an autoanalyzer (Dir-chem 4000i, Fujifilm, Tokyo, Japan) by standard methods.
Isolation of PBMC: Peripheral blood mononuclear cells were prepared from whole blood by Ficoll-Paque PLUS (GE Healthcare Biosciences). Briefly, EDTA-treated blood was diluted to 1:1 with phosphate buffered saline (PBS) plus 2% fetal bovine serum (FBS) and layered onto Ficoll-Paque PLUS with a ratio of blood plus PBS:Ficoll maintained at 2:1. The blood was centrifuged at 400 × g for 40 min at room temperature. The buffy coat layer was removed carefully, and washing steps were followed: the cells were suspended in a balanced salt solution (provided by the manufacturer), and then, the sample was centrifuged at 100 × g for 10 min at room temperature. The pellet was collected after removal of supernatant, and this washing step was repeated twice more to remove any contaminant. The PBMC samples were then kept at −70°C until use. The isolation of PBMC from rat blood was verified by a flow cytometry analysis using anti-pan B-cells antibody (clone number of 68-IB3, Santa Cruz).
Sample preparations and 2D-PAGE: The PBMC samples were homogenized in lysis buffer (40 mM Tris-HCl (pH 7.4), 8 M urea and 0.4% 3[(3-Cholamidopropyl) dimethylammonio]-propanesulfonic acid). For isoelectric focusing (IEF), 60 µg proteins were diluted with the rehydration solution (GE Healthcare Biosciences), and the sample was spread over the bottom of the 7-cm IPGphor holder. The 7-cm immobilized pH gradient (IPG, pH 3–10) dry-strips were then overlaid onto the sample. After IEF, the IPG strip was stored at −70°C until analysis by SDS-PAGE. The individual strips were incubated at room temperature in the equilibrium solution A (50 mM Tris-HCl (pH 8.8), 6 M urea, 30% (v/v) glycerol, 2% SDS, bromophenol blue trace and 20 mM DTT), followed by solution B (solution A except that DTT was replaced by 20 mM iodoacetamide), for 15 min each. The IPG strips were then electrophoresed by 12% SDS-PAGE. These steps for 2D-PAGE were repeated with each PBMC sample to obtain statistical significance.
Mass Spectrometry analysis of gel spots: After silver staining of the electrophoresed gel, the target spots were excised and then destained with 1:1 mixture of 30 mM potassium ferricynide and 100 mM sodium thiosulfate solution. Destained gel spots were subjected to in-gel trypsin digestion using the European Molecular Biology Laboratory protocol. After the in gel digestion, the digest was concentrated and desalted using the Ziptipc18 procedure (Millipore, Bedford, MA, U.S.A.), as suggested by the manufacturer, and then eluted with 50% acetonitrile containing 0.1% trifluoroacetic acid. One microliter of the eluted sample was mixed with an equal volume of saturated α-cyano-4-hydrocinnamic acid, spotted on a plate and followed by MALDI-TOF measurement using Voyager MALDI-TOF DETM PRO mass spectrometry (Applied Biosystems, Foster City, CA, U.S.A.) in the ACTH reflector mode. Protein identification was performed using MS-Fit (http://prospector.ucsf.edu).
Western blot analysis after 2D-PAGE: Electrophoretically separated extracts of PBMC were transferred onto nitrocellulose membranes with 0.2 µm pore size (Whatman, Maidstone, U.K.). The membrane was incubated overnight at 4°C with monoclonal antibody against albumin and then anti-mouse IgG conjugated with peroxidase (Sigma-Aldrich, Oakville, Canada) for 1 hr at room temperature, respectively. The Enhanced chemiluminescence detection kit (GE Healthcare Biosciences) was used with a scientific imaging film (Kodak, Rochester, NY, U.S.A.) to visualize albumin.
Quantitative real-time PCR (qRT-PCR): Total RNA was extracted from intact PBMC (~2 × 106 cells) using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The extracted RNA was subjected to first-strand cDNA synthesis using the Reverse Transcription System (Promega, Madison, WI, U.S.A.) according to the manufacturer’s protocol. qRT-PCR was performed using Rotor-Gene 6000 (Qiagen), and the 2−ΔΔCT method was used for relative quantitation. Threshold cycle number and reaction efficiency were determined using Rotor-Gene 6000 series software version 2.7. The primers used were as follows: 5′-ATACACCCAGAAAGCACCTC-3′ (forward) and 5′-CACGAATTGTGCGAATGTCAC-3′ (reverse) for albumin, and 5′-CTAGAAGCATTTGCGGTGGACGATGGACCC-3′ (forward) and 5′TGACGGGGTCACCCACACACTGTGCCCATCTA-3′ (reverse) for GAPDH as a housekeeping gene.
Measurement of oxidative stresses: The amount of H2O2 was measured spectrofluorimetrically using the Amplex® Red (AR) assay kit according to the manufacturer’s instructions. Emission fluorescence for the AR assay was recorded at 585 nm under an excitation wavelength of 571 nm. Lipid peroxidation (LPO) was estimated by measuring thiobarbituric acid-reactive substances, using malondialdehyde as a standard, as described [15]. Reduced glutathione (GSH) levels were determined by the sulfhydryl content using Ellman’s reagent [5]. The ratio of GSH/GSSG (oxidized glutathione) was also determined by a GSH/GSSG ratio assay kit (Abcam, Cambridge, MA, U.S.A.). The amounts of H2O2 and GSH were measured with cytosolic fractions of PBMC. LPO was estimated with microsomal fractions of PBMC.
Statistical analysis: Data are expressed as a mean ± S.E. of ten animals per group. Statistical analyses were performed using Student’s t-test for 2 groups. A P-value<0.05 was considered significant.
RESULTS
Blood biochemistry: To evaluate the development of STZ-induced DM in rats, eleven biochemical parameters were measured with serum samples. In addition to hyperglycemia (approximately 3.3-fold increase), Table 1 shows the elevated levels of TG and hepatic enzyme activities (AST, ALT and ALP) as well as decreased TP content in STZ-treated rats compared with those of normal group, which are common characteristic features of diabetes. With blood sample of STZ-treated rats, the lower and higher amounts of CRE and BUN were also measured, respectively. Thereby, when BUN/CRE ratio was calculated with each individual rat, the ratio was on average 138.7 and this value was approximately 2-fold higher than that of normal rats (~65.6). The BUN/CRE ratio is considered to be a useful index to evaluate renal insufficiency [10]. It has also been suggested that acute renal insufficiency, which is developed in STZ-induced DM, causes an increase in the BUN/CRE ratio [20]. Therefore, these results suggest that diabetic status was developed in STZ-treated rats. However, hypoalbuminemia, another condition of diabetes, was not severe, and albumin concentration was slightly decreased in diabetic rats. The concentration of T-CHO did not show statistically significant difference between two groups as well.
Table 1. Biochemical values of serum obtained from normal (Control) and STZ-treated (STZ) rats.
| AST (IU/l) | ALT (IU/l) | ALP (IU/l) | ALB (g/dl) | TP (g/dl) | BUN (mg/dl) | |
|---|---|---|---|---|---|---|
| Control | 77.2 ± 7.0 | 33.0 ± 5.7 | 187.5 ± 34.2 | 3.6 ± 0.1 | 5.1 ± 0.3 | 18.4 ± 2.2 |
| STZ | 169.8 ± 22.7* | 94.5 ± 19.3* | 349.8 ± 28.6* | 3.2 ± 0.2* | 4.0 ± 0.2* | 26.1 ± 4.3* |
| GLU (mg/dl) | CRE (mg/dl) | T-CHO (mg/dl) | T-BIL (mg/dl) | TG (mg/dl) | BUN/CRE | |
| Control | 160.2 ± 13.5 | 0.3 ± 0.1 | 92.6 ± 7.6 | 0.4 ± 0.1 | 109.2 ± 28.1 | 65.6 ± 10.8 |
| STZ | 524.3 ± 47.2* | 0.2 ± 0.1* | 100.8 ± 13.5 | 0.5 ± 0.1* | 220.8 ± 59.4* | 138.7 ± 23.5* |
Data are mean ± S.E., n=10 (per each group). * P<0.05 vs. control.
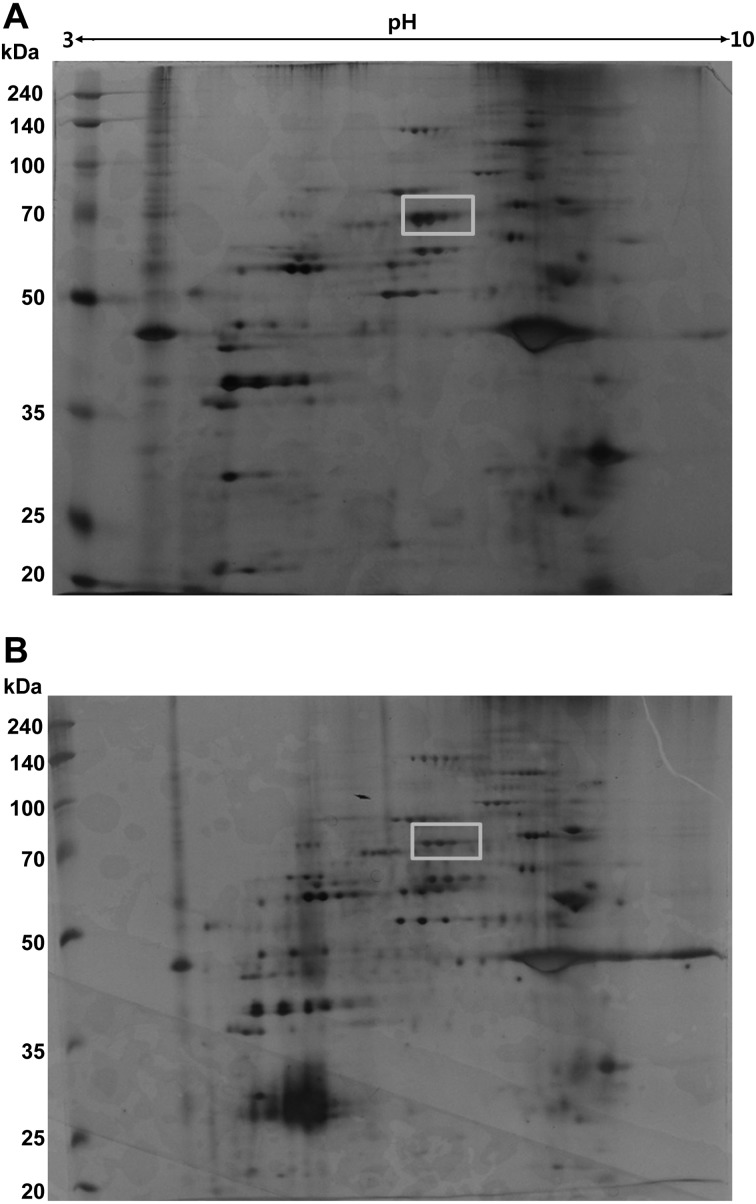
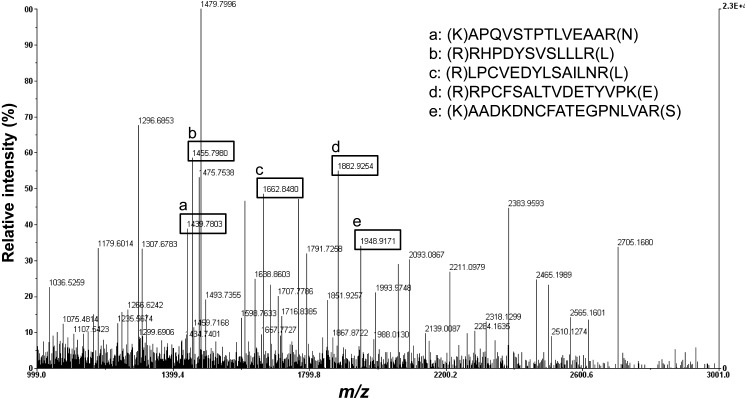
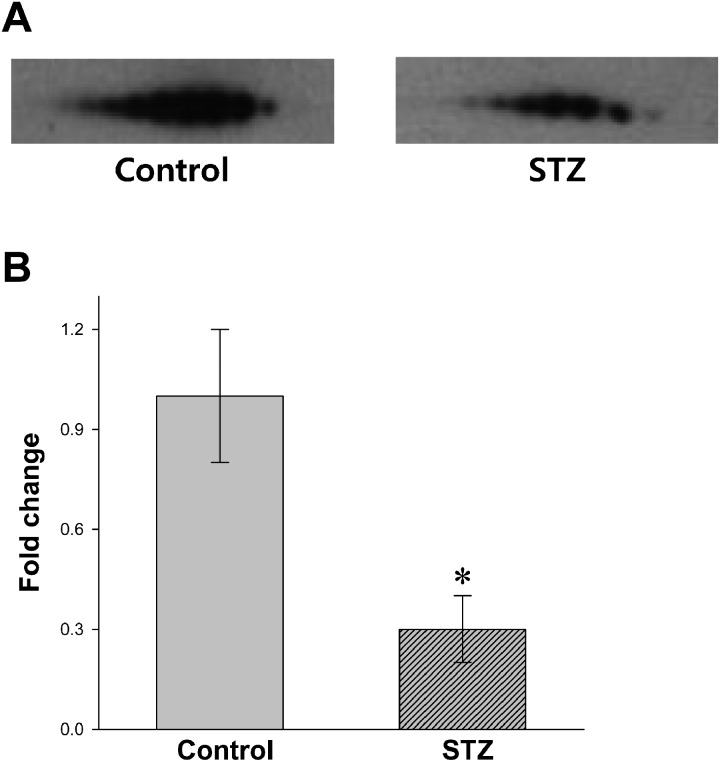
Decreased level of PBMC albumin in STZ-diabetic rats: Protein compositions of normal and STZ-induced diabetic PBMC were analyzed using 2D-PAGE, and the results are shown in Fig. 1. Using an image software, more than 100 protein spots were observed on the silver stained gel within a pI range of 3−10 and a molecular weight range of 20−240 kDa using marker proteins. About 5 protein spots showing differential expression levels were found between diabetic and normal rat PBMC extracts. These proteins had similar molecular weights (~70 kDa) and different pI values. The protein spots were picked from the gels and digested subsequently with trypsin after destaining the gel. The protein fragments were then analyzed by MALDI-TOF mass spectroscopy (Fig. 2). The 5 spots were all identified as albumin by MS-Fit searching with 5 matching peptides (a, b, c, d and e in Fig. 2). To confirm that the albumin content was decreased in diabetic rat PBMC compared to the normal sample, immunoblots were performed after 2D-PAGE with each PBMC extract. Figure 3A shows that the protein spots identified as albumin by MALDI-TOF were detected by anti-albumin antibody, and the decreased expression level of albumin was confirmed in diabetic rat PBMC. Taken together, these results suggest that STZ induces the decreased expression of albumin in rats. In addition, all PBMC samples (n=10) consistently showed decreased albumin content in 2D-PAGE and immunoblot analysis, although the degrees of reduction were different among samples and exact quantification could not be performed (results not shown).
Fig. 1.
2D-PAGE analysis of PBMC extracts. Protein extracts (60 µg) from normal (A) and STZ-induced diabetic PBMC (B) were separated by IEF on Immobiline™ DryStirp pH 3–10 NL and then by SDS-PAGE. The gel was stained with silver staining. Rectangle represents the protein spot analyzed by MALDI-TOF mass spectrometry.
Fig. 2.
Identification of protein spots by peptide mass fingerprinting using MALDI-TOF. The protein spots from 2D-PAGE were subjected to in-gel digestion with trypsin, and the extracted peptides were analyzed by MALDI-TOF mass spectroscopy. MS-Fit searching was used to identify the protein with 5 matching peptides. The m/z in y-axis represents mass to charge ratio.
Fig. 3.
Immunoblot (A) and qRT-PCR (B) analyses with PBMC proteins and RNAs, respectively. 2D-PAGE followed by immunoblot was performed with the protein extracts (10 µg) of normal (Control) and STZ-induced diabetic PBMC (STZ). The ECL detection kit was used to visualize the protein spots. The transcriptional level of the albumin gene was expressed by relative fold change after quantitation of cDNA. The transcription level of normal PBMC was normalized to be one (1) in the figure. * P<0.05, n=10 (per each group).
It was found in an earlier study that diabetic patents contain heterogeneous albumins with broad ranges of pI caused by different levels of glycosylation [8]. However, direct evidence for the albumin isoforms on 2D-PAGE could not be obtained from the peptide mass-fingerprinting as shown in Fig. 2. STZ-induced decreased expression of PBMC albumin was also observed in RNA level using qRT-PCR (Fig. 3B); the transcription was reduced in diabetic PBMC by about 70% compared with that of the normal group. This result strongly suggests that STZ resulted in the decreased expression of albumin in PBMC, although there is still a possibility that the results for 2D-PAGE (Fig. 2) might be affected by FBS during the isolation steps of PBMC.
Increased oxidative stress in STZ-diabetic PBMC: We have suggested previously that the production of oxidative stress is elevated in tissues, such as the liver, kidney, brain and pancreas of STZ-induced diabetic rats [1]. To obtain insight into the correlation between decreased albumin expression and oxidative stress, the generation of H2O2 and LPO was measured in diabetic and normal PBMC. Table 2 shows that H2O2 and LPO levels were significantly enhanced by approximately 55% and 104%, respectively, in diabetic PBMC compared with those of the control sample. The amount of GSH, one of the antioxidant defense materials that function as a free radical scavenger, was also measured. In parallel with the elevated production of oxidative stress, a decrease in the GSH content, which was approximately 46% of the control value, was observed in diabetic PBMC. The ratio of GSH/GSSG, a useful indicator of oxidative stress, was also determined. Normal rats showed the ratio of 0.84 implying that most of glutathione pool is in the reduced form. However, the ratio was decreased to 0.43 in diabetic PBMC. Therefore, the current results suggest that STZ exerts similar effects on PBMC to other cells and tissues in rats. Although a direct correlation between PBMC albumin and oxidative stresses is not present, and the functional role of the protein is still unknown, STZ-induced decreased expression of albumin in PBMC may act as one of the reasons resulting in enhanced oxidative stress in rats. In contrast to the measurement of H2O2, a significant level of superoxide was not detected using Amplex® Red assay combined with superoxide dismutase (results not shown).
Table 2. Measurement of oxidative stresses with PBMC extracts of normal (Control) and STZ-treated (STZ) rat.
| H2O2 (pmol/mg protein) |
LPO (nmol/mg protein) |
GSH (nmol/min/mg protein) |
GSH/GSSG ratio |
|
|---|---|---|---|---|
| Control | 4.7 ± 0.5 | 2.4 ± 0.4 | 26.8 ± 5.4 | 0.84 ± 0.15 |
| STZ | 7.3 ± 0.7* | 4.9 ± 0.5* | 12.5 ± 4.2* | 0.43 ± 0.12* |
Data are mean ± S.E., n=10 (per each group). * P<0.05 vs. control.
DISCUSSION
The present study provides evidence that the amount of PBMC albumin was decreased in STZ-diabetic rats when compared to that of control. ROS production, such as hydrogen peroxide and lipid peroxidation, was also elevated, and the ratio of GSH/GSSG was reduced in diabetic PBMC. These results coincide well with the previous suggestion that STZ induces increased oxidative stress [6, 9] and depletes the antioxidant pool in cells, making them more susceptible to oxidative damage [12]. As these changes in oxidative stress are common phenomena in diabetic serum [3], we suggest that similar effects were exerted on diabetic PBMC, although precise cause (s) for the current results is still unclear in a molecular level.
It has been well known that serum albumin has several important physiological and pharmacological functions: The protein affects vascular permeability [19] and transports various molecules. Moreover, one of the most prominent functions of albumin is its antioxidant activities, such as free radical-trapping [4, 21]. Therefore, any factor inducing decreased level of albumin may act as one of reasons for pathogenesis in a number of oxidative stress-related diseases, such as diabetes, neuro-degeneration, cardiovascular dysfunction and even cancer. In addition to oxidative stress, decreased level of serum albumin per se is another complication of diabetes, because albumin plays a decisive role in modulating osmotic pressure of plasma. One possible explanation for this hypoalbuminemia may be the increased urinary excretion of albumin as a result of diabetic nephropathy [24]. Low hepatic synthesis of albumin has also been suggested as another reason for the decreased albumin level in diabetes [18]. As mentioned above, lymphocytes have membrane-bound albumin, although its physiological functions including the origin are still unclear. Therefore, when judged by the present results, it is reasonable that the stimulation of oxidative stress in diabetic PBMC may be attributed to the decreased albumin levels. Conversely, further investigations should be also performed to reveal the functional effects of lymphocytic albumin on the regulation of oxidative stress.
Albumin is mainly synthesized in the liver, representing about 25% of total hepatic protein synthesis, and thus, serum albumin is the most abundant circulating protein in the plasma accounting for approximately 60% of total plasma protein. Non-hepatic transcription of albumin has also been reported in several tissues, such as the kidney, pancreas, intestine and lymph gland [13, 23]. However, despite these suggestions, non-hepatic synthesis of albumin has rarely been reported at the protein level. In the case of lymphocytes (and macrophages), there has been a possibility that cell surface-bound albumin may originate from tissue culture medium in vitro or from serum in vivo [7]. However, when the possibility has been tested by an immunostaining method using anti-albumin antibody as described previously [2], no significant fluorescent signal was detected in normal and diabetic PBMC (results not shown). Therefore, this suggests that the albumin detected by the current study is a ‘cryptic’ component of PBMC rather than from serum as suggested previously [22]. At present, it is not clear whether albumin is synthesized in PBMC and/or is transported from other tissues, such as the liver, into the cells. When judged by the results obtained from qRT-PCR, the former may be plausible; the reduced albumin content in diabetic PBMC may result from decreased protein expression. Other point to be addressed is whether the change in transcription level is specific to PBMC. If it is (or is not) tissue-specific, it should be also revealed what cause (s) affects the gene expression of albumin and what effect oxidative stress exerts. At the moment, we do not have any satisfactory explanation for the points, and the relevant results are rarely available. Therefore, further studies should be undertaken to solve these issues.
Several studies have been performed to reveal the relation between diabetic status and lymphocytes. Among them, it is especially implicative with the present investigations that diabetes induces apoptosis in lymphocytes of rats and humans accompanying a reduced number of blood-circulating lymphocytes [17]. Diabetes-induced impairment of lymphocyte function has also been suggested previously [16]. Consequently, these results can explain the impaired immune system and enhanced susceptibility to infections in a poorly controlled diabetic state [11].
In summary, we propose for the first time that type 1 diabetes may induce albumin-mediated PBMC injuries as well as etiological damages of other tissues and cells.
Acknowledgments
This work was financially supported by Chonnam National University, 2011.
REFERENCES
- 1.Ahn T., Yun C. H., Oh D. B.2006. Tissue-specific effect of ascorbic acid supplementation on the expression of cytochrome P450 2E1 and oxidative stress in streptozotocin-induced diabetic rats. Toxicol. Lett. 166: 27–36. doi: 10.1016/j.toxlet.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 2.Ahn S. M., Byun K., Cho K., Kim J. Y., Yoo J. S., Kim D., Paek S. H., Kim S. U., Simpson R. J., Lee B.2008. Human microglial cells synthesize albumin in brain. PLoS ONE 3: e2829. doi: 10.1371/journal.pone.0002829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baynes J. W., Thorpe S. R.1999. Role of oxidative stress in development of complications in diabetes: a new perspective on an old paradigm. Diabetes 48: 1–9. doi: 10.2337/diabetes.48.1.1 [DOI] [PubMed] [Google Scholar]
- 4.Bourdon E., Blache D.2001. The importance of proteins in defense against oxidation. Antioxid. Redox Signal. 3: 293–311. doi: 10.1089/152308601300185241 [DOI] [PubMed] [Google Scholar]
- 5.Buttar H. S., Chow A. Y. K., Downie R. H.1977. Glutathione alterations in rat liver after acute and subacute oral administration of paracetamol. Clin. Exp. Pharmacol. Physiol. 4: 1–6. doi: 10.1111/j.1440-1681.1977.tb02371.x [DOI] [PubMed] [Google Scholar]
- 6.Desco M. C., Asensi M., Marquez R., Martinez-Valls J., Vento M., Pallardo F. V., Sastre J., Vina J.2002. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51: 1118–1124. doi: 10.2337/diabetes.51.4.1118 [DOI] [PubMed] [Google Scholar]
- 7.Dziarski R.1994. Cell-bound albumin is the 70-kDa peptidoglycan-, lipopolysaccharide-, and lipoteichoic acid-binding protein on lymphocytes and macrophages. J. Biol. Chem. 269: 20431–20436 [PubMed] [Google Scholar]
- 8.Ghiggeri G. M., Candiano G., Delfino G., Queirolo C.1985. Electrical charge of serum and urinary albumin in normal and diabetic humans. Kidney Int. 28: 168–177. doi: 10.1038/ki.1985.137 [DOI] [PubMed] [Google Scholar]
- 9.Hunt J. V., Dean R. T., Wolff S. P.1988. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem. J. 256: 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouki T., Komiya I., Masuzaki H.2010. The ratio of the blood urea nitrogen/creatinine index in patients with acute renal failure is decreased due to dextran or mannitol. Intern. Med. 49: 223–226. doi: 10.2169/internalmedicine.49.2681 [DOI] [PubMed] [Google Scholar]
- 11.Kraine M. R., Tisch R. M.1999. The role of environmental factors in insulin-dependent diabetes mellitus: an unresolved issue. Environ. Health Perspect. 107: 777–781. doi: 10.1289/ehp.99107s5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low P. A., Nickander K. K., Tritschler H. J.1997. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes 46 Suppl. 2: S38–S42. doi: 10.2337/diab.46.2.S38 [DOI] [PubMed] [Google Scholar]
- 13.Nahon J. L., Tratner I., Poliard A., Presse F., Poiret M., Gal A., Sala-Trepat J. M., Legrès L., Feldmann G., Bernuau D.1988. Albumin and α-fetoprotein gene expression in various nonhepatic rat tissues. J. Biol. Chem. 263: 11436–11442 [PubMed] [Google Scholar]
- 14.Nicholson J. P., Wolmarans M. R., Park G. R.2000. The role of albumin in critical illness. Br. J. Anaesth. 85: 599–610. doi: 10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H., Ohishi N., Yagi K.1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 16.Otton R., Mendoça J. R., Curi R.2002. Diabetes causes marked changes in lymphocyte metabolism. J. Endocrinol. 174: 55–61. doi: 10.1677/joe.0.1740055 [DOI] [PubMed] [Google Scholar]
- 17.Otton R., Soriano F. G., Verlengia R., Curi R.2004. Diabetes induces apoptosis in lymphocytes. J. Endocrinol. 182: 145–156. doi: 10.1677/joe.0.1820145 [DOI] [PubMed] [Google Scholar]
- 18.Peavy D. E., Taylor J. M., Jefferson L. S.1985. Time course of changes in albumin synthesis and mRNA in diabetic and insulin-treated diabetic rats. Am. J. Physiol. 248: E656–663 [DOI] [PubMed] [Google Scholar]
- 19.Peters T., Jr1977. Serum albumin: recent progress in the understanding of its structure and biosynthesis. Clin. Chem. 23: 5–12 [PubMed] [Google Scholar]
- 20.Reynolds R. M., Padfield P. L., Seckl J. R.2006. Disorders of sodium balance. BMJ 332: 702–705. doi: 10.1136/bmj.332.7543.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roche M., Rondeau P., Singh N. R., Tarnus E., Bourdon E.2008. The antioxidant properties of serum albumin. FEBS Lett. 582: 1783–1787. doi: 10.1016/j.febslet.2008.04.057 [DOI] [PubMed] [Google Scholar]
- 22.Sidman C. L.1981. Lymphocyte surface receptors and albumin. J. Immunol. 127: 1454–1458 [PubMed] [Google Scholar]
- 23.Shamay A., Homans R., Fuerman Y., Levin I., Barash H., Silanikove N., Mabjeesh S. J.2005. Expression of albumin in nonhepatic tissues and its synthesis by the bovine mammary gland. J. Dairy Sci. 88: 569–576. doi: 10.3168/jds.S0022-0302(05)72719-3 [DOI] [PubMed] [Google Scholar]
- 24.Stehouwer C. D., Gall M. A., Twisk J. W., Knudsen E., Emeis J. J., Parving H. H.2002. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 51: 1157–1165. doi: 10.2337/diabetes.51.4.1157 [DOI] [PubMed] [Google Scholar]
- 25.Vincent A. M., Brownlee M., Russell J. W.2002. Oxidative stress and programmed cell death in diabetic neuropathy. Ann. N. Y. Acad. Sci. 959: 368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x [DOI] [PubMed] [Google Scholar]