Interestingly, for the research on the role of platelets in contact activation of coagulation, 2 articles on the topic using the same polyphosphate (polyP) preparation have been published.1,2 In our paper in Blood, “Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII,”1 we found no evidence for platelet-induced activation of factor XII (FXII). Conversely, the paper in Cell2 concluded the following: “PolyP represents the long sought ‘foreign’ surface that triggers fibrin formation by activated platelets linking primary to secondary hemostasis and critically contributing to ‘procoagulant’ platelet activity.” Obviously, these 2 papers contradict each other, and it is useful for progress in the field to consider why. To support their position that platelet-derived polyP activates FXII, some of the authors of the Cell paper2 tried to explain our negative findings in a letter to the editor.3 Unfortunately, their letter contains statements based on factual errors and unfounded assumptions. Therefore, we wish to correct these errors and present possible explanations to the discrepancies that could be valuable for researchers in the field.
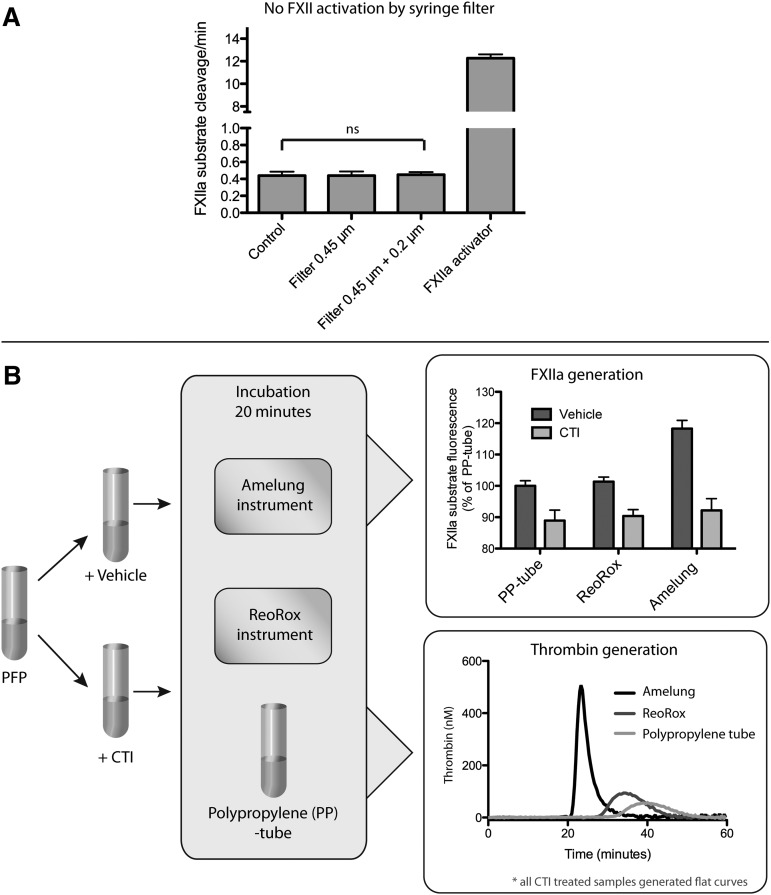
To support their claim that platelet-derived polyP activates FXII,2 it is incorrectly assumed in the letter3 that lack of FXII activation in our paper1 can be explained by use of an old preparation and thus degraded polyphosphates. However, it is important to note that all activity measurements were performed within 1.5 months from receiving the substance donated by the Renné laboratory. It is also incorrectly stated that our findings were a result of using polytetrafluoroethylene filters, with the claim that such filters activate FXII. However, this claim is based on a citation that mentions neither contact activation nor FXII.4 In fact, polytetrafluoroethylene is extensively used in blood-contacting biomaterials and shows very low procoagulant activity.5 Regardless, polytetrafluoroethylene filters were not used in our paper. Figure 1A demonstrates that plasma filtration with the Minisart filters used1 does not generate detectable FXIIa.
Figure 1.
Contact activation in vitro. (A) Filtration with Minisart filters does not cause significant contact activation: FXIIa generation was measured before (control) and after filtration of plasma with the filters used in our Blood paper,1 and kaolin (100 μg/mL) was used as positive control (n = 4, ns, nonsignificant Student t test). (B) Amelung KC4 causes contact activation, leading to substantial thrombin generation: pooled citrated plasma samples with or without CTI corn trypsin inhibitor (CTI) were incubated for 20 minutes at 37°C in Amelung KC4, ReoRox4, or polypropylene (PP) tubes. FXIIa generation was measured as described previously1 with the fluorescent substrate present during incubation in the instrument. Bars represent FXIIa substrate fluorescence as percentage of the PP tubes (n = 8, background fluorescence of inhibitor/vehicle additions were compensated). Aliquots from incubated plasma were used for subsequent real-time thrombin generation measurements in the presence of 10 μM phospholipids. Curves represent means of 4 independent experiments.
Their letter3 further dismisses our results that were obtained using the sensitive fluorogenic substrate Boc-Gln-Gly-Arg-AMC (7-Amino-4-methylcoumarin) to measure FXIIa, claiming potential unspecific cleavage by other proteases. However, when we measured FXIIa generation in citrated plasma with activated platelets or platelet-derived polyphosphate, we found little if any substrate cleavage compared with well-known contact activators.1 Aristotelian logic tells us that, given the observations that A (FXIIa) and B (other proteases) give C (substrate cleavage), the deduction that absence of C necessarily entails the absence of A must hold true, regardless of potential activities of other proteases (B). Thus, the argument put forward is logically invalid. Additionally, FXII-deficient plasma or FXIIa inhibitor was always used as controls.
It is also claimed in the letter3 that we are unable to reproduce our own data, citing an article where platelet agonists shortened clotting times more dramatically6 than what was reported by us recently.1 Crucially, however, they do not inform the reader that 0.6 pM tissue factor (TF) was added in the experiments reported in our Blood paper,1 whereas no TF was added in the previous study.6 Obviously, this difference in the conditions accounts for the discrepant results between the experiments.
Notably, there are no comments on our critique of their use of the Amelung instrument in their coagulation experiments. In fact, Figures 5D and S3A in their Cell paper2 show that their coagulation assay is afflicted with artifactual contact activation, because “spontaneous coagulation” was significantly more rapid in normal than in FXII-deficient plasma. Recent publications have highlighted how low-grade contact activation is greatly amplified by the presence of phospholipids simulating the procoagulant membranes of activated platelets.7,8 Thus, the dramatic effects on recalcification times observed with preactivation of platelets in their study2 likely stem from platelet-dependent amplification of material-induced contact activation and not from FXIIa generation by platelets per se. In Figure 1B, we show that the Amelung instrument they used2 generates far more FXIIa than comparable assays. Incubating plasma in the Amelung generates sufficient FXIIa to induce robust thrombin generation in the presence of phospholipids, indicating that artifactual contact activation likely affects clotting times in this instrument. In this debate, it is essential to recognize that any kind of material can induce some degree of FXII activation, including plastics. In fact, this is also demonstrated in Figure 1B in their letter,3 where normal plasma without activator still causes thrombin generation compared with the flat curve in FXII-deficient plasma.
To minimize the detrimental effects of material-induced contact activation, we used 0.6 pM TF in one of our experiments (Figure 4 of our Blood paper).1 The letter claims that the addition of TF conceals the effects of platelet activation and platelet PolyP on coagulation times, basing these conclusions on thrombin generation measurements in plasma.3 However, the differences in clotting times caused by the use of different TF/liposome preparations and instruments with varying degree of contact activation potential (Figure 1B) makes direct comparisons between the experiments impossible. Indeed, in Figure S2B in our Blood paper,1 we demonstrate that the ReoRox instrument used in our study is superior for detecting the effects of contact activation on coagulation compared with the Amelung, even when 0.6 pM TF was used with ReoRox compared with no TF in the Amelung.
Most regrettably, no mention was made in their letter3 of the questions regarding the experiment wherein infusion of thrombin receptor activating peptide-6 (Trap-6) induced death by pulmonary embolism in 13 of 15 mice.2 When repeating this pivotal experiment, we found no signs of pulmonary embolism or circulatory distress in challenged mice, which seems more reasonable, as PAR1, the receptor responsible for platelet activation by Trap-6, is not expressed by murine platelets. For the field to trust their Cell paper,2 it is important that the authors can explain their results with Trap-6.
We want to emphasize that our results do not dispute that platelet polyP may play a role in coagulation in vivo, as was recently suggested in an article on inositol hexakisphosphate kinase 1 knockout mice.9 However, that paper contains no evidence that the reported hemostasis defects are connected to FXII. Likewise, we do not exclude a role for FXII in thrombosis in vivo. However, we hold that currently there is no valid evidence to support a direct cause-effect relationship between platelets and the physiologically relevant activation of FXII.
Authorship
Contribution: N.B. and L.F. performed experiments, designed the study, carried out interpretation of data and wrote the manuscript; T.L.L. and S.R. contributed to design of the study, interpretation of data and wrote the manuscript; E.T. wrote and revised the manuscript; and J.O.S. and P.T. contributed with critical revision of the manuscript.
Conflict-of-interest disclosure: T.L.L. and E.T. are members of the Board and are minor shareholders of Medirox (E.T. since April 25, 2013). The remaining authors declare no competing financial interests.
Correspondence: Tomas L. Lindahl, Department of Clinical and Experimental Medicine, Linköping University, SE-58185 Linköping, Sweden; e-mail: tomas.lindahl@liu.se.
References
- 1.Faxälv L, Boknäs N, Ström JO, et al. Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII. Blood. 2013;122(23):3818–3824. doi: 10.1182/blood-2013-05-499384. [DOI] [PubMed] [Google Scholar]
- 2.Müller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickel KF, Spronk HM, Mutch NJ, Renné T. Time-dependent degradation and tissue factor addition mask the ability of platelet polyphosphates in activating factor XII-mediated coagulation. Blood. 2013;122(23):3847–3849. doi: 10.1182/blood-2013-09-525840. [DOI] [PubMed] [Google Scholar]
- 4.Onder S, Kazmanli K, Kok FN. Alteration of PTFE surface to increase its blood compatibility. J Biomater Sci Polym Ed. 2011;22(11):1443–1457. doi: 10.1163/092050610X510551. [DOI] [PubMed] [Google Scholar]
- 5.Fink H, Faxälv L, Molnár GF, et al. Real-time measurements of coagulation on bacterial cellulose and conventional vascular graft materials. Acta Biomater. 2010;6(3):1125–1130. doi: 10.1016/j.actbio.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Ramström S, Rånby M, Lindahl TL. Platelet phosphatidylserine exposure and procoagulant activity in clotting whole blood—different effects of collagen, TRAP and calcium ionophore A23187. Thromb Haemost. 2003;89(1):132–141. [PubMed] [Google Scholar]
- 7.Boknäs N, Faxälv L, Lindahl TL, Ramström S. Contact activation: important to consider when measuring the contribution of tissue factor-bearing microparticles to thrombin generation using phospholipid-containing reagents. J Thromb Haemost. 2014;12(4):515–518. doi: 10.1111/jth.12503. [DOI] [PubMed] [Google Scholar]
- 8.Ollivier V, Wang J, Manly D, et al. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res. 2010;125(1):90–96. doi: 10.1016/j.thromres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh S, Shukla D, Suman K, et al. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122(8):1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]