ABSTRACT
Serine proteases elicit cellular responses via protease-activated receptor-2 (PAR-2) which is known to regulate inflammation and the immune response. Although the gastrointestinal tract is exposed to large amounts of proteolytic enzymes, the role of PAR-2 in canine inflammatory bowel disease (IBD) remains unclear. The objective of this study was to investigate the effects of PAR-2 activation on inflammatory cytokine/chemokine gene expression in canine intestine and the expression of intestinal PAR-2 and fecal serine protease activity in dogs with IBD. Duodenal biopsies from healthy dogs were cultured and treated ex vivo with trypsin or PAR-2 agonist peptide, and inflammatory cytokine/chemokine gene expression in the tissues was then quantified by real-time PCR. PAR-2 mRNA and protein expression levels in the duodenal mucosa were examined by real-time PCR and immunohistochemistry, respectively. Fecal serine protease activity was determined by azocasein assay. In ex vivo-cultured duodenum, trypsin and PAR-2 agonist peptide induced significant up-regulation of mRNA expression levels of interleukin-1 β (IL-1β), IL-8, mucosae-associated epithelial chemokine (MEC) and fractalkine, and this up-regulation was inhibited by a serine protease inhibitor. Duodenal PAR-2 mRNA and protein expression levels were higher in dogs with IBD than in healthy control dogs. Fecal serine protease activity was significantly elevated in dogs with IBD, and the level of activity correlated positively with the clinical severity score. These results suggest that PAR-2 may contribute to the pathogenesis of canine IBD by inducing expression of inflammatory mediators in response to luminal serine proteases.
Keywords: canine, chemokine, chronic enteropathy, cytokine, PAR-2
Inflammatory bowel disease (IBD) in dogs is a heterogeneous gastrointestinal disorder of undetermined etiology that is defined by its clinical and histopathological features and response to treatment [1, 10, 19]. The histological classification is based on the predominant type of infiltrating inflammatory cell and the area of the gut mucosa affected, and lymphocytic-plasmacytic enteritis (LPE) is the most common form of canine IBD [10]. Various histologic and immunophenotypic studies have shown that the inflamed mucosa contains elevated numbers of CD3+ and CD4+ T cells, immunoglobulin-containing plasma cells, macrophages and neutrophils [11, 18, 20, 23, 45]. Recently, we observed that the expression levels of several cytokines and chemokines in the intestinal mucosa were higher in dogs with IBD than in healthy dogs [28,29,30], suggesting that these inflammatory mediators may be involved in the development of chronic enteritis. However, the mechanism responsible for the elevated expression of these cytokines and chemokines in canine IBD lesions remains unknown.
Although the proteases in the intestinal lumen are traditionally considered to be digestive enzymes, recent observations suggest that luminal proteases also act as signaling molecules by cleaving and activating protease-activated receptors (PARs) [13, 36]. PARs, which are novel members of the G-protein-coupled, 7-transmembrane-domain receptor family, are activated by a unique mechanism. Proteases cleave within the extracellular N-terminus of PARs, thereby exposing tethered ligand domains that bind and activate the cleaved receptors [5, 21]. PARs can be activated pharmacologically in the absence of proteolytic cleavage by the use of synthetic peptides corresponding to the sequences of the specific tethered ligand domains (selective PAR agonist peptides). Four PAR family members (PAR-1, -2, -3 and -4) have been identified to date. PAR-1, PAR-3 and PAR-4 are activated by thrombin, whereas PAR-2 is activated by serine proteases, such as trypsin and tryptase. In humans and mice, PAR-2 is highly expressed in the gastrointestinal tract and may contribute to the pathogenesis of IBD [47, 48]. Colonic administration of PAR-2 agonist peptide induces colitis in wild-type mice, but not in PAR-2-deficient mice [4]. Moreover, the development of experimental colitis induced by 3 different methods, dextran sodium sulfate (DSS), trinitrobenzene sulfonic acid (TNBS) and oxazolone, is suppressed in PAR-2 deficient mice [16]. PAR-2 expression in the intestinal tissues is higher in human patients with IBD than in healthy subjects [22]. Furthermore, the PAR-2 activators trypsin and tryptase are present at elevated levels in the colons of human patients with IBD [2, 3, 9, 35]. These findings suggest that the interactions between PAR-2 and luminal serine proteases may be associated with the pathogenesis of IBD. As in humans and rodents, PAR-2 is prominently expressed in the small intestine and colon in dogs [27], which indicates that PAR-2 may also play a role in gut inflammation in this species. However, little is known about the pathophysiological roles of PAR-2 and luminal serine proteases in canine IBD. We speculated that PAR-2 activation may induce inflammatory cytokine/chemokine expression in canine intestine and that intestinal PAR-2 expression and serine protease activity may be increased in dogs with IBD. The aim of this study was to examine the effects of PAR-2 activation on inflammatory cytokine/chemokine gene expression in ex vivo-cultured duodenal tissues. Furthermore, we aimed to investigate the duodenal PAR-2 expression and fecal serine protease activity in dogs with IBD.
MATERIALS AND METHODS
Study population: Forty dogs with IBD were enrolled in this prospective study; these comprised 18 females (6 intact and 12 neutered) and 22 males (17 intact and 5 neutered) aged 38−170 months (median, 92 months) and weighing from 1.7 to 12.6 kg (median, 5.6 kg). The client-owned dogs were recruited from the Veterinary Medical Center of the University of Tokyo (VMC-UT) between January 2010 and October 2012. Informed consent was obtained from all owners, and the study protocol was approved by the animal care committee of VMC-UT. Case selection criteria of IBD were as described previously [29, 31]. Dogs that had been treated with corticosteroids within the 2 weeks prior to the beginning of the study were excluded. The clinical severity of IBD was scored in all dogs according to the canine chronic enteropathy clinical activity index (CCECAI) [1]. All cases had evidence of inflammation within intestinal mucosa and a histopathologic diagnosis of LPE. A summary of clinical and histopathologic data of the IBD dogs is shown in Table S1 and S2, respectively.
Twenty-two dogs with self-limiting (duration<7 days) acute diarrhea were used as “other gastrointestinal disease” controls; these comprised 16 females (9 intact and 7 neutered) and 6 males (all intact) aged 2−167 months (median, 31 months) and weighing from 0.6 to 21 kg (median, 4.9 kg). None of these dogs received any drugs in the 2 weeks prior to the sampling. The breeds represented were Miniature Dachshund (n=4), French Bulldog (n=3), Papillon (n=3), mixed breed (n=3), Yorkshire Terrier (n=2), Pembroke Welsh Corgi (n=2), Toy Poodle (n=1), Chihuahua (n=1), Chinese Crested Dog (n=1), Shih Tzu (n=1) and Maltese (n=1). These dogs were diagnosed with acute diarrhea of unknown etiology (n=10), hemorrhagic gastroenteritis (n=6), fecal spore-forming bacteria (n=4), fecal infection with a Campylobacter-like organism (n=1) and foreign body ingestion (n=1).
Twenty-five beagles were used as healthy controls; these comprised 9 females (1 intact and 8 neutered) and 16 males (10 intact and 6 neutered) aged 43−104 months (median, 61 months) and weighing from 8.3 to 15 kg (median, 11 kg). These dogs were healthy, exhibited no clinical signs of gastrointestinal disease, such as diarrhea, vomiting, anorexia or weight loss, and were not treated with any drugs. Routine urinalysis and blood examinations, including a complete blood count and measurements of blood urea nitrogen, creatinine, alanine aminotransferase and alkaline phosphatase levels, showed no abnormalities. Moreover, parasitic and bacterial analyses of the fecal samples revealed no abnormal findings. The use of dogs in this study was approved by the animal care committee of the University of Tokyo (approval no. P11-530).
Sample collection and histopathology: Fresh rectal feces were obtained from all dogs and stored at −80°C for later analysis. Duodenal tissue samples were collected by endoscopic biopsy from both healthy dogs and dogs with IBD under general anesthesia. Dogs were fasted for 12−18 hr prior to endoscopy. Six mucosal biopsy specimens were obtained from the stomach, duodenum, ileum and colon for histopathology. The samples were placed in 10% formalin for 48 hr and then embedded in paraffin, and hematoxylin and eosin (HE)-stained sections were prepared. A histopathologic diagnosis of gastrointestinal inflammation was made according to the World Small Animal Veterinary Association (WSAVA) criteria [6, 28, 31]. At least 1 biopsy specimen from each site was used for PCR for antigen receptor gene rearrangement [7]. Duodenal samples for RNA extraction were immediately submerged in RNAlater (Qiagen, Valencia, CA, U.S.A.) and stored at −20°C.
Ex vivo-cultured tissue and PAR-2 stimulation: Endoscopic biopsy specimens from 10 healthy beagles were cultured ex vivo as described in a previous study [41] with a slight modification. Duodenal biopsies (20 from each dog) were collected and immediately transferred into ice-cold Hank’s balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO, U.S.A.) containing 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich) and then rinsed 3 times. The duodenal tissues were seeded into 48-well tissue culture plates and incubated in RPMI 1640 medium (Invitrogen, Carlsbad, CA, U.S.A.) containing 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C under 5% CO2 for 1 hr. After pre-incubation, the tissues were exposed to 25 U/ml trypsin (Wako Pure Chemical Industries, Osaka, Japan), 250 µM human PAR-2 agonist peptide (SLIGKV-NH2; Bachem, Bubendorf, Switzerland) or 250 µM inverse control peptide (LSIGKV-NH2; Bachem) to activate PAR-2. The ability of the human PAR-2 agonist to activate canine PAR-2 was confirmed in a previous study [26]. HBSS alone was added to the tissues of each dog as an “unstimulated” control. For the inhibition study, the tissues were pretreated with a serine protease inhibitor, phenylmethyl sulphonyl fluoride (PMSF; Sigma-Aldrich) at 1.5 mM for 30 min and then exposed to 25 U/ml trypsin. The samples were incubated at 37°C under 5% CO2 for the indicated period (0, 3, 6, 12 or 24 hr), and total RNA was then extracted from the duodenal tissues. The mRNA expression levels of interleukin-1 β (IL-1β), tumour necrosis factor α (TNF-α), macrophage inflammatory protein-3 α (MIP-3α), monocyte chemotactic protein-1 (MCP-1), IL-8, thymus-expressed chemokine (TECK), mucosae-associated epithelial chemokine (MEC) and fractalkine in the ex vivo-cultured samples were determined by two-step real-time PCR, as described below. These cytokines and chemokines have been shown to be up-regulated in the inflamed mucosa in dogs with IBD [28,29,30]. The expression levels were compared between stimulated and unstimulated samples, and the fold change in expression of each target mRNA was calculated as the ratio of the level in the stimulated samples to the level in the unstimulated samples.
Quantitative real-time PCR: Total RNA was extracted from the duodenal samples using a commercially available kit (RNAspin Mini RNA Isolation Kit; GE Healthcare, Buckinghamshire, U.K.). Genomic DNA was eliminated from the samples using the TURBO DNA-free Kit (Applied Biosystems, Foster City, CA, U.S.A.), and the samples were then stored at −80°C for later use. Various cytokine/chemokine expressions in the ex vivo-cultured samples and duodenal PAR-2 expression in healthy dogs and dogs with IBD were determined by 2-step real-time RT-PCR as described previously [28, 29]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TATA-box binding protein (TBP) and succinate dehydrogenase complex subunit A (SDHA) were used as reference genes. These three housekeeping genes were chosen on the basis of published data [34]. The primer pair sequences used in this study are shown in Table 1. After quantification, the representative PCR products were sequenced by the dideoxy chain termination method (ABI prism BigDye Terminator v3.1 Cycle Sequencing Kit; Applied Biosystems) to confirm the amplification of the specific target genes.
Table 1. Sequences of the oligonucleotide primers used for quantitative real-time PCR.
| Primer set | Primer sequence (5′-3′) | Position of the primers | GenBank accession number | |
|---|---|---|---|---|
| PAR-2 | Forward | TGA AGA TCG CCT ACC ACA TCC A | 389–525 | AB458680 |
| Reverse | CCA ATA CCG TTG CAC ACT GA | |||
| IL-1β | Forward | ACC CGA ACT CAC CAG TGA AAT G | 12–121 | NM_001037971 |
| Reverse | GGT TCA GGT CTT GGC AGC AG | |||
| TNF-α | Forward | CCC AAG TGA CAA GCC AGT AGC TC | 249–394 | NM_001003244 |
| Reverse | ACA ACC CAT CTG ACG GCA CTA TC | |||
| MCP-1 | Forward | CAC CTG CTG CTA TAC ACT CAC C | 97–196 | NM_001003297 |
| Reverse | GAT CAC AGC TTC TTT GGG ACA | |||
| MIP-3α | Forward | ATC ATG GGC TTC ACA CAA CA | 139–256 | NM_001005254 |
| Reverse | TCC GTT TCA CCC ATT TCT TC | |||
| IL-8 | Forward | CTT CCA AGC TGG CTG TTG CTC | 11–183 | NM_001003200 |
| Reverse | TGG GCC ACT GTC AAT CAC TCT C | |||
| TECK | Forward | GCT GCT TAG CCT ACC ACC AC | 86–228 | NM_001005259 |
| Reverse | TGG GTT CAC ACA CAG CAT CT | |||
| MEC | Forward | CAG ACA GGA CTC ACT CTC GCT CTC | 7–113 | NM_001005257 |
| Reverse | TGT GAA ACC TCA GTG CAA CAG CTA | |||
| Fractalkine | Forward | CTT CCT TGG CCT CCT CTT CT | 1119–1265 | AB648939 |
| Reverse | GGC ACC AGG ACA TAC GAG TT | |||
| TBP | Forward | CTA TTT CTT GGT GTG CAT GAG G | 72–167 | XM_849432 |
| Reverse | CCT CGG CAT TCA GTC TTT TC | |||
| GAPDH | Forward | CAT TGC CCT CAA TGA CCA CT | 894–998 | NM_001003142 |
| Reverse | TCC TTG GAG GCC ATG TAG AC | |||
| SDHA | Forward | GCC TTG GAT CTC TTG ATG GA | 855–946 | XM535807 |
| Reverse | TTC TTG GCT CTT ATG CGA TG |
PAR-2: Protease-activated receptor-2, IL-1β: Interleukine-1 beta, TNF-α: Tumor necrosis factor alpha, MCP-1: Monocyte chemotactic protein-1, MIP-3α: Macrophage inflammatory protein-3 α, TECK: Thymus-expressed chemokine, MEC: Mucosae-associated epithelial chemokine, TBP: TATA box binding protein, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, SDHA: Succinate dehydrogenase complex subunit A.
Immunohistochemistry: Immunohistochemistry was conducted on 4-µm-thick paraffin-embedded sections of the duodenal samples obtained by endoscopic biopsy. Heat-induced antigen retrieval was performed by autoclaving the sections for 5 min at 121°C in 10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked by incubation with REAL Peroxidase-Blocking Solution (Dako, Glostrup, Denmark) at room temperature for 10 min. The sections were blocked with 5% skim milk in Tris-buffered saline (TBS) at room temperature for 60 min and then incubated with a mouse anti-human PAR-2 mAb (SAM 11; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) at 4°C overnight. The cross-reactivity of this mAb with canine PAR-2 was confirmed in a previous study [26]. The slides were washed with TBS and then incubated with EnVision polymer reagent (Dako) at room temperature for 45 min. The reaction products were visualized with 3,3′-diaminobenzidine. Negative control slides were processed similarly, except that an isotype-matched antibody (R&D Systems, Minneapolis, MN, U.S.A.) was used as the primary antibody. PAR-2+ epithelial cells were quantified in different compartments (villus and basal crypt area) with a software (ImageJ) [42]. Five appropriate sites were chosen for each compartment, and positively stained epithelial cells were counted (each of 200−300 epithelial cells).
Measurement of fecal serine protease activity: Fecal serine protease activity was measured by the azocasein assay (Sigma-Aldrich) as previously described [9, 40] with a slight modification. Fecal samples from dogs with IBD, dogs with acute diarrhea and healthy controls were thawed at 4°C. Each fecal sample (0.1−0.5 g) was added to 1 ml of reaction buffer (0.5% W/V NaHCO3, pH 8.3) and homogenized. The homogenate was centrifuged at 1,800 g for 10 min at 4°C, and any remaining coarse particles were removed by passing the supernatant through a 0.8-µm pore size syringe filter. Trypsin was serially diluted in the reaction buffer to various concentrations (364−46,600 U/ml) to build the standard curve. Aliquots of the trypsin solution and the fecal homogenate supernatants were incubated with 100 µl of reaction buffer and 100 µl of azocasein solution (0.5% W/V azocasein in reaction buffer) at 40°C for 20 min. Each reaction was terminated with 100 µl of 10% V/V trichloroacetic acid. After centrifugation at 1,800 g for 10 min at 4°C, the absorbance values at 450 nm of the clear supernatants were measured using a microplate reader (Bio-Rad Laboratories, Hercules, CA, U.S.A.). All of the samples were analyzed in duplicate, and the mean OD was calculated and reported. The total protein concentrations of the samples were determined by the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories), and the serine protease activity was expressed as units of trypsin activity per mg of total protein (U/mg).
Statistics: Statistical analyses were performed using JMP version 9 (SAS Institute, Cary, NC, U.S.A.). The mRNA expression levels of cytokines and chemokines in ex vivo tissues among baseline and other time points of each treatment were compared using the Kruskal–Wallis test with the Dunn’s test. The Mann-Whitney U test was used to compare the expression levels of cytokine and chemokine mRNA between trypsin treatment and trypsin and PMSF treatment groups. The mRNA and protein expression levels of PAR-2 were compared between healthy dogs and dogs with IBD using the Mann-Whitney U test. Fecal serine protease activity was compared among healthy dogs, dogs with acute diarrhea and dogs with IBD using the Kruskal–Wallis test with the Dunn’s test. The relationship was evaluated using the Spearman rank correlation coefficient. Statistical significance was defined as P<0.05.
RESULTS
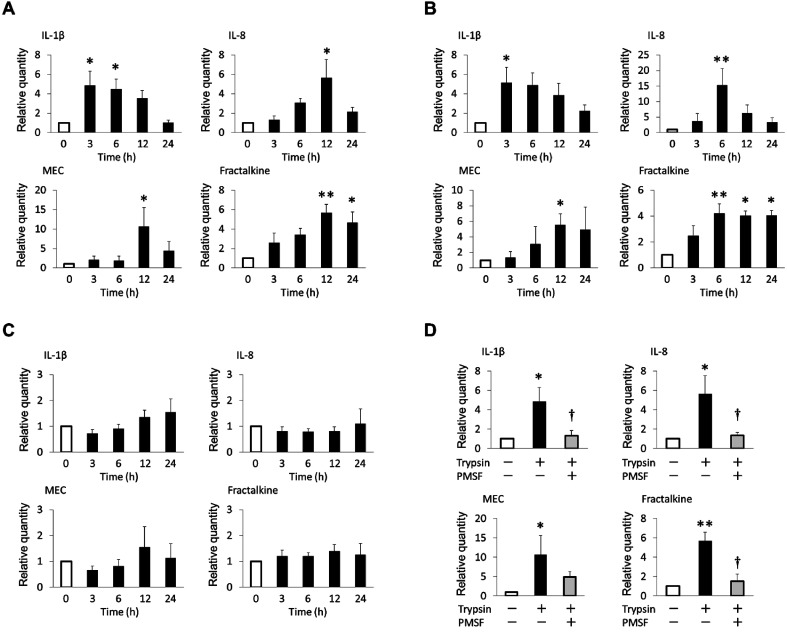
Effect of PAR-2 activation on mRNA expression levels of inflammatory cytokines and chemokines in ex vivo-cultured duodenal tissue: Each primer pair successfully amplified the appropriate cytokine or chemokine mRNA, as confirmed by sequencing analysis (data not shown). Expression of all cytokines and chemokines mRNA was observed in all ex vivo-cultured samples. Of the cytokines and chemokines investigated in this study, IL-1β, IL-8, MEC and fractalkine exhibited significantly elevated relative mRNA expression in ex vivo-stimulated tissues (Fig. 1). As shown in Fig. 1A, trypsin stimulation significantly increased the mRNA expression levels of IL-1β (P<0.05), IL-8 (P<0.05), MEC (P<0.05) and fractalkine (P<0.01). Similar effects were observed in tissues treated ex vivo with PAR-2 agonist peptide (Fig. 1B). In contrast, the inverse control peptide did not alter the mRNA expression levels of these cytokines and chemokines (Fig. 1C).
Fig. 1.
Effects of PAR-2 stimulation on the mRNA expression levels of inflammatory cytokines and chemokines in ex vivo-cultured tissues from healthy dogs (n=10). The mRNA expression levels of IL-1β, IL-8, MEC and fractalkine in tissues stimulated ex vivo with (A) 25 U/ml trypsin, (B) 250 µM PAR-2 agonist peptide (SLIGKV-NH2) and (C) 250 µM inverse control peptide (LSIGKV-NH2). (D) The mRNA expression levels of IL-1β, IL-8, MEC and fractalkine in tissues stimulated ex vivo with 25 U/ml trypsin for 3 hr (IL-1β) and 12 hr (IL-8, MEC and fractalkine) with or without pretreatment with 1.5 mM PMSF. TBP, SDHA and GAPDH were used as internal controls. Because the results were similar for the control genes, only the data standardized by TBP are shown. The mean relative expression represents the ratio of the level of the stimulated samples to that of the unstimulated samples at each time point. The error bars represent the SEM. *P<0.05, **P<0.01 vs. 0 hr unstimulated samples. †P<0.05 vs. trypsin-stimulated samples.
Next, we assessed the effect of pretreatment with a serine protease inhibitor, PMSF, on trypsin-induced up-regulation of cytokine expression to determine whether this up-regulation was due to the proteolytic activity. As shown in Fig. 1D, pretreatment with PMSF significantly inhibited the induction of mRNA expression of IL-1β, IL-8 and fractalkine in response to trypsin stimulation (P<0.05). PMSF also tended to abolish trypsin-induced MEC expression, but this inhibitory effect was not significant (Fig. 1D).
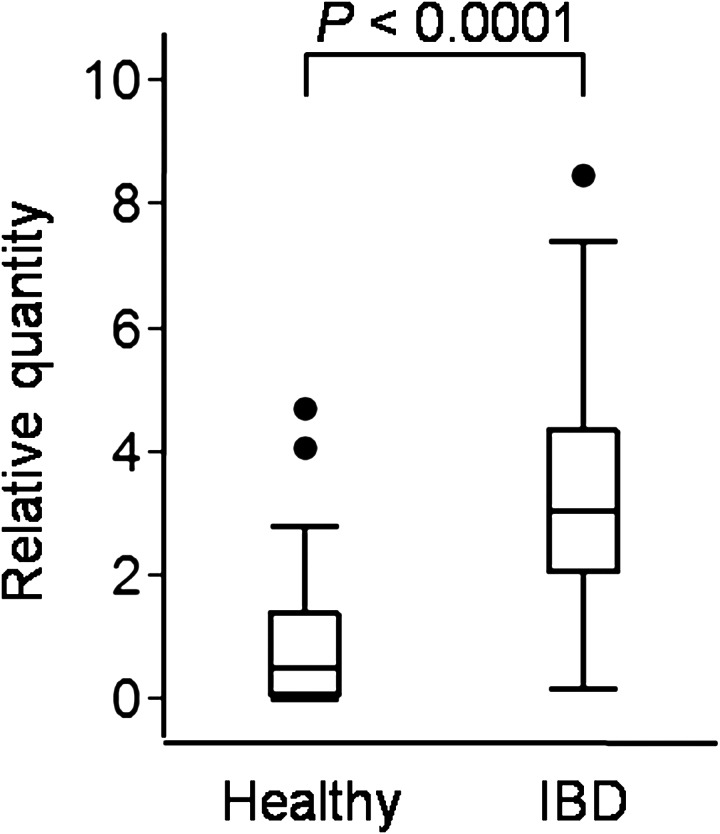
PAR-2 expression in duodenal mucosa: Canine PAR-2 mRNA was successfully amplified by PCR, as confirmed by sequencing analysis (data not shown). The duodenal PAR-2 mRNA expression levels were significantly higher in dogs with IBD than in healthy control dogs (P<0.0001; Fig. 2). However, the PAR-2 mRNA expression level did not correlate significantly with the CCECAI (rs=−0.1041, P=0.5157) and WSAVA score (rs=−0.1351, P=0.3989) in dogs with IBD.
Fig. 2.
The mRNA expression levels of PAR-2 in the duodenal mucosa of healthy dogs (n=25) and dogs with IBD (n=40). TBP, SDHA and GAPDH were used as internal controls. Because the results were similar for the control genes, only the data standardized by TBP are shown. Data are presented as the median with the 25th and 75th quartiles in each box plot. The whiskers indicate the highest and lowest data points within 1.5 times the length of the quartiles. The circles represent outliers.
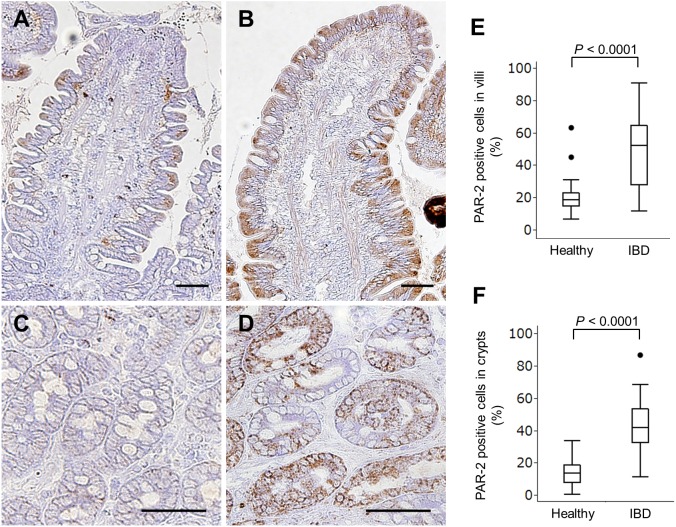
Immunohistochemistry was performed to investigate the localization of PAR-2 in canine intestinal mucosa. The isotype-matched negative controls indicated no non-specific staining (data not shown). Although duodenal villous and crypt epithelial cells expressed PAR-2 in healthy dogs and dogs with IBD, the immunoreactivity in dogs with IBD was more widespread and denser than that observed in healthy dogs (Fig. 3A–3D). The proportion of PAR-2+ epithelial cells in the villi (Fig. 3E) and crypts (Fig. 3F) was significantly higher in dogs with IBD than in healthy dogs (P<0.0001). However, the proportion of PAR-2+ epithelial cells did not correlate significantly with the CCECAI (villi, rs=0.0317, P=0.843; crypts, rs=0.032, P=0.8413) and WSAVA score (villi, rs=0.1069, P=0.5041; crypts, rs=−0.0854, P=0.5936) in dogs with IBD.
Fig. 3.
Detection of PAR-2 in the duodenal mucosa by immunohistochemistry. Representative results for PAR-2 expression in the villi (A) and crypts (C) of the duodenum from a healthy dog. Representative results for PAR-2 expression in the villi (B) and crypts (D) of the duodenum from a dog with IBD. Bar=50 µm. The proportions of PAR-2+ epithelial cells in the duodenal villi (E) and crypts (F) of healthy dogs (n=25) and dogs with IBD (n=40). Data are presented as the median with the 25th and 75th quartiles in each box plot. The whiskers indicate the highest and lowest data points within 1.5 times the length of the quartiles. The circles represent outliers.
Fecal serine protease activity: The median fecal serine protease activity in healthy dogs was 19.2 U/mg of total protein (range, 0.1−162.8), while the fecal serine protease activity in dogs with IBD was significantly higher (median, 44.1 U/mg of total protein, range, 1.5−394.2, P<0.01); however, no significant difference was identified between healthy dogs and dogs with acute diarrhea (median, 25.3 U/mg of total protein, range, 1.2−234; Fig. 4A). In addition, the fecal serine protease activity did not differ significantly between dogs with acute diarrhea and those with IBD (Fig. 4A). Further analysis showed that the fecal serine protease activity in dogs with IBD correlated significantly with the clinical severity (rs=0.3812, P=0.0173; Fig. 4B). Moreover, the fecal serine protease activity in healthy dogs and dogs with IBD correlated significantly with the intestinal PAR-2 mRNA (rs=0.2941, P=0.0186; Fig. 4C) and protein expression (villi, rs=0.3806, P=0.0023, crypts, rs=0.4035, P=0.0012; Fig. 4D and 4E). However, the fecal serine protease activity did not correlate significantly with the WSAVA score (rs=−0.0084, P=0.9581).
Fig. 4.
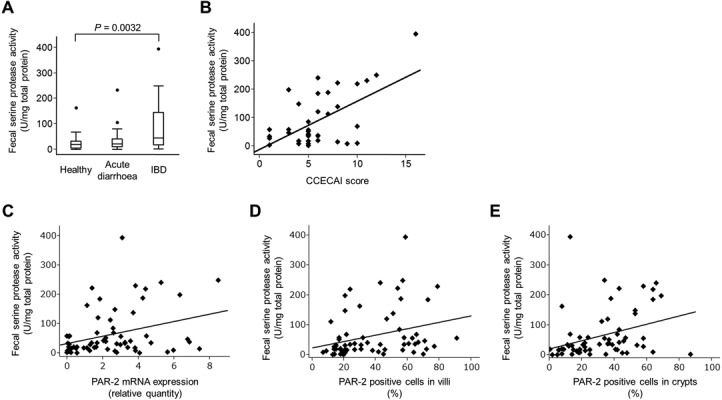
Fecal serine protease activity. (A) Fecal serine protease activity in healthy dogs (n=25), dogs with acute diarrhea (n=22) and dogs with IBD (n=40). The fecal serine protease activity was normalized to the total protein concentration of each sample. Data are presented as the median with the 25th and 75th quartiles in each box plot. The whiskers indicate the highest and lowest data points within 1.5 times the length of the quartiles. The circles represent outliers. (B) Significant correlation between the CCECAI score (x-axis) and the fecal serine protease activity (y-axis) in dogs with IBD (rs=0.3812, P=0.0173). (C) Significant correlation between the duodenal PAR-2 mRNA expression (x-axis) and the fecal serine protease activity (y-axis) in healthy dogs and dogs with IBD (rs=0.2941, P=0.0186). (D) Significant correlation between the proportion of PAR-2+ epithelial cells in the duodenal villi (x-axis) and the fecal serine protease activity (y-axis) in healthy dogs and dogs with IBD (rs=0.3806, P=0.0023). (E) Significant correlation between the proportion of PAR-2+ epithelial cells in the duodenal crypts (x-axis) and the fecal serine protease activity (y-axis) in healthy dogs and dogs with IBD (rs=0.4035, P=0.0012).
DISCUSSION
The present study shows that intestinal PAR-2 expression is up-regulated in dogs with IBD and that PAR-2 activation induces inflammatory cytokine/chemokine mRNA expression in canine intestine. As canine intestinal epithelial cell lines were unavailable, we adopted an ex vivo culture system [41] in this study. This ex vivo model includes epithelial cells, endothelial cells, lymphocytes, macrophages, neutrophils, eosinophils and probably other cell types as well. The immunohistochemical analysis performed in this study revealed that canine PAR-2 was selectively expressed in the intestinal epithelial cells; therefore, we believe that the increased expression levels of cytokines and chemokines in ex vivo-stimulated tissues were due to activation of the PAR-2 expressed in the epithelium. Previous reports have shown that PAR-2 activation induces IL-8 expression in human intestinal epithelial cells lines via the MAP kinase pathway [8, 46, 49]. In this study, we observed that both trypsin and the PAR-2 agonist peptide enhanced mRNA expression of IL-1β, IL-8, MEC and fractalkine in ex vivo-cultured intestinal tissues. These cytokines and chemokines have been shown to be up-regulated in the inflamed mucosa of dogs with IBD [28,29,30]. Furthermore, this study showed that PAR-2 mRNA expression was significantly increased within the duodenal mucosa of dogs with IBD. We also observed that the number of PAR-2+ epithelial cells was significantly increased in dogs with IBD. Combining our present and previous data, PAR-2 activation may be involved in the elevated expression of the inflammatory cytokines and chemokines in canine IBD lesions. However, the ex vivo tissues subjected to PAR-2 stimulation in this study were only derived from healthy dogs. Further investigation using ex vivo tissues from dogs with IBD is needed.
Subsequently, we found that fecal serine protease activity was elevated in dogs with IBD, but not in dogs with acute diarrhea. This finding suggests that the elevated levels of serine proteases in the intestinal lumen may induce over-activation of the PAR-2 expressed by the intestinal epithelial cells of dogs with IBD. This hypothesis is consistent with previous reports concerning human IBD [9, 40]. However, it is unclear whether fecal serine protease activity accurately reflects luminal protease activity. Further studies including the measurement of serine protease activity in the duodenal contents will be necessary to clarify this point. Several serine proteases, such as trypsin, elastase and mast cell tryptase, are elevated in the colons and feces of human patients with IBD [3, 9, 35]. In addition to endogenous proteases, PAR-2 can also be activated by various bacterial proteases [14, 15]. Although this study did not investigate the origin of the elevated serine protease activity in dogs with IBD, both endogenous and exogenous serine proteases may be involved. Endogenous protease inhibitors control the protease activity to prevent the proteolytic injury of intestinal mucosa in healthy conditions [33, 44]. The high fecal serine protease activity observed in dogs with IBD may be associated with decreased endogenous protease inhibitors.
The mechanism of the increased PAR-2 expression in dogs with IBD remains unknown. A possible explanation for the increased PAR-2 expression in canine IBD lesions is the regulation of inflammatory cytokines. In human endothelial cells, inflammatory cytokines, such as IL-1β and TNF-α, induce PAR-2 expression [32, 38]. Both IL-1β and TNF-α are increased in canine IBD lesions [12, 29, 37]; therefore, these inflammatory cytokines may induce PAR-2 expression. Other possible explanation is the regulation of serine proteases. In mice, PAR-2 expression is regulated by luminal serine protease activity. Treatment with antibiotics reduced the luminal serine protease activity coupled with a decrease in intestinal PAR-2 expression. Under the antibiotic treatment, trypsin administration restored the PAR-2 expression, indicating that the effect on PAR-2 expression is specific for the protease activity [39]. In this study, we found the positive correlation between the fecal serine protease activity and the intestinal PAR-2 expression, suggesting that elevated serine protease activity in the lumen may lead to the up-regulation of PAR-2 in the epithelium.
Inhibition of PAR-2 activation by a novel PAR-2 antagonist (GB88) ameliorated disease activity in rodent models of arthritis and colitis [24, 25], suggesting that antagonism of PAR-2 protects against several inflammatory diseases. Furthermore, treatment with serine protease inhibitors, namely nafamostat mesilate and camostat mesilate, improved clinical and histological findings in mice with TNBS-induced colitis and human patients with IBD [17, 43, 50]. In this study, the fecal serine protease activity correlated positively and significantly with the clinical severity of IBD in affected dogs. Moreover, a serine protease inhibitor blocked trypsin-induced cytokine up-regulation in ex vivo-stimulated tissues. These findings suggest that blockade of the luminal protease−PAR-2 pathway by serine protease inhibitors may be an effective approach to treating canine IBD.
This study may be limited by the potential bias introduced by the different dog breeds represented in each group. As it was impossible to obtain normal duodenal samples that were breed-matched to the clinical cases, all healthy control dogs were of the same breed (Beagle). More extensive studies will be required to investigate breed differences in PAR-2 expression and fecal serine protease activity.
In summary, we have demonstrated that PAR-2 activation induces mRNA expression of IL-1β, IL-8, MEC and fractalkine in canine intestine. We also show that the duodenal PAR-2 expression and fecal serine protease activity are increased in dogs with IBD compared to healthy dogs. Serine protease−PAR-2 pathway may mediate intestinal inflammation in canine IBD through cytokine and chemokine production.
Supplementary table
Acknowledgments
This study was supported by a Grant-in-Aid for Science Research and JSPS Fellows of Japan Society for the Promotion of Science.
REFERENCES
- 1.Allenspach K., Wieland B., Grone A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 2.Bustos D., Negri G., De Paula J. A., Di Carlo M., Yapur V., Facente A., De Paula A.1998. Colonic proteinases: increased activity in patients with ulcerative colitis. Medicina (B. Aires) 58: 262–264 [PubMed] [Google Scholar]
- 3.Cenac N., Andrews C. N., Holzhausen M., Chapman K., Cottrell G., Andrade-Gordon P., Steinhoff M., Barbara G., Beck P., Bunnett N. W., Sharkey K. A., Ferraz J. G., Shaffer E., Vergnolle N.2007. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 117: 636–647. doi: 10.1172/JCI29255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenac N., Coelho A. M., Nguyen C., Compton S., Andrade-Gordon P., MacNaughton W. K., Wallace J. L., Hollenberg M. D., Bunnett N. W., Garcia-Villar R., Bueno L., Vergnolle N.2002. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 161: 1903–1915. doi: 10.1016/S0002-9440(10)64466-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlin S. R.1994. Protease-activated receptors start a family. Proc. Natl. Acad. Sci. U.S.A. 91: 9200–9202. doi: 10.1073/pnas.91.20.9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day M. J., Bilzer T., Mansell J., Wilcock B., Hall E. J., Jergens A., Minami T., Willard M., Washabau R.2008. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 138 (Suppl 1): S1–43. doi: 10.1016/j.jcpa.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Fukushima K., Ohno K., Koshino-Goto Y., Uchida K., Nomura K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2009. Sensitivity for the detection of a clonally rearranged antigen receptor gene in endoscopically obtained biopsy specimens from canine alimentary lymphoma. J. Vet. Med. Sci. 71: 1673–1676. doi: 10.1292/jvms.001673 [DOI] [PubMed] [Google Scholar]
- 8.Fyfe M., Bergstrom M., Aspengren S., Peterson A.2005. PAR-2 activation in intestinal epithelial cells potentiates interleukin-1beta-induced chemokine secretion via MAP kinase signaling pathways. Cytokine 31: 358–367. doi: 10.1016/j.cyto.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Gecse K., Roka R., Ferrier L., Leveque M., Eutamene H., Cartier C., Ait-Belgnaoui A., Rosztoczy A., Izbeki F., Fioramonti J., Wittmann T., Bueno L.2008. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57: 591–599. doi: 10.1136/gut.2007.140210 [DOI] [PubMed] [Google Scholar]
- 10.German A. J., Hall E. J., Day M. J.2003. Chronic intestinal inflammation and intestinal disease in dogs. J. Vet. Intern. Med. 17: 8–20. doi: 10.1111/j.1939-1676.2003.tb01318.x [DOI] [PubMed] [Google Scholar]
- 11.German A. J., Hall E. J., Day M. J.2001. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J. Vet. Intern. Med. 15: 14–25. doi: 10.1111/j.1939-1676.2001.tb02292.x [DOI] [PubMed] [Google Scholar]
- 12.German A. J., Helps C. R., Hall E. J., Day M. J.2000. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig. Dis. Sci. 45: 7–17. doi: 10.1023/A:1005436721798 [DOI] [PubMed] [Google Scholar]
- 13.Hansen K. K., Oikonomopoulou K., Baruch A., Ramachandran R., Beck P., Diamandis E. P., Hollenberg M. D.2008. Proteinases as hormones: targets and mechanisms for proteolytic signaling. Biol. Chem. 389: 971–982. doi: 10.1515/BC.2008.120 [DOI] [PubMed] [Google Scholar]
- 14.Hansen K. K., Sherman P. M., Cellars L., Andrade-Gordon P., Pan Z., Baruch A., Wallace J. L., Hollenberg M. D., Vergnolle N.2005. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc. Natl. Acad. Sci. U.S.A. 102: 8363–8368. doi: 10.1073/pnas.0409535102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzhausen M., Spolidorio L. C., Ellen R. P., Jobin M. C., Steinhoff M., Andrade-Gordon P., Vergnolle N.2006. Protease-activated receptor-2 activation: a major role in the pathogenesis of Porphyromonas gingivalis infection. Am. J. Pathol. 168: 1189–1199. doi: 10.2353/ajpath.2006.050658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyun E., Andrade-Gordon P., Steinhoff M., Vergnolle N.2008. Protease-activated receptor-2 activation: a major actor in intestinal inflammation. Gut 57: 1222–1229. doi: 10.1136/gut.2008.150722 [DOI] [PubMed] [Google Scholar]
- 17.Isozaki Y., Yoshida N., Kuroda M., Handa O., Takagi T., Kokura S., Ichikawa H., Naito Y., Okanoue T., Yoshikawa T.2006. Anti-tryptase treatment using nafamostat mesilate has a therapeutic effect on experimental colitis. Scand. J. Gastroenterol. 41: 944–953. doi: 10.1080/00365520500529470 [DOI] [PubMed] [Google Scholar]
- 18.Jergens A. E., Gamet Y., Moore F. M., Niyo Y., Tsao C., Smith B.1999. Colonic lymphocyte and plasma cell populations in dogs with lymphocytic-plasmacytic colitis. Am. J. Vet. Res. 60: 515–520 [PubMed] [Google Scholar]
- 19.Jergens A. E., Moore F. M., Haynes J. S., Miles K. G.1992. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987–1990). J. Am. Vet. Med. Assoc. 201: 1603–1608 [PubMed] [Google Scholar]
- 20.Jergens A. E., Moore F. M., Kaiser M. S., Haynes J. S., Kinyon J. M.1996. Morphometric evaluation of immunoglobulin A-containing and immunoglobulin G-containing cells and T cells in duodenal mucosa from healthy dogs and from dogs with inflammatory bowel disease or nonspecific gastroenteritis. Am. J. Vet. Res. 57: 697–704 [PubMed] [Google Scholar]
- 21.Kawabata A.2002. PAR-2: structure, function and relevance to human diseases of the gastric mucosa. Expert Rev. Mol. Med. 4: 1–17. doi: 10.1017/S1462399402004799 [DOI] [PubMed] [Google Scholar]
- 22.Kim J. A., Choi S. C., Yun K. J., Kim D. K., Han M. K., Seo G. S., Yeom J. J., Kim T. H., Nah Y. H., Lee Y. M.2003. Expression of protease-activated receptor 2 in ulcerative colitis. Inflamm. Bowel Dis. 9: 224–229. doi: 10.1097/00054725-200307000-00002 [DOI] [PubMed] [Google Scholar]
- 23.Kleinschmidt S., Meneses F., Nolte I., Hewicker-Trautwein M.2007. Characterization of mast cell numbers and subtypes in biopsies from the gastrointestinal tract of dogs with lymphocytic-plasmacytic or eosinophilic gastroenterocolitis. Vet. Immunol. Immunopathol. 120: 80–92. doi: 10.1016/j.vetimm.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 24.Lohman R. J., Cotterell A. J., Barry G. D., Liu L., Suen J. Y., Vesey D. A., Fairlie D. P.2012. An antagonist of human protease activated receptor-2 attenuates PAR2 signaling, macrophage activation, mast cell degranulation, and collagen-induced arthritis in rats. FASEB J. 26: 2877–2887. doi: 10.1096/fj.11-201004 [DOI] [PubMed] [Google Scholar]
- 25.Lohman R. J., Cotterell A. J., Suen J., Liu L., Do A. T., Vesey D. A., Fairlie D. P.2012. Antagonism of protease-activated receptor 2 protects against experimental colitis. J. Pharmacol. Exp. Ther. 340: 256–265. doi: 10.1124/jpet.111.187062 [DOI] [PubMed] [Google Scholar]
- 26.Maeda S., Maeda S., Ohno K., Kaji N., Hori M., Fujino Y., Tsujimoto H.2013. Protease-activated receptor-2 induces proinflammatory cytokine and chemokine gene expression in canine keratinocytes. Vet. Immunol. Immunopathol. 153: 17–25. doi: 10.1016/j.vetimm.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 27.Maeda S., Maeda S., Shibata S., Chimura N., Fukata T.2009. Molecular cloning of canine protease-activated receptor-2 and its expression in normal dog tissues and atopic skin lesions. J. Vet. Med. Sci. 71: 577–582. doi: 10.1292/jvms.71.577 [DOI] [PubMed] [Google Scholar]
- 28.Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Nakajima M., Goto-Koshino Y., Fujino Y., Tsujimoto H.2012. Increased expression of fractalkine and its receptor CX(3)CR1 in canine inflammatory bowel disease and their possible role in recruitment of intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 148: 226–235. doi: 10.1016/j.vetimm.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 29.Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Tsukamoto A., Goto-Koshino Y., Fujino Y., Tsujimoto H.2012. Mucosal imbalance of interleukin-1beta and interleukin-1 receptor antagonist in canine inflammatory bowel disease. Vet. J. 194: 66–70. doi: 10.1016/j.tvjl.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 30.Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Tsukamoto A., Goto-Koshino Y., Fujino Y., Tsujimoto H.2011. Quantification of chemokine and chemokine receptor gene expression in duodenal mucosa of dogs with inflammatory bowel disease. Vet. Immunol. Immunopathol. 144: 290–298. doi: 10.1016/j.vetimm.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 31.Maeda S., Ohno K., Uchida K., Nakashima K., Fukushima K., Tsukamoto A., Nakajima M., Fujino Y., Tsujimoto H.2013. Decreased immunoglobulin a concentrations in feces, duodenum, and peripheral blood mononuclear cells of dogs with inflammatory bowel disease. J. Vet. Intern. Med. 27: 47–55. doi: 10.1111/jvim.12023 [DOI] [PubMed] [Google Scholar]
- 32.Nystedt S., Ramakrishnan V., Sundelin J.1996. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 271: 14910–14915. doi: 10.1074/jbc.271.25.14910 [DOI] [PubMed] [Google Scholar]
- 33.Nyström M., Westin U. P., Linder C., Ohlsson K.2001. Secretory leukocyte protease inhibitor in punch biopsies from human colonic mucosa. Mediators Inflamm. 10: 269–272. doi: 10.1080/09629350120093740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters I. R., Peeters D., Helps C. R., Day M. J.2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol. 117: 55–66. doi: 10.1016/j.vetimm.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 35.Raithel M., Winterkamp S., Pacurar A., Ulrich P., Hochberger J., Hahn E. G.2001. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand. J. Gastroenterol. 36: 174–179. doi: 10.1080/003655201750065933 [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran R., Hollenberg M. D.2008. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br. J. Pharmacol. 153 Suppl. 1: S263–S282. doi: 10.1038/sj.bjp.0707507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridyard A. E., Nuttall T. J., Else R. W., Simpson J. W., Miller H. R.2002. Evaluation of Th1, Th2 and immunosuppressive cytokine mRNA expression within the colonic mucosa of dogs with idiopathic lymphocytic-plasmacytic colitis. Vet. Immunol. Immunopathol. 86: 205–214. doi: 10.1016/S0165-2427(02)00039-9 [DOI] [PubMed] [Google Scholar]
- 38.Ritchie E., Saka M., Mackenzie C., Drummond R., Wheeler-Jones C., Kanke T., Plevin R.2007. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br. J. Pharmacol. 150: 1044–1054. doi: 10.1038/sj.bjp.0707150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Róka R., Demaude J., Cenac N., Ferrier L., Salvador-Cartier C., Garcia-Villar R., Fioramonti J., Bueno L.2007. Colonic luminal proteases activate colonocyte proteinase-activated receptor-2 and regulate paracellular permeability in mice. Neurogastroenterol. Motil. 19: 57–65. doi: 10.1111/j.1365-2982.2006.00851.x [DOI] [PubMed] [Google Scholar]
- 40.Róka R., Rosztoczy A., Leveque M., Izbeki F., Nagy F., Molnar T., Lonovics J., Garcia-Villar R., Fioramonti J., Wittmann T., Bueno L.2007. A pilot study of fecal serine-protease activity: a pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 5: 550–555. doi: 10.1016/j.cgh.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 41.Sauter S. N., Allenspach K., Gaschen F., Grone A., Ontsouka E., Blum J. W.2005. Cytokine expression in an ex vivo culture system of duodenal samples from dogs with chronic enteropathies: modulation by probiotic bacteria. Domest. Anim. Endocrinol. 29: 605–622. doi: 10.1016/j.domaniend.2005.04.006 [DOI] [PubMed] [Google Scholar]
- 42.Schneider C. A., Rasband W. S., Eliceiri K. W.2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senda S., Fujiyama Y., Bamba T., Hosoda S.1993. Treatment of ulcerative colitis with camostat mesilate, a serine protease inhibitor. Intern. Med. 32: 350–354. doi: 10.2169/internalmedicine.32.350 [DOI] [PubMed] [Google Scholar]
- 44.Si-Tahar M., Merlin D., Sitaraman S., Madara J. L.2000. Constitutive and regulated secretion of secretory leukocyte proteinase inhibitor by human intestinal epithelial cells. Gastroenterology 118: 1061–1071. doi: 10.1016/S0016-5085(00)70359-3 [DOI] [PubMed] [Google Scholar]
- 45.Stonehewer J., Simpson J. W., Else R. W., Macintyre N.1998. Evaluation of B and T lymphocytes and plasma cells in colonic mucosa from healthy dogs and from dogs with inflammatory bowel disease. Res. Vet. Sci. 65: 59–63. doi: 10.1016/S0034-5288(98)90028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka Y., Sekiguchi F., Hong H., Kawabata A.2008. PAR2 triggers IL-8 release via MEK/ERK and PI3-kinase/Akt pathways in GI epithelial cells. Biochem. Biophys. Res. Commun. 377: 622–626. doi: 10.1016/j.bbrc.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 47.Vergnolle N.2005. Clinical relevance of proteinase activated receptors (pars) in the gut. Gut 54: 867–874. doi: 10.1136/gut.2004.048876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vergnolle N.2008. Proteinase-activated receptors (PARs) in infection and inflammation in the gut. Int. J. Biochem. Cell Biol. 40: 1219–1227. doi: 10.1016/j.biocel.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 49.Wang H., Moreau F., Hirota C. L., MacNaughton W. K.2010. Proteinase-activated receptors induce interleukin-8 expression by intestinal epithelial cells through ERK/RSK90 activation and histone acetylation. FASEB J. 24: 1971–1980. doi: 10.1096/fj.09-137646 [DOI] [PubMed] [Google Scholar]
- 50.Yoshida N., Yoshikawa T.2008. Basic and translational research on proteinase-activated receptors: implication of proteinase/proteinase-activated receptor in gastrointestinal inflammation. J. Pharmacol. Sci. 108: 415–421. doi: 10.1254/jphs.08R31FM [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.