Abstract
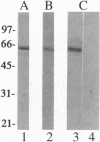
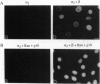
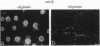
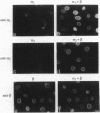
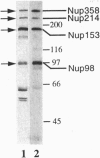
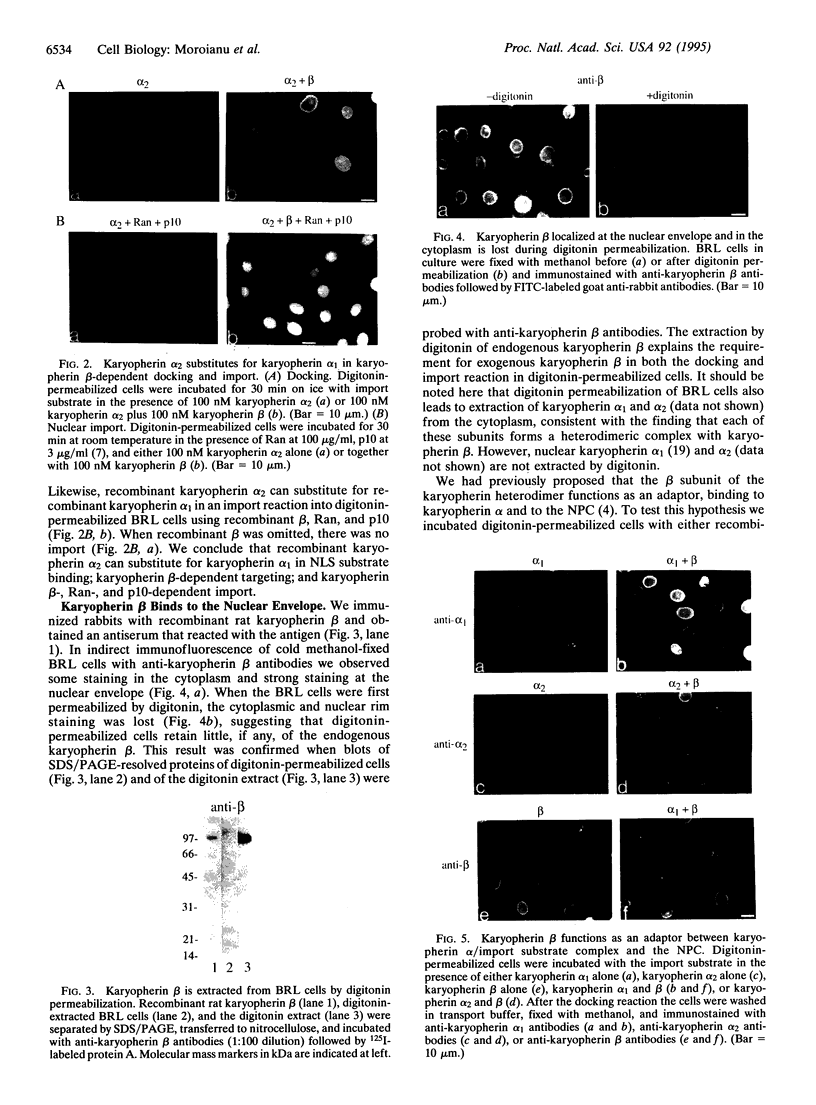
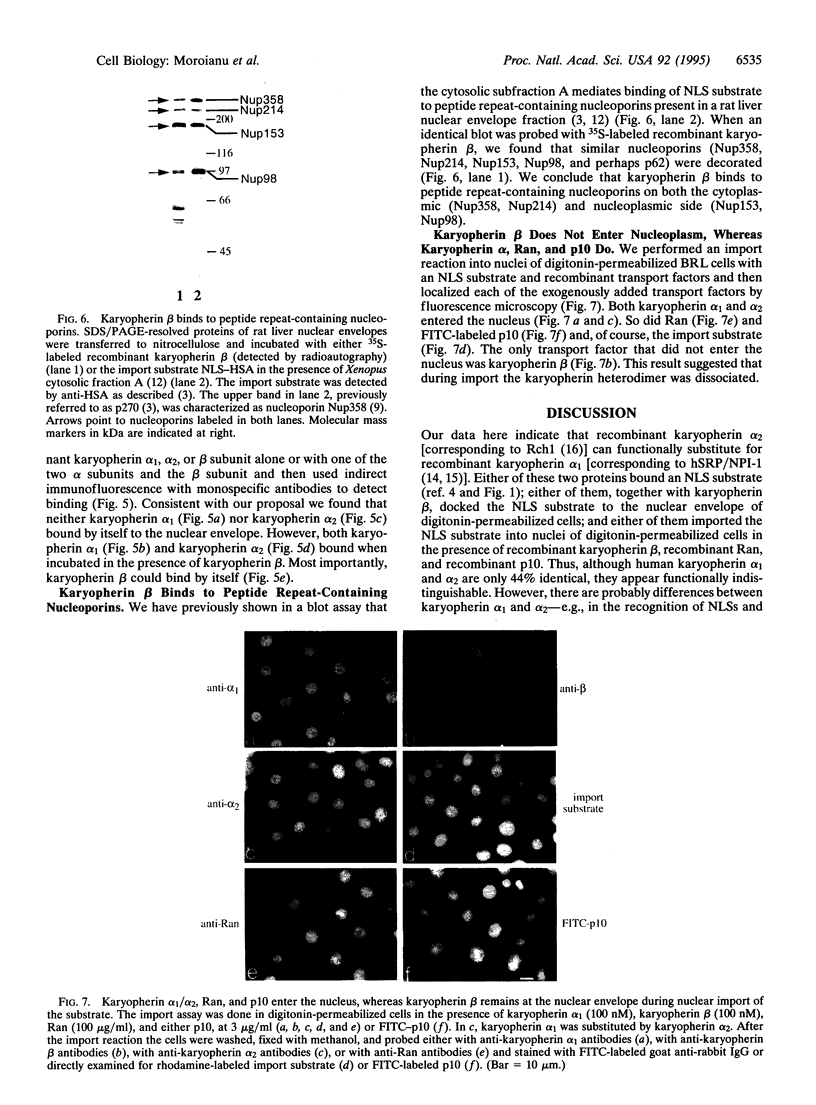
Although only 44% identical to human karyopherin alpha 1, human karyopherin alpha 2 (Rch1 protein) substituted for human karyopherin alpha 1 (hSRP-1/NPI-1) in recognizing a standard nuclear localization sequence and karyopherin beta-dependent targeting to the nuclear envelope of digitonin-permeabilized cells. By immunofluorescence microscopy of methanol-fixed cells, karyopherin beta was localized to the cytoplasm and the nuclear envelope and was absent from the nuclear interior. Digitonin permeabilization of buffalo rat liver cells depleted their endogenous karyopherin beta. Recombinant karyopherin beta can bind directly to the nuclear envelope of digitonin-permeabilized cells at 0 degree C (docking reaction). In contrast, recombinant karyopherin alpha 1 or alpha 2 did not bind unless karyopherin beta was present. Likewise, in an import reaction (at 20 degrees C) with all recombinant transport factors (karyopherin alpha 1 or alpha 2, karyopherin beta, Ran, and p10) import depended on karyopherin beta. Localization of the exogenously added transport factors after a 30-min import reaction showed karyopherin beta at the nuclear envelope and karyopherin alpha 1 or alpha 2, Ran, and p10 in the nuclear interior. In an overlay assay with SDS/PAGE-resolved and nitrocellulose-transferred proteins of the nuclear envelope, 35S-labeled karyopherin beta bound to at least four peptide repeat-containing nucleoporins--Nup358, Nup214, Nup153, and Nup98.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E. J., Adam S. A. Identification of cytosolic factors required for nuclear location sequence-mediated binding to the nuclear envelope. J Cell Biol. 1994 May;125(3):547–555. doi: 10.1083/jcb.125.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J., Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995 Apr 7;81(1):1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Cortes P., Ye Z. S., Baltimore D. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7633–7637. doi: 10.1073/pnas.91.16.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutavas E., Ren M., Oppenheim J. D., D'Eustachio P., Rush M. G. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993 Dec 9;366(6455):585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Cuomo C. A., Kirch S. A., Gyuris J., Brent R., Oettinger M. A. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6156–6160. doi: 10.1073/pnas.91.13.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann U., Nerlich C., Rein T., Lottspeich F., Küpper H. A. Isolation of cDNA coding for the placental protein 15 (PP15). Nucleic Acids Res. 1988 May 25;16(10):4721–4721. doi: 10.1093/nar/16.10.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Laskey R. A., Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994 Dec 2;79(5):767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Kraemer D., Wozniak R. W., Blobel G., Radu A. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1519–1523. doi: 10.1073/pnas.91.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992 Jul 10;70(1):127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- Melchior F., Paschal B., Evans J., Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993 Dec;123(6 Pt 2):1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. Purification of a Ran-interacting protein that is required for protein import into the nucleus. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10212–10216. doi: 10.1073/pnas.91.21.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993 Oct 14;365(6447):661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Moroianu J., Blobel G. Protein export from the nucleus requires the GTPase Ran and GTP hydrolysis. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4318–4322. doi: 10.1073/pnas.92.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J., Blobel G., Radu A. Previously identified protein of uncertain function is karyopherin alpha and together with karyopherin beta docks import substrate at nuclear pore complexes. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. E., Palese P. NPI-1, the human homolog of SRP-1, interacts with influenza virus nucleoprotein. Virology. 1995 Jan 10;206(1):116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Radu A., Blobel G., Moore M. S. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout M. P., Wente S. R. Pores for thought: nuclear pore complex proteins. Trends Cell Biol. 1994 Oct;4(10):357–365. doi: 10.1016/0962-8924(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Sukegawa J., Blobel G. A nuclear pore complex protein that contains zinc finger motifs, binds DNA, and faces the nucleoplasm. Cell. 1993 Jan 15;72(1):29–38. doi: 10.1016/0092-8674(93)90047-t. [DOI] [PubMed] [Google Scholar]