Abstract
[Purpose] VO2 is expressed as the product of cardiac output and O2 extraction by the Fick equation. During the incremental exercise test and constant high-intensity exercise test, VO2 results in the attainment of maximal O2 uptake at exhaustion. However, the differences in the physiological components, cardiac output and muscle O2 extraction, have not been fully elucidated. We tested the hypothesis that constant exercise would result in higher O2 extraction than incremental exercise at exhaustion. [Subjects] Twenty-five subjects performed incremental exercise and constant exercise at 80% of their peak work rate. [Methods] Ventilatory, cardiovascular, and muscle oxygenation responses were measured using a gas analyzer, Finapres, and near-infrared spectroscopy, respectively. [Results] VO2 was not significantly different between the incremental exercise and constant exercise. However, cardiac output and muscle O2 saturation were significantly lower for the constant exercise than the incremental exercise at the end of exercise. [Conclusion] These findings indicate that if both tests produce a similar VO2 value, the VO2 in incremental exercise would have a higher ratio of cardiac output than constant exercise, and VO2 in constant exercise would have a higher ratio of O2 extraction than incremental exercise at the end of exercise.
Key words: Cardiopulmonary exercise test, Fick equation, Near-infrared spectroscopy
INTRODUCTION
The cardiopulmonary exercise test (CPX) is the gold standard for evaluating the cause of exercise intolerance in patients with pulmonary and cardiac disease1). Various tests are available, each being more or less suitable as a stressor of a particular component of the patient’s pathophysiology. According to the American Thoracic Society/American College of Chest Physicians statement on CPX, the incremental exercise test (IET) is most widely used in clinical practice, but the constant high-intensity exercise test (CET) is gaining popularity because of its clinical applicability, particularly for monitoring responses to a spectrum of therapeutic interventions2).
The IET is used to calculate the aerobic threshold (AT)3), respiratory compensation point, and O2 at peak work rate (PW; VO2 peak or VO2 max)4). On the other hand, the CET is useful for the analysis of gas exchange kinetics5) and dynamic hyperinflation6). In addition, the change in time to the limit of tolerance in CET (also known as endurance time [ET]) at 75–80% load of PW has been shown to provide a more sensitive index of improvement than PW, or VO2 peak, in IET1, 7,8,9,10). Most recent studies of CET have used an 80% load of PW7, 8, 11,12,13).
VO2 is determined by the variables in the Fick equation:
| VO2 = Q × (CaO2 − CvO2) |
where Q is the cardiac output (CO; the product of heart rate [HR] and stroke volume [SV]), and CaO2 and CvO2 are the O2 contents of arterial and mixed venous blood, respectively. Thus, VO2 is expressed as the product of CO and O2 extraction. The VO2 value during CET reaches VO2 max, as assessed by IET, in the case of symptom limit and load (higher critical power) during CET14, 15). However, we conjectured that even if both tests reach VO2 peak at exhaustion, the component ratios are different because of the different exercise load patterns. We hypothesized that CET would reach a higher O2 extraction at exhaustion because of progressive fatigue due to a higher ATP turnover rate16, 17). Therefore, the purpose of this study was to compare the physiological changes during IET and CET at 80% load of PW in healthy human subjects.
SUBJECTS AND METHODS
Twenty-five young men (mean ± standard deviation [SD]; age = 23.3 ± 1.48 years, height = 169.4 ± 5.9 cm, body mass = 61.6 ± 6.4 kg) participated in this study. None of the subjects had routine fitness habits or were smokers. The subjects were informed of the potential risks and discomfort associated with the experiments before giving their written consent to participate in this study, which was approved by the ethical review committee of Kio University (Koryo-cho, Japan) and performed in accordance with the ethical principles of the Declaration of Helsinki.
Subjects reported to the laboratory on two separate occasions. An IET (work rate of 25 W/min, pedal revolution rate of 60 rpm/min) was performed using a braked cycle ergometer (Lode; Corival, Groningen, The Netherlands) on the first day of testing for the determination of estimated PW and other respiratory and metabolic factors. On a separate day, subjects returned to the laboratory for a CET at 80% of PW (pedal revolution rate of 60 rpm/min).
During both tests, we measured physiological responses, namely, ventilatory, metabolic, and cardiovascular responses, and muscle oxygenation. Ventilatory and metabolic variables were recorded breath-by-breath with a gas analyzer; cardiovascular responses were recorded with Finapres; and muscle oxygenation was recorded by near-infrared spectroscopy (NIRS).
Ventilatory and metabolic variables were recorded breath-by-breath with a computerized metabolic cart (MetaMax3B; Cortex, Leipzig, Germany). Oxygen uptake (VO2), carbon dioxide output (VCO2), minute ventilation (VE), the ventilator equivalent for carbon dioxide (VE/VCO2), the ratio of dead space to tidal volume (VD/VT), end-tidal carbon dioxide pressure (PETCO2), the respiratory exchange ratio (RER), and HR were determined from values averaged every 30 s.
Noninvasive continuous arterial systolic blood pressure (SBP) was measured beat-to-beat using the Finapres finger cuff (PORTEPRESS; FMS, Amsterdam, The Netherlands) on the index finger along with a height correction system attached to the top of the finger. The Finapres finger cuff uses the volume-clamp technique to measure finger arterial pressure18). Together with Beatscope software (Beatscope Easy; FMS, Amsterdam, The Netherlands), the Finapres finger cuff determines left ventricular SV; SV and HR values are used to calculate CO.
Oxygenation changes in the vastus lateralis muscle were evaluated by NIRS (BOM-L1TRW; Omegawave, Tokyo, Japan) at rest and during exercise. The NIRS probe was placed on the muscle approximately 14–20 cm above the knee joint. This instrument uses 3 light-emitting diodes (wavelengths: 780, 810, and 830 nm) and calculates the relative tissue levels of oxygenated hemoglobin (O2Hb), deoxygenated hemoglobin (HHb), and total hemoglobin (THb) according to the Beer-Lambert law. The absorption coefficients of hemoglobin (Hb) are based on previously reported data19), and individual values are proportional to the Hb levels. Levels of O2Hb, HHb, and THb are expressed in µmol/L, but Hb concentrations are expressed in arbitrary units (AU) because they do not represent actual physical volumes. We calculated muscle O2 saturation (SmO2) from the O2Hb and THb values with the following formula: SmO2 (%) = (O2Hb/THb) × 100.
Data analysis was performed using SPSS® version 19.0 statistical software (SPSS Inc., Chicago, IL, USA). The peak value for each of the measurements was defined as the mean of values acquired during the last 30 s of the test. All data are presented as the mean ± SD. Comparisons between the IET and CET results were analyzed using the paired t-test. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Table 1 shows the exercise capacity, and Table 2 shows the results of the comparison of end-exercise responses of the IET and CET. All subjects completed the IET, reaching exhaustion levels; HRmax was 94.2 ± 4.23% of the age-predicted value, and RER was 1.17 ± 0.0120). The VO2 max of IET was 2.55 ± 0.47 L/min, and there was no significant difference in VO2 between the IET and CET. SBP and CO were significantly higher in the IET than in the CET (p < 0.05). HHb was significantly higher and SmO2 was significantly lower in the CET than in the IET (p < 0.01).
Table 1. The results of exercise capacity.
| Variables | |
|---|---|
| Incremental exercise test (IET) | |
| VO2max (L/min) | 2.55±0.47 |
| Peak Watt: PW (W) | 202.9±22.6 |
| HR max (bpm) | 185.3±8.58 |
| VO2AT (L/min) | 1.32±0.22 |
| AT (W) | 98.7±18.8 |
| Constant high-intensity exercise test (CET) | |
| Work rate; 80% of PW (W) | 162.3±18.1 |
| Exercise tolerance time (sec) | 634.3±73.7 |
Variables are presented as mean±SD. VO2: oxygen uptake, HR: heart rate, AT: anaerobic threshold
Table 2. Comparison of exercise testing variables at end of exercise between IET and CET.
| Variables | IET | CET |
|---|---|---|
| VO2 (L/min) | 2.55±0.47 | 2.63±0.41 |
| VCO2 (L/min) | 2.92±0.43 | 2.71±0.37* |
| VE (L/min) | 68.9±12.3 | 67.5±12.1 |
| VE/VCO2 | 24.8±3.18 | 26.7±2.92* |
| VD/VT | 0.11±0.03 | 0.13±0.04* |
| PETCO2 | 45.2±6.26 | 42.5±4.30* |
| RER | 1.17±0.10 | 1.21±0.12 |
| HR (bpm) | 185.3±8.58 | 186.5±5.01 |
| SBP (mmHg) | 199.09±16.67 | 186.01±24.27* |
| SV (ml) | 96.93±17.85 | 82.2±20.07 |
| CO (L/min) | 16.1±3.23 | 13.23±3.02* |
| TPR | 0.68±0.28 | 0.57±0.25 |
| O2Hb (A.U.) | 7.63±1.68 | 7.24±1.88 |
| HHb (A.U.) | 9.07±2.86 | 9.53±2.74** |
| THb (A.U.) | 16.8±2.90 | 16.77±3.21 |
| SmO2 (%) | 46.4±0.09 | 43.6±0.09** |
p value of the paired t-test: *p<0.05, **p<0.01. Variables are presented as mean±SD. Each value was obtained at the end of exercise. IET: incremental exercise test, CET: constant high-intensity exercise test, VO2: oxygen uptake, VCO2: carbon dioxide output, VE: minute ventilation, VE/VCO2: ventilator equivalent for carbon dioxide, VD/VT: ratio of dead space to tidal volume, PETCO2: end-tidal carbon dioxide pressure, RER: respiratory exchange ratio, HR: heart rate, SBP: arterial blood pressure, SV: stroke volume, CO: cardiac output, O2Hb: oxygenated hemoglobin, HHb: deoxygenated hemoglobin, THb: total hemoglobin, SmO2: muscle oxygen saturation
DISCUSSION
In the present study, all subjects completed the IET and reached exhaustion levels20). Therefore, we believe that the IET was performed until exhaustion and that VO2 reached VO2 max. In addition, there were no significant differences in VO2 between the IET and CET. Therefore, we believe that VO2 during the CET also reached VO2 max.
SBP and CO in the IET were significantly higher than in the CET. These results suggest that the IET might have a higher cardiac load than the CET. This hypothesis is supported by the fact that VO2 max during the IET is mainly limited by the cardiovascular system in normal healthy subjects21) and that muscle blood flow is correlated linearly and positively with work rate22, 23). Further, the IET would demand a greater O2 delivery than the CET.
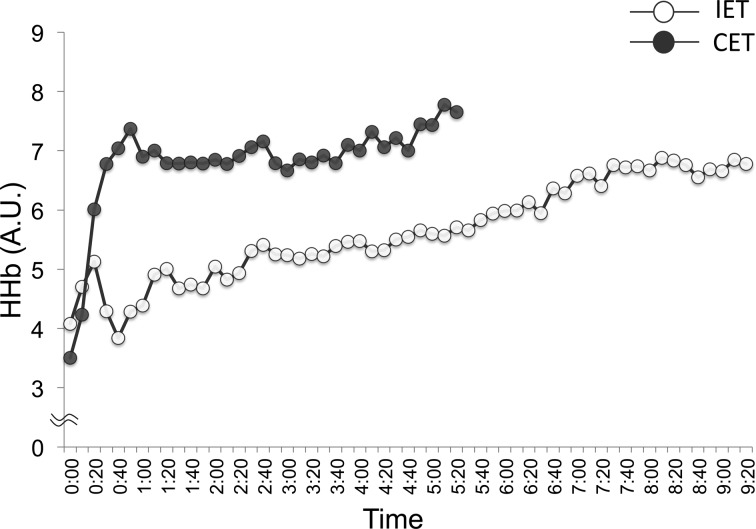
By the end of the exercises, HHb in the CET was higher than in the IET. HHb is considered to be similar to O2 extraction24, 25), and the increasing rate of HHb in venous occlusion can be used as a muscle O2 consumption index26). In addition, SmO2 was significantly lower in the CET than in the IET. SmO2 signals provide a reliable estimate of the dynamic balance between O2 supply and O2 consumption in the area of investigation27). These findings suggest that the CET has a higher muscle O2 extraction rate than the IET at the end of exercise. Interestingly, the findings may be explained by HHb kinetics at the end of the tests. At the end of the IET, the HHb response displays a slowdown or plateau point28,29,30), whereas at the end of the CET, the HHb response displays a progressive rise following the HHb “slow component”31, 32). We found that the HHb showed these trends in the present study (Fig. 1). The HHb slow component has been reported to be strongly correlated with lactate concentrations during running33). Lactic acidosis favors a greater release of O2 from Hb due to the rightward displacement of the oxyhemoglobin dissociation curve (Bohr effect), consequently facilitating O2 extraction from the blood. The physiological mechanisms of these HHb responses in relation to the IET and CET are still not fully understood and cannot be explained using the results of the present study, but it is likely that metabolic changes in the muscles play a role.
Fig. 1.
An example of deoxygenated hemoglobin (HHb) kinetics during exercise tests in a representative subject.
In conclusion, the present study demonstrated that there are differences in physiological responses between the IET and CET at the end of exercise. Although both exercise tests induced similar responses with respect to VO2, the factors determining VO2 changes were different: cardiac load was lower and muscle O2 extraction was higher in CET than IET. These results suggest that if both tests produce a similar VO2 value, VO2 in the IET would have a higher ratio of cardiac output than the CET, and VO2 in the CET would have a higher ratio of O2 extraction than the IET at the end of exercise. These findings may be useful for further assessment of individual exercise-limiting factors.
REFERENCES
- 1.Palange P, Ward SA, Carlsen KH, et al. ERS task force: recommendations on the use of exercise testing in clinical practice. Eur Respir J, 2007, 29: 185–209 [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic SocietyAmerican College of Chest Physicians: ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med, 2003, 167: 211–277 [DOI] [PubMed] [Google Scholar]
- 3.Wasserman K, McIlroy MB: Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am J Cardiol, 1964, 14: 844–852 [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Eisen H, Kussmaul W, et al. : Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation, 1991, 83: 778–786 [DOI] [PubMed] [Google Scholar]
- 5.Whipp BJ, Wasserman K: Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol, 1972, 33: 351–356 [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell DE: Breathlessness in patients with chronic airflow limitation. Mechanisms and management. Chest, 1994, 106: 904–912 [DOI] [PubMed] [Google Scholar]
- 7.Arizono S, Taniguchi H, Nishiyama O, et al. : Improvements in quadriceps force and work efficiency are related to improvements in endurance capacity following pulmonary rehabilitation in COPD patients. Intern Med, 2011, 50: 2533–2539 [DOI] [PubMed] [Google Scholar]
- 8.Oga T, Nishimura K, Tsukino M, et al. : The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. A comparison of three different exercise tests. Am J Respir Crit Care Med, 2000, 161: 1897–1901 [DOI] [PubMed] [Google Scholar]
- 9.Casaburi R: Factors determining constant work rate exercise tolerance in COPD and their role in dictating the minimal clinically important difference in response to interventions. COPD, 2005, 2: 131–136 [DOI] [PubMed] [Google Scholar]
- 10.Whipp BJ, Ward SA: Quantifying intervention-related improvements in exercise tolerance. Eur Respir J, 2009, 33: 1254–1260 [DOI] [PubMed] [Google Scholar]
- 11.Laviolette L, Bourbeau J, Bernard S, et al. : Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax, 2008, 63: 115–121 [DOI] [PubMed] [Google Scholar]
- 12.Ong KC, Chong WF, Soh C, et al. : Comparison of different exercise tests in assessing outcomes of pulmonary rehabilitation. Respir Care, 2004, 49: 1498–1503 [PubMed] [Google Scholar]
- 13.Vivodtzev I, Gagnon P, Pepin V, et al. : Physiological correlates of endurance time variability during constant-workrate cycling exercise in patients with COPD. PLoS ONE, 2011, 6: e17007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill DW, Poole DC, Smith JC: The relationship between power and the time to achieve. VO(2max). Med Sci Sports Exerc, 2002, 34: 709–714 [DOI] [PubMed] [Google Scholar]
- 15.Sloniger MA, Cureton KJ, Carrasco DI, et al. : Effect of the slow-component rise in oxygen uptake on VO2max. Med Sci Sports Exerc, 1996, 28: 72–78 [DOI] [PubMed] [Google Scholar]
- 16.Hogan MC, Richardson RS, Haseler LJ: Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol 1985, 1999, 86: 1367–1373 [DOI] [PubMed] [Google Scholar]
- 17.Vanhatalo A, Fulford J, DiMenna FJ, et al. : Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol, 2010, 95: 528–540 [DOI] [PubMed] [Google Scholar]
- 18.Hodgson Y, Choate J: Continuous and noninvasive recording of cardiovascular parameters with the Finapres finger cuff enhances undergraduate student understanding of physiology. Adv Physiol Educ, 2012, 36: 20–26 [DOI] [PubMed] [Google Scholar]
- 19.Matcher SJ, Elwell CE, Cooper CE, et al. : Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal Biochem, 1995, 227: 54–68 [DOI] [PubMed] [Google Scholar]
- 20.Poole DC, Wilkerson DP, Jones AM: Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur J Appl Physiol, 2008, 102: 403–410 [DOI] [PubMed] [Google Scholar]
- 21.Stickland MK, Butcher AJ, Marciniuk DD, et al. : Assessing exercise limitation using cardiopulmonary exercise testing. Pulm Med, 2012, 2012: 824091. [DOI] [PMC free article] [PubMed]
- 22.Osada T, Rådegran G: Femoral artery inflow in relation to external and total work rate at different knee extensor contraction rates. J Appl Physiol, 1985, 2002: 1325–1330 [DOI] [PubMed] [Google Scholar]
- 23.Saltin B, Rådegran G, Koskolou MD, et al. : Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand, 1998, 162: 421–436 [DOI] [PubMed] [Google Scholar]
- 24.DeLorey DS, Kowalchuk JM, Paterson DH: Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 1985, 2003, 95: 113–120 [DOI] [PubMed] [Google Scholar]
- 25.Grassi B, Pogliaghi S, Rampichini S, et al. : Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 1985, 2003, 95: 149–158 [DOI] [PubMed] [Google Scholar]
- 26.Homma S, Eda H, Ogasawara S, et al. : Near-infrared estimation of O2 supply and consumption in forearm muscles working at varying intensity. J Appl Physiol 1985, 1996, 80: 1279–1284 [DOI] [PubMed] [Google Scholar]
- 27.Ferrari M, Muthalib M, Quaresima V: The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys. Eng Sci, 2011, 369: 4577–4590 [DOI] [PubMed] [Google Scholar]
- 28.Boone J, Koppo K, Barstow TJ, et al. : Pattern of deoxy [Hb+Mb] during ramp cycle exercise: influence of aerobic fitness status. Eur J Appl Physiol, 2009, 105: 851–859 [DOI] [PubMed] [Google Scholar]
- 29.Spencer MD, Murias JM, Paterson DH: Characterizing the profile of muscle deoxygenation during ramp incremental exercise in young men. Eur J Appl Physiol, 2012, 112: 3349–3360 [DOI] [PubMed] [Google Scholar]
- 30.Osawa T, Kime R, Hamaoka T, et al. : Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc, 2011, 43: 1406–1413 [DOI] [PubMed] [Google Scholar]
- 31.DiMenna FJ, Wilkerson DP, Burnley M, et al. : Priming exercise speeds pulmonary O2 uptake kinetics during supine “work-to-work” high-intensity cycle exercise. J Appl Physiol 1985, 2010, 108: 283–292 [DOI] [PubMed] [Google Scholar]
- 32.Jones AM, Berger NJ, Wilkerson DP, et al. : Effects of “priming” exercise on pulmonary O2 uptake and muscle oxygenation kinetics during heavy-intensity cycle exercise in the supine and upright positions. J Appl Physiol, 1985, 2006: 1432–1441 [DOI] [PubMed] [Google Scholar]
- 33.Ferri A, Adamo S, La Torre A, et al. : Determinants of performance in 1,500-m runners. Eur J Appl Physiol, 2012, 112: 3033–3043 [DOI] [PubMed] [Google Scholar]