Key Points
XLF-deficient mice recapitulate the lymphocytopenia of XLF-deficient patients.
Premature aging of hematopoietic stem cells underlies the severe and progressive lymphocytopenia in XLF-deficient mice.
Abstract
XRCC4-like factor (XLF/Cernunnos) is a component of the nonhomologous end-joining (NHEJ) pathway of double-strand DNA break repair. XLF-deficient patients develop a severe progressive lymphocytopenia. Although NHEJ is required for V(D)J recombination and lymphocyte development, XLF-deficient mice have normal V(D)J recombination, highlighting the need for an alternative mechanism for the lymphocytopenia. Here, we report that XLF-deficient mice recapitulate the age-dependent lymphocytopenia of patients. We show that XLF deficiency leads to premature aging of hematopoietic stem cells (HSCs), measured by decreased functional capacity in transplantation assays, preferential myeloid reconstitution, and reduced self-renewal at a young age. We propose that premature aging of HSCs, together with previously reported defects in class-switch recombination and memory immune response, underlies the progressive and severe lymphocytopenia in XLF-deficient patients in the absence of measurable V(D)J recombination defects.
Introduction
Nonhomologous end-joining (NHEJ) is a major DNA double-strand break (DSB) repair pathway. During lymphocyte development, NHEJ is required to rejoin programmed DSBs generated during V(D)J recombination. XRCC4-like factor (XLF, also called Cernunnos or NHEJ1) is 1 of 7 known NHEJ factors. Patients with germline homozygous inactivation mutations of XLF develop progressive lymphocytopenia, microcephaly, and occasional myeloproliferative disease.1,2 Although the role of XLF in NHEJ and V(D)J recombination was thought to explain the lymphocytopenia in XLF-deficient patients, we and others showed that XLF-deficient mice have no measurable defect in V(D)J recombination,3-6 highlighting the need for alternative mechanisms.
Hematopoietic stem cells (HSCs) differentiate to multipotent progenitors and then common lymphoid progenitors that eventually give rise to lymphocytes.7,8 DNA repair maintains the genomic stability throughout hematopoiesis and lymphocyte development beyond V(D)J recombination. Aged HSCs accumulate γ-H2AX foci, an indicator of DSB formation,9,10 lose self-renewal and reconstitution capacities, and preferentially commit to myeloid lineage at the price of lymphoid differentiation.11,12 Given the role of XLF in maintaining genomic stability in murine embryonic stem cells and human induced pluripotent stem cells,5,13 we investigated whether XLF preserves lymphogenesis by preventing premature aging of HSCs.
Study design
XLF-deficient mice were previously described.3 Recipient B6.SJL-PtprcaPep3b/BoyJ and competitor B6.Cg-Tg(CAG-DsRed*MST)1Nagy/J mice were purchased from Jackson Laboratories. To sort HSCs, bone marrow (BM) cells were depleted of lineage+ cells using MACS columns (Miltenyi Biotec) after staining with anti-lineage biotin-conjugated antibodies (CD3ε, CD4, CD8α, B220, Mac1, Gr1, and Ter119) and then with fluorophore-conjugated streptavidin, anti-Sca-1, anti-c-Kit, anti-CD150, and anti-CD48 antibodies, and then sorted using FACSAriaII (Becton Dickinson). Flow cytometry data were collected using LSRII (BD) and analyzed with FlowJo. For transplantations, donor (CD45.2+) BM cells (1 × 106) or HSCs were injected into lethally irradiated (1200 cGy in 2 doses) recipient mice (CD45.1+), with 1 × 106 CD45.2+dsRed+ competitor BM cells. Peripheral blood (PB) mononuclear cells (MNCs) were analyzed for CD45.2, dsRed, and lineage markers (Thy-1.2, Mac1, Gr1, and B220) at 4, 10, and ≥17 weeks after transplantations. Ratio of donor to competitor percentages was used to determine reconstitutive capacity. Serial transplantations were performed at >20 weeks by injecting 1-2 × 106 BM cells from primary recipients of BM cells into lethally irradiated CD45.1+ secondary recipients. After 8 weeks, PB was analyzed as above, and serial reconstitution ability was measured by δ log ratio, which is the difference between the common logarithmic transformations of the average donor/competitor ratio in secondary recipients and the corresponding primary recipient.14 Alkaline comet assay was performed on purified lineagenegSca-1+c-Kit+ cells (Trevigen).
Results and discussion
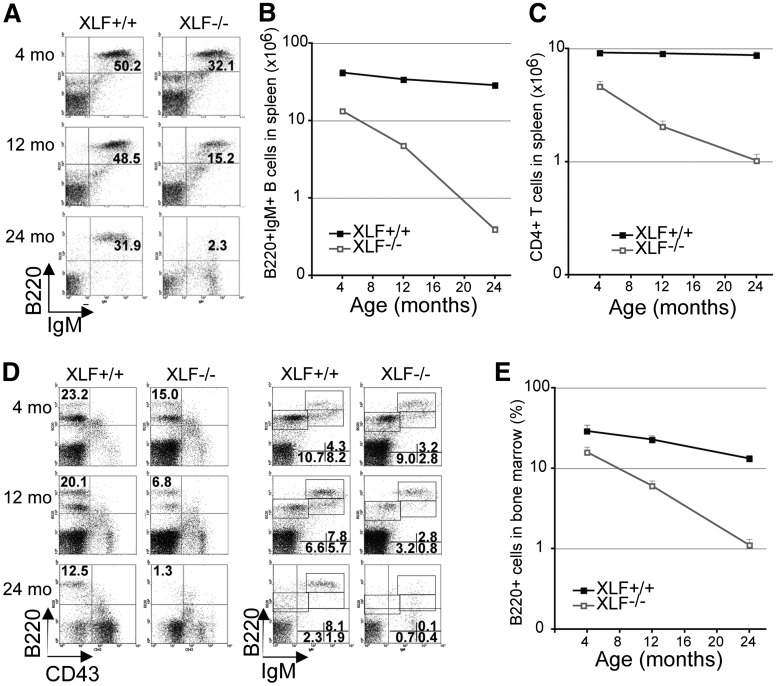
Although lymphocyte count is only moderately reduced in 4-week-old XLF−/− mice,3 we found that peripheral B- and T-cell counts declined significantly faster in 4-, 12- and 24-month-old XLF−/− mice than in XLF+/+ littermates (Figure 1A-C). In BM, newly generated naïve B220lowIgM+ B-cells decreased earlier and faster in XLF−/− mice than in controls, leading to faster decline of total B220+ B cells in BM (Figure 1D-E). While in wild-type mice age-dependent reduction in de novo lymphogenesis is accompanied by accumulation of antigen-experienced B220highIgM+ B-cells, XLF−/− mice failed to accumulate B220highIgM+ B cells, further exacerbating the B-cell lymphocytopenia (Figure 1D). The frequency of BM red blood cell progenitors (Ter119+) and PB myeloid cells (Mac1+/Gr1+) did not decrease in aged XLF−/− mice (supplemental Figure 1B-C available at the Blood Web site), consistent with myeloid bias of aged HSCs and strict requirement for red blood cells for survival. These findings show that XLF-deficient mice recapitulate the severe progressive lymphocytopenia in XLF-deficient patients.
Figure 1.
Progressive lymphocytopenia in XLF-deficient mice. (A) Representative flow cytometric analysis of B cells in the spleen of XLF−/− mice and XLF+/+ littermates. Absolute number of mature B220+IgM+ B cells (B) and CD4+CD8− T cells (C) in the spleen of XLF−/− mice and XLF+/+ littermates; P < 2 × 10−7. (D) Representative flow cytometric analysis of B cells in the BM in XLF−/− mice and XLF+/+ littermates. (E) Percentage of total B220+ B cells in the BM of XLF−/− mice and XLF+/+ littermates; P = 1.4 × 10−7. n ≥ 3 per age group per genotype. Data represent mean ± standard deviation in all graphs. The P values in this figure were calculated by comparing the slope of regression lines between XLF+/+ and XLF−/− mice with Student t test. IgM, immunoglobulin M.
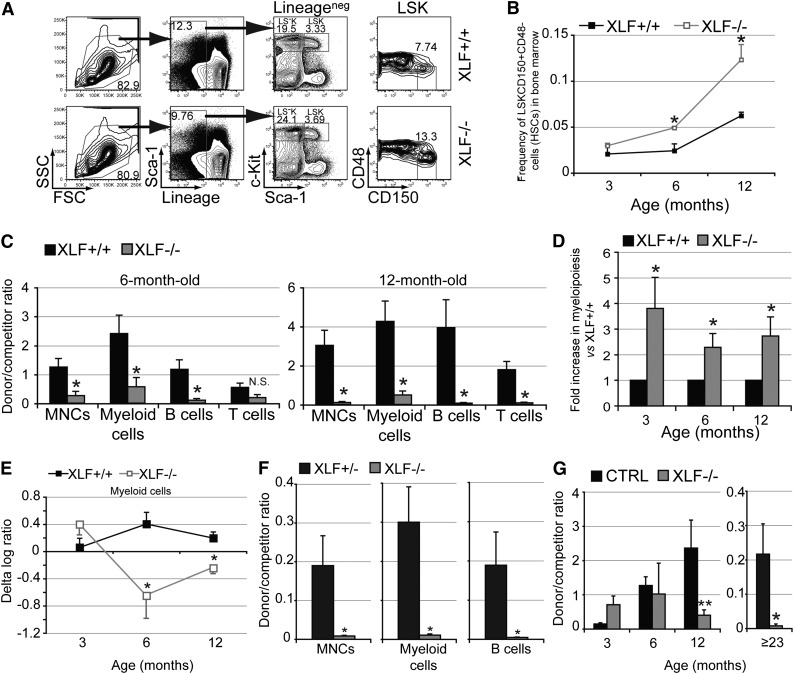
To determine whether HSC dysfunction contributes to the lymphocytopenia associated with XLF deficiency, we analyzed HSCs in XLF−/− mice using well-established markers (Figure 2A).15 Increased HSC number with declined function is a hallmark of HSC aging.16-18 Although the frequency and number of lineagenegSca-1+c-Kit+CD150+CD48− HSCs increased with age in both XLF−/− and XLF+/+ mice, XLF−/− mice harbored >2-fold more HSCs than controls at 6 and 12 months of age (Figure 2A-B). We then evaluated HSC function in competitive transplantations with BM cells from 3-, 6-, or 12-month-old XLF−/− mice or age-matched controls. Compared with controls, BM cells from 6- and 12-month-old, but not 3-month-old, XLF−/− mice had greatly reduced repopulation ability in MNCs, myeloid and B lineages (Figure 2C and supplemental Figure 2C). T-cell reconstitution was significantly lower in recipients of 12-month-old XLF−/− BM cells, likely due to the long half-life of T cells in PB (Figure 2C). Donor-derived myeloid vs B-cell ratio was consistently higher in recipients of XLF−/− BM cells than in recipients of control BM cells, indicating myeloid bias (Figure 2D). Together these data documented premature aging of XLF−/− HSCs, measured by their increased number, reduced trilineage reconstitution, and myeloid bias at a younger age.
Figure 2.
Functional decline of XLF-deficient HSCs with aging. (A) Representative flow cytometric analysis of lineagenegSca-1+c-Kit+CD150+CD48− HSCs in the BM in XLF−/− and control mice. (B) Frequency of HSCs in the BM at various ages of XLF−/− (n ≥ 3 per age group) and control mice (n ≥ 2 per age group). (C) Ratio of donor to competitor contribution within total MNCs and mature lineages in PB of primary recipients of BM cells from XLF−/− and XLF+/+ mice 10 weeks after transplantation; n ≥ 6 per age group per genotype. XLF−/− and XLF+/+ BM cells within each age group were transplanted with BM cells from a common competitor mouse for easy comparison. Different age groups (3, 6, and 12 months old) were not transplanted at the same time; therefore, absolute chimerism between various age groups of the same genotype cannot be directly compared. (D) Myeloid donor/competitor ratio vs B-cell donor/competitor ratio in primary recipients of XLF−/− BM cells normalized to that of controls; n ≥ 6 per age group per genotype. (E) The δ log ratio within peripheral myeloid cells between secondary and primary transplantations for recipients of XLF+/+ or XLF−/− BM cells; n ≥ 4 per age group per genotype. (F) Ratio of donor to competitor contribution within PB MNCs, myeloid and B cells of primary recipients of purified HSCs from ≥23-month-old XLF+/− or XLF−/− mice with competitor BM cells 10 weeks after transplantation; n ≥ 6 per age group per genotype. (G) Donor-to-competitor ratio within BM lineagenegSca-1+c-Kit+CD150+ HSCs of primary recipients of 3-, 6-, and 12-month-old XLF+/+ (CTRL) and XLF−/− BM cells ≥20 weeks after transplantation, and primary recipients of ≥23-month-old XLF+/− (CTRL) and XLF−/− HSCs ≥17 weeks after transplantation; n ≥ 5 per age group per genotype. Data represent mean ± standard error of the mean. *P < .05, **P < .01. The P values in this figure were calculated using two-tailed Student t test assuming equal variance. FSC, forward scatter; N.S., not significant; SSC, side scatter.
Reconstitution in primary recipients of BM cells reflects a combined function of progenitor cells and HSCs. To determine the long-term repopulating capacity of HSCs specifically, we performed serial transplantations. Further reduction of reconstitution ability in secondary recipients relative to the corresponding primary recipient indicates a long-term repopulation defect in HSCs and is reflected as negative δ log ratio. Myeloid cells, the most short-lived cells in PB, are used as a readout of ongoing HSC function.19 Δ log ratio in myeloid cell reconstitution decreased significantly in the 6- and 12-month-old XLF−/− donor group, but not in age-matched controls (Figure 2E). Finally, in competitive transplantations with purified lineagenegSca-1+c-Kit+CD150+CD48− HSCs from ≥23-month-old XLF−/− mice and age-matched XLF+/− controls, XLF−/− HSCs had 27- and 41-fold lower reconstitution in myeloid and B-cell lineages, respectively (Figure 2F). Together, these data provide strong evidence for cell-intrinsic long-term repopulation defects in XLF−/− HSCs that worsen over time.
To determine whether XLF deficiency affects HSC self-renewal, we analyzed HSC chimerism in primary recipients in BM cells and HSC transplantations. The donor HSC chimerism decreased significantly in recipients of 12-month-old XLF−/− BM cells and aged HSCs, but not in those of 3- and 6-month-old XLF−/− BM cells, implying reduced self-renewal at advanced age (Figure 2G).
Ligase4 or Ku80, 2 other NHEJ factors, have been previously linked to HSC aging in murine models.9,20 However, Ku80 and Ligase4 are essential for V(D)J recombination, so the contribution of HSC dysfunction to lymphocytopenia cannot be evaluated in Ku80- or Ligase4-deficient mice. XLF is the only known NHEJ factor that is not strictly required for V(D)J recombination. XLF-deficient patients are diagnosed later in childhood or adolescence,1,21 compared with other NHEJ factor deficiencies, which are apparent in infancy. Given that most XLF mutations are truncation mutations,1,21 this delay cannot be simply explained by hypomorphism.
Here, we show that XLF-deficient mice display severe and progressive lymphocytopenia, reminiscent of the lymphocytopenia of XLF-deficient patients. XLF deficiency leads to accumulation of DNA damage in HSCs (supplemental Figure 3C) and premature aging of HSCs, evidenced by reduced trilineage reconstitution, self-renewal, and myeloid bias at young ages. In wild-type mice, memory lymphocytes accumulate throughout the lifetime to functionally compensate for the reduced de novo lymphogenesis at advanced age. However, the establishment and maintenance of memory immune response in XLF-deficient mice is compromised, likely due to defects in class switch recombination, a critical step in B-cell memory response, and in T-cell renewal.3,4,21 We conclude that premature aging of HSCs, combined with defective immune memory response, leads to severe progressive lymphocytopenia in XLF deficiency and potentially in other DNA-repair protein deficiency, such as WRN or ATM, independent of V(D)J recombination. In addition to age, the clinical presentation of XLF deficiency may also be influenced by genetic background, especially by the availability of redundant DNA-repair pathways.6,22,23
Acknowledgments
The authors thank Dr Richard Baer for his critical review of the manuscript.
This research was supported by National Institutes of Health grants 1R21AI103826 (S.Z., S.M.) from the National Institute of Allergy and Infectious Diseases, and 1RO1CA158073 (S.Z.) from the National Cancer Institute. S.Z. was a St Baldrick’s Scholar for Pediatric Cancer and is a Leukemia Lymphoma Society Scholar. J.L.C. is supported in part by National Institutes of Health grant TL1TR000082 from the National Cancer Institute.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.Z. and S.M. conceived the study; S.A. and S.Z. designed and performed the experiments, analyzed the data, and wrote the manuscript; K.Y. performed the comet assay; M.C. and J.L.C. performed preliminary experiments; C.L. and B.J.L. maintained the XLF-deficient mouse colony; and T.Z. performed statistical analysis in Figure 1.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shan Zha, Institute for Cancer Genetics, Columbia University Medical Center, 1130 St Nicholas Ave, Room 505, New York, NY 10032; e-mail: sz2296@columbia.edu.
References
- 1.Buck D, Malivert L, de Chasseval R, et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124(2):287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 2.Dai Y, Kysela B, Hanakahi LA, et al. Nonhomologous end joining and V(D)J recombination require an additional factor. Proc Natl Acad Sci USA. 2003;100(5):2462–2467. doi: 10.1073/pnas.0437964100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Alt FW, Cheng HL, et al. Lymphocyte-specific compensation for XLF/cernunnos end-joining functions in V(D)J recombination. Mol Cell. 2008;31(5):631–640. doi: 10.1016/j.molcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vera G, Rivera-Munoz P, Abramowski V, et al. Cernunnos deficiency reduces thymocyte life span and alters the T cell repertoire in mice and humans. Mol Cell Biol. 2013;33(4):701–711. doi: 10.1128/MCB.01057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zha S, Alt FW, Cheng HL, Brush JW, Li G. Defective DNA repair and increased genomic instability in Cernunnos-XLF-deficient murine ES cells. Proc Natl Acad Sci USA. 2007;104(11):4518–4523. doi: 10.1073/pnas.0611734104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zha S, Guo C, Boboila C, et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nature. 2011;469(7329):250–254. doi: 10.1038/nature09604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akashi K, Reya T, Dalma-Weiszhausz D, Weissman IL. Lymphoid precursors. Curr Opin Immunol. 2000;12(2):144–150. doi: 10.1016/s0952-7915(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 8.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 9.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 10.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171(5):2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106(4):1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilgner K, Neganova I, Singhapol C, et al. Brief report: a human induced pluripotent stem cell model of cernunnos deficiency reveals an important role for XLF in the survival of the primitive hematopoietic progenitors. Stem Cells. 2013;31(9):2015–2023. doi: 10.1002/stem.1456. [DOI] [PubMed] [Google Scholar]
- 14.Langer JC, Henckaerts E, Orenstein J, Snoeck HW. Quantitative trait analysis reveals transforming growth factor-beta2 as a positive regulator of early hematopoietic progenitor and stem cell function. J Exp Med. 2004;199(1):5–14. doi: 10.1084/jem.20030980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 16.de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93(10):3294–3301. [PubMed] [Google Scholar]
- 17.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 19.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 20.Nijnik A, Woodbine L, Marchetti C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 21.Du L, Peng R, Björkman A, et al. Cernunnos influences human immunoglobulin class switch recombination and may be associated with B cell lymphomagenesis. J Exp Med. 2012;209(2):291–305. doi: 10.1084/jem.20110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oksenych V, Kumar V, Liu X, et al. Functional redundancy between the XLF and DNA-PKcs DNA repair factors in V(D)J recombination and nonhomologous DNA end joining. Proc Natl Acad Sci USA. 2013;110(6):2234–2239. doi: 10.1073/pnas.1222573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Jiang W, Dubois RL, Yamamoto K, Wolner Z, Zha S. Overlapping functions between XLF repair protein and 53BP1 DNA damage response factor in end joining and lymphocyte development. Proc Natl Acad Sci USA. 2012;109(10):3903–3908. doi: 10.1073/pnas.1120160109. [DOI] [PMC free article] [PubMed] [Google Scholar]