Figure 7.
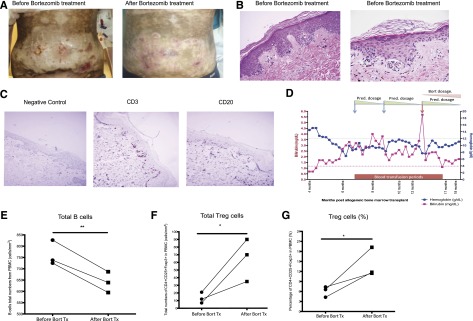
Treatment effects of bortezomib on clinical cGVHD human patients. A single institution pilot study of bortezomib was initiated in patients with steroid-dependent, -intolerant, or -refractory cGVHD. (A) Patient 4 showed extensive grade III skin sclerodermatous GVHD covering >0% of the body. The abdominal region before and after bortezomib treatments are shown. (B) Representative images of the pretreatment skin biopsies taken from the patient shown in A. (C) Immunohistochemical staining for CD3 and CD20 in pretreatment skin biopsy samples from patient 4. (D) CBC and biochemistry data from patient 5 were collected through the trial period. (E) Total numbers of peripheral blood B cells (CD45+CD19+) from 3 patients were analyzed by flow cytometry before and after bortezomib treatment. (F-G) Treg cell populations (CD4+CD25+Foxp3+) were analyzed by flow cytometry and shown as total numbers and percentage. All the data were collected from individual cGVHD patients that underwent bortezomib treatment. The data are shown as mean ± SEM and analyzed by Student t test to compare pre- and postbortezomib treatments. *P < .05 was considered significant.
