Abstract
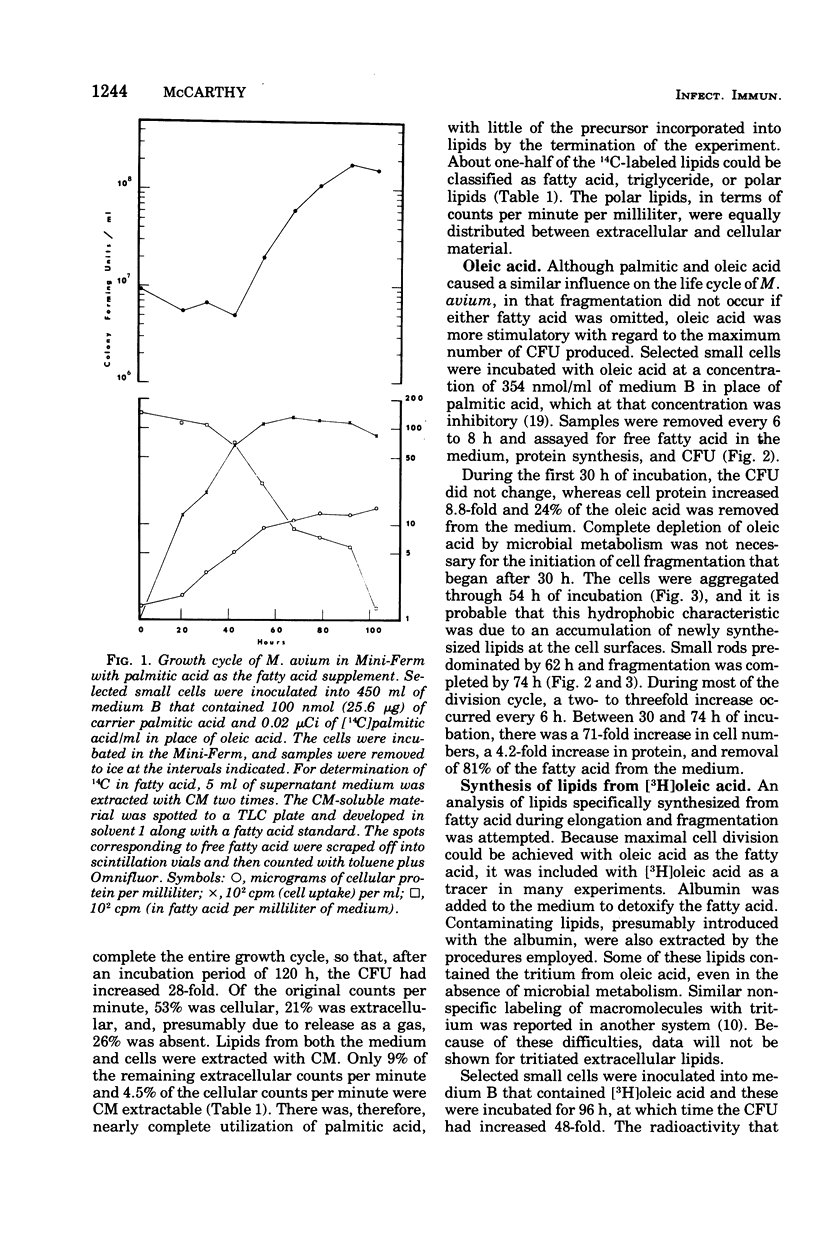
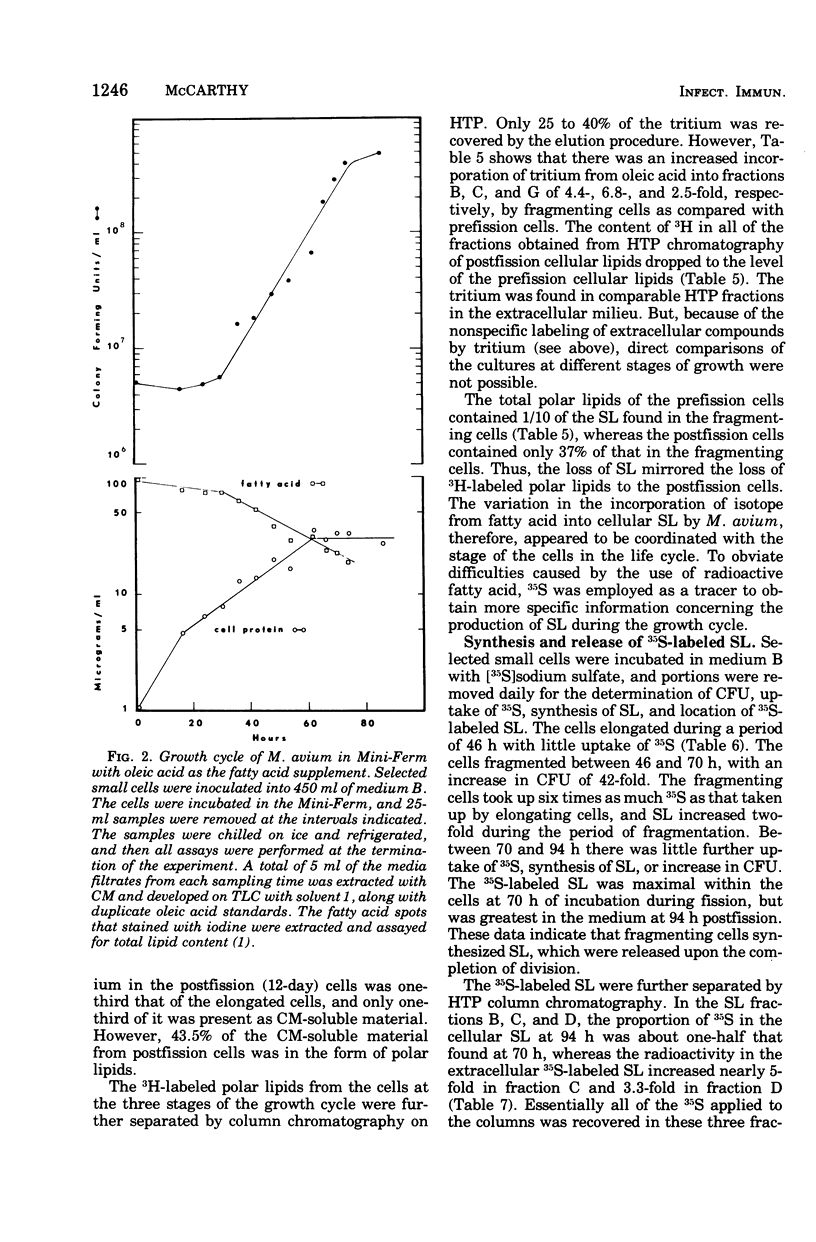
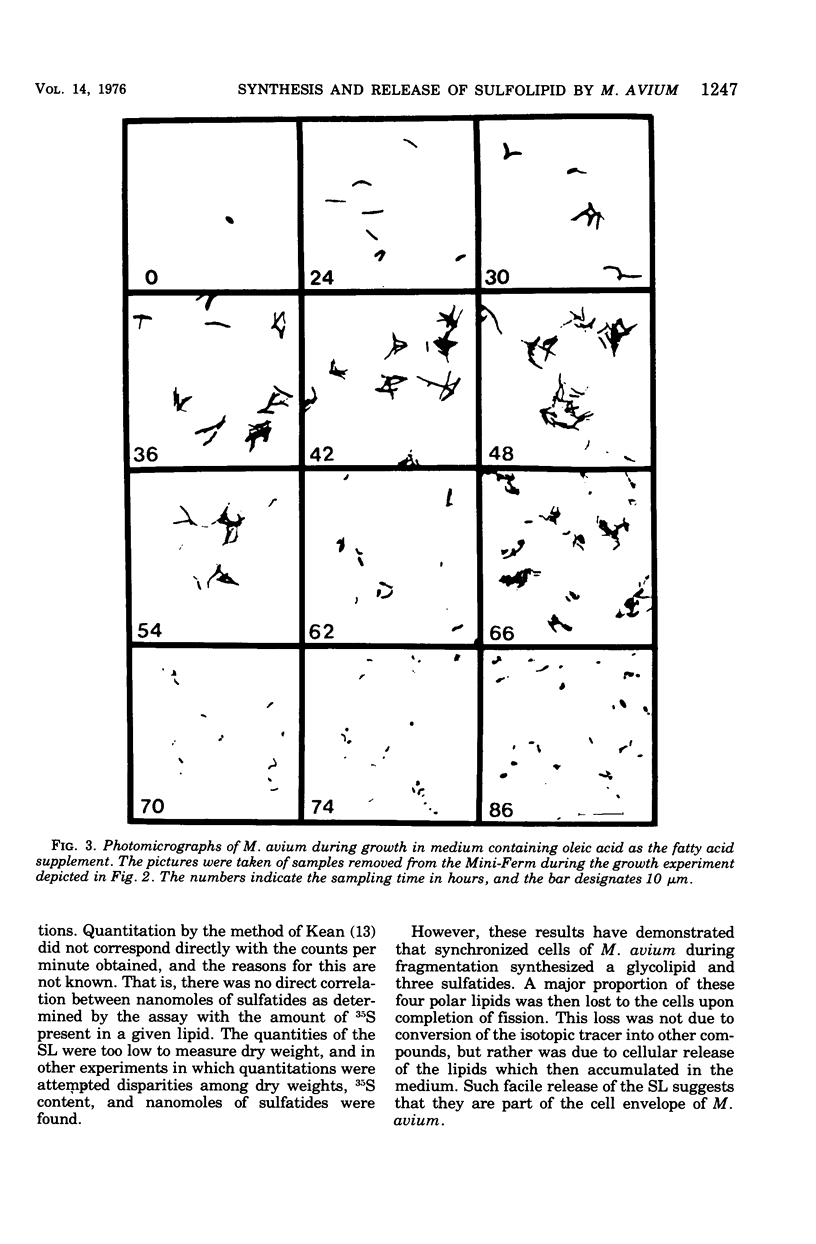
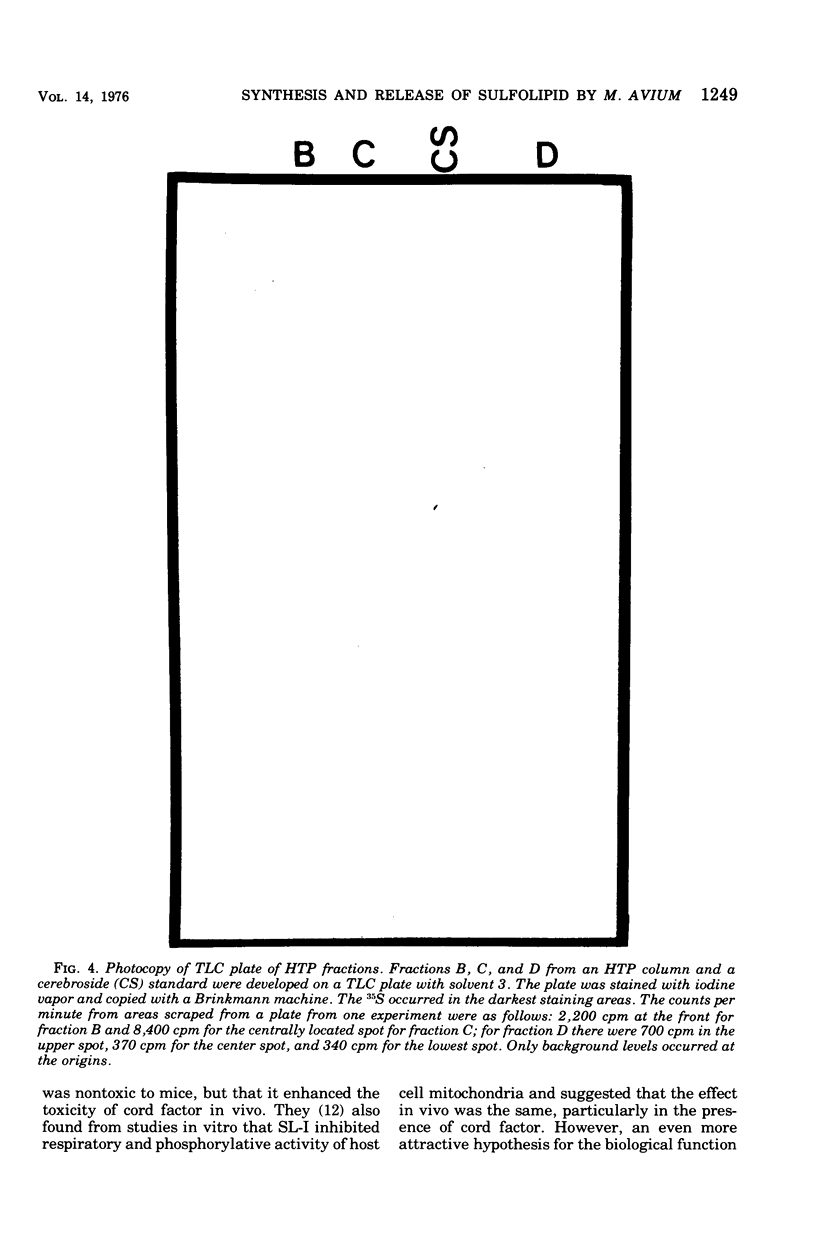
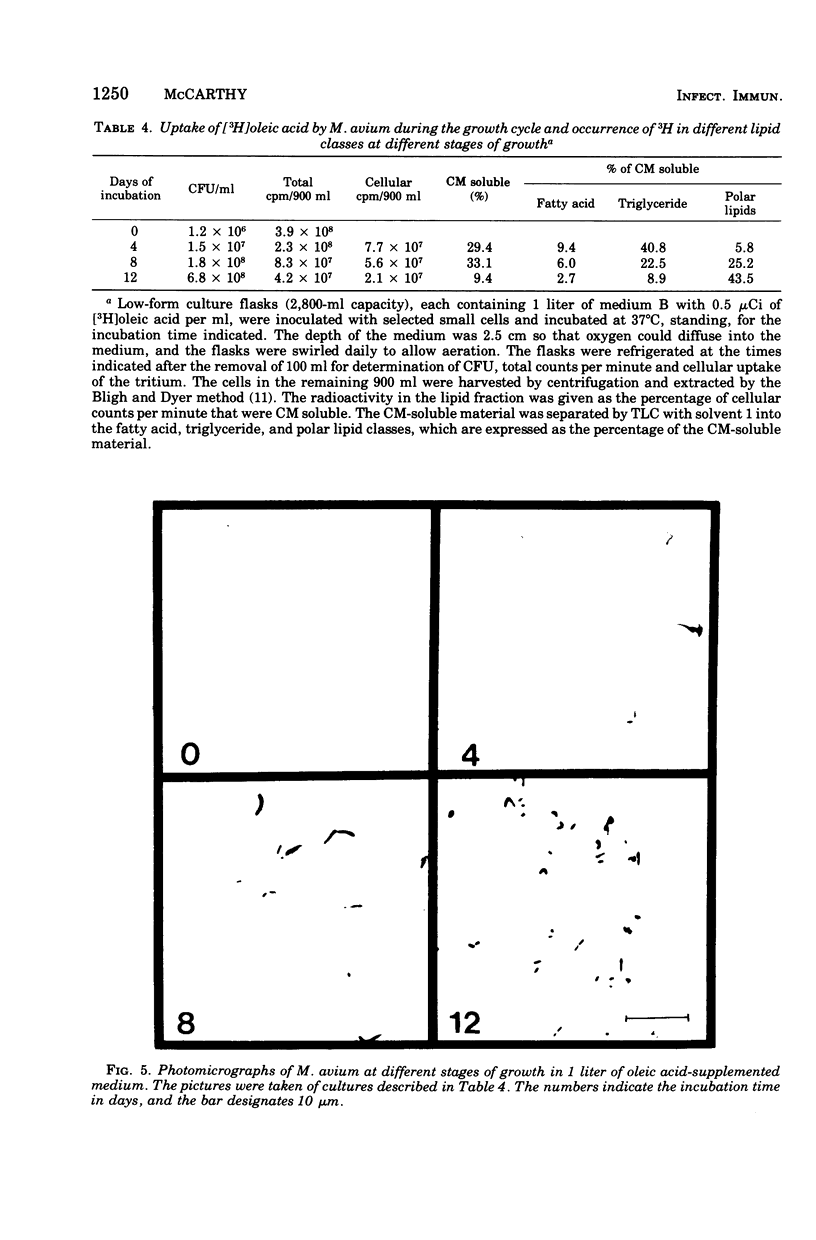
Mycobacterium avium exhibits a life cycle wherein small cells elongate to form filaments. The life cycle is unique in that elongated cells will undergo rapid division by fragmentation only if fatty acid is present. The utilization of [14C]palmitic acid and [3H]oleic acid by M. avium during the life cycle was assessed. Four glycolipids, identifiable by elution patterns from hydroxylapatite columns, were associated with postfission cells and contained isotope from the precursor fatty acid. The incorporation of 3H from oleic acid into the cellular glycolipids was maximal during cell division, but as much as 73% of the radioactivity was lost to the lipids from cells in the postfission status. Three of the glycolipids were sulfatides into which 36S was incorporated by M. avium. The [35]sulfatides were synthesized by cells undergoing fragmentation and were recovered from the medium at the termination of cell fission. These results demonstrated that the isotope was not lost to the cells because of turnover, but rather that the labeled compounds were released, intact, from the cells after fission. Because of the facile release of the sulfolipids, it was suggested that they were part of the cell envelope of M. avium cells during the division process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMENTA J. S. A RAPID CHEMICAL METHOD FOR QUANTIFICATION OF LIPIDS SEPARATED BY THIN-LAYER CHROMATOGRAPHY. J Lipid Res. 1964 Apr;5:270–272. [PubMed] [Google Scholar]
- AUBERT E. Cold stain for acid-fast bacteria. Can J Public Health. 1950 Jan;41(1):31–31. [PubMed] [Google Scholar]
- Engbaek H. C., Vergmann B., Baess I. Non-photochromogenic mycobacteria serotype Davis. The inhomogeneity within the serological group and the relationship to Mycobacterium avium. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(5):619–631. [PubMed] [Google Scholar]
- Falk G. A., Hadley S. J., Sharkey F. E., Liss M., Muschenheim C. Mycobacterium avium infections in man. Am J Med. 1973 Jun;54(6):801–810. doi: 10.1016/0002-9343(73)90069-7. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Schaefer W. B. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect Immun. 1974 Jan;9(1):142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Mycobacterial lipids: selected topics. Bacteriol Rev. 1972 Mar;36(1):33–64. doi: 10.1128/br.36.1.33-64.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 9;210(1):116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. II. Structural studies. Biochim Biophys Acta. 1970 Jun 9;210(1):127–138. doi: 10.1016/0005-2760(70)90068-8. [DOI] [PubMed] [Google Scholar]
- Herrmann H. Nonenzymatic tight binding of radioactivity to macromolecular fractions as a source of error in labeling experiments. Anal Biochem. 1974 May;59(1):293–301. doi: 10.1016/0003-2697(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Kato M., Goren M. B. Synergistic action of cord factor and mycobacterial sulfatides on mitochondria. Infect Immun. 1974 Oct;10(4):733–741. doi: 10.1128/iai.10.4.733-741.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides. J Lipid Res. 1968 May;9(3):319–327. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakshminarayan S., Sahn S. Disseminated infection caused by Mycobacterium avium. Report of a case with associated leukopenia. Am Rev Respir Dis. 1973 Jul;108(1):123–126. doi: 10.1164/arrd.1973.108.1.123. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Effect of palmitic acid utilization on cell division in Mycobacterium avium. Infect Immun. 1974 Feb;9(2):363–372. doi: 10.1128/iai.9.2.363-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Electronic counting in growth studies of Mycobacterium avium. Appl Microbiol. 1971 Oct;22(4):546–551. doi: 10.1128/am.22.4.546-551.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Utilization of palmitic acid by Mycobacterium avium. Infect Immun. 1971 Sep;4(3):199–204. doi: 10.1128/iai.4.3.199-204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Schröder K. H., Amadio G. E., Anz W., Chaparas S., Engel H. W., Jenkins P. A., Käppler W., Kleeberg H. H., Kubala E. A co-operative numerical analysis of nonscoto- and nonphotochromogenic slowly growing mycobacteria. J Gen Microbiol. 1974 Aug;83(2):207–235. doi: 10.1099/00221287-83-2-207. [DOI] [PubMed] [Google Scholar]
- Moore T. D., Allen A. M., Ganaway J. R., Sevy C. E. A fatal infection in the opossum due to Mycobacterium intracellulare. J Infect Dis. 1971 Jun;123(6):569–578. doi: 10.1093/infdis/123.6.569. [DOI] [PubMed] [Google Scholar]
- Reznikov M., Stranger R. S., Leggo J. H., Young A. V. Mycobacterial lymphadenitis in pigs on the Darling Downs. Aust Vet J. 1973 May;49(5):264–265. doi: 10.1111/j.1751-0813.1973.tb05225.x. [DOI] [PubMed] [Google Scholar]
- Runyon E. H. Pathogenic mycobacteria. Bibl Tuberc. 1965;21:235–287. [PubMed] [Google Scholar]
- Saito H., Tasaka H., Osasa S., Yamura T., Fukuhara T. Disseminated Mycobacterium intracellulare infection. Am Rev Respir Dis. 1974 May;109(5):572–576. doi: 10.1164/arrd.1974.109.5.572. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Horowitz M. I. Separation of polar lipids by column chromatography on hydroxylapatite. J Chromatogr. 1970 Jun 24;49(3):455–461. doi: 10.1016/s0021-9673(00)93659-8. [DOI] [PubMed] [Google Scholar]
- Wijsmuller G., Erickson P. The reaction to PPD-Battey. A new look. Am Rev Respir Dis. 1974 Jan;109(1):29–40. doi: 10.1164/arrd.1974.109.1.29. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P. THE PATHOGENIC "ATYPICAL" MYCOBACTERIA. Annu Rev Microbiol. 1963;17:473–494. doi: 10.1146/annurev.mi.17.100163.002353. [DOI] [PubMed] [Google Scholar]