Abstract
Background and Aims:
Neuraxial anaesthesia has become popular for the renal surgeries during the last few years. This study was aimed at comparing general anaesthesia (GA) with epidural anaesthesia in patients undergoing renal surgeries.
Methods:
One hundred American Society of Anaesthesiologists (ASA) physical status-I and II adult consenting patients of both gender in the age group of 25-55 years undergoing renal surgeries were randomly assigned to two groups of 50 patients each: Group G and Group E. Group G patients were administered conventional GA while Group E received epidural anaesthesia (EA) with 3 mg/kg of ropivacaine and 1 μg/kg of dexmedetomidine. Besides cardio-respiratory parameters, surgeon's satisfaction, patient's satisfaction and side effects were observed. Parametric data were analysed by ANOVA while non-parametric data were compared with Mann–Whitney U-test and Wilcoxon test. Value of P < 0.05 was considered statistically significant.
Results:
The demographic profile, total anaesthesia time, surgical time and haemodynamic parameters and surgeon's satisfaction scores were comparable in both groups. Patient's satisfaction scores were better in Group E during the post-operative period. Incidence of side-effects such as nausea and vomiting and shivering were higher in Group G (P < 0.001) while the incidence of dry mouth was higher in Group E (P < 0.001).
Conclusion:
Epidural anaesthesia with ropivacaine and dexmedetomidine can be safely and effectively used in patients undergoing renal surgeries.
Keywords: Dexmedetomidine, epidural, general anaesthesia, renal surgery, ropivacaine
INTRODUCTION
Various regional and general anaesthesia (GA) techniques have been tried and used with success for renal surgeries. GA is usually preferred by the anaesthesiologists and surgeons because of the discomfited body position during prolonged renal surgical procedures.[1] However, this is avoided by the use of sedative agents along with regional anaesthesia (RA). GA is considered to provide superior muscle relaxation and controlled diaphragmatic motion during the surgery.[1]
Recent investigations have revealed that RA can be safely used for renal surgeries including donor nephrectomy and renal transplantation as well.[1,2] RA provides better haemodynamic stability with minimal blood loss during surgery. Moreover, minimal need for blood transfusion, lower incidence of toxicity from anaesthetic agents, good post-operative pain relief and fewer post-operative complications make RA a safer option as compared to GA.[3]
Dexmedetomidine, the α2-adrenoceptor agonist is increasingly being used in the clinical practice. Its distinctive properties render it suitable for premedication, as an anaesthetic adjuvant for general and regional anaesthesia, as well as for post-operative sedation and analgesia.[4,5,6,7,8] Dexmedetomidine has been found to be a better epidural adjuvant with more stable cardio-respiratory parameters and higher sedation scores as compared with clonidine.[6,7] The use of α2-adrenergic agonist agents as adjuncts to local anaesthetics in neuraxial anaesthesia improves the quality of the block, provides good intra-operative sedation and prolongs post-operative analgesia.[6,7] α2-adrenergic agonists are also free of the side-effects commonly associated with the use of opioids in neuraxial anaesthesia such as pruritis, nausea and vomiting, urinary retention, respiratory depression and so on.[4] Dexmedetomidine undergoes almost complete biotransformation through direct glucuronidation and cytochrome P450 metabolism in the liver. Very little amount of the drug is excreted unchanged in the urine or faeces.[8] Keeping in view the safety profile of epidural anaesthesia and remarkable properties of dexmedetomidine as epidural adjunct, a prospective, comparative study was designed in patients undergoing elective renal surgeries under GA or epidural anaesthesia with ropivacaine and dexmedetomidine in a randomised manner. The aim of the study was to compare the surgical conditions, surgeon's satisfaction intra-operatively and patient's satisfaction in the post-operative period in the two groups. The secondary outcome was to compare the haemodynamic parameters and the side-effects associated with the two anaesthesia techniques.
METHODS
After obtaining permission from the Institutional Ethical Committee, 100 American Society of Anaesthesiologists (ASA) Class-I and II adult patients of either gender in the age group of 25-55 years undergoing renal surgeries (pyelo-lithotomy, uretero-lithotomy, and nephrectomy) were enrolled in the study [Figure 1]. A written informed consent was obtained from all the patients. Exclusion criteria included patients with diabetes mellitus, uncontrolled hypertension, cardiac rhythm disturbances, obesity, severe pulmonary disease, hepatic impairment, deranged coagulation profile, cerebrovascular disorder and refusal for epidural anaesthesia. The patients were randomly assigned using sealed envelope technique into two groups of 50 patients each: Group G and Group E. Group G patients were administered conventional GA while Group E received epidural anaesthesia.
Figure 1.
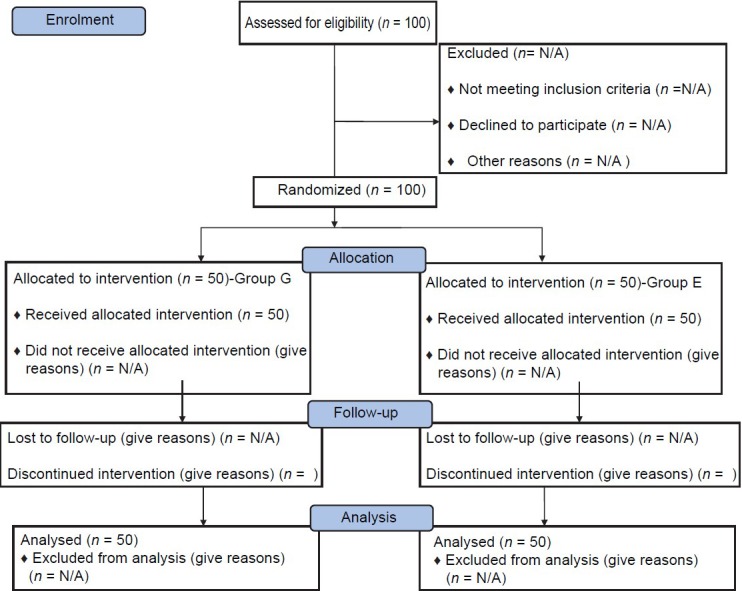
Consolidated standards of reporting trials flow diagram
All patients received ranitidine 150 mg as premedication a night before and on the morning of surgery with a sip of water. In the operation theatre, intravenous (IV) access was secured with 18G cannula and all patients were pre-loaded with 10 ml/kg of Ringer lactate solution. Standard monitoring included electrocardiogram, pulse oximetry (SpO2), non-invasive blood pressure, urinary output and respiratory rate (RR).
In Group G, induction of anaesthesia was achieved with propofol 2 mg/kg, butorphanol 0.02 mg/kg, isoflurane, oxygen and vecuronium 0.1 mg/kg as a muscle relaxant to facilitate endotracheal intubation with appropriate sized endotracheal tube. Thereafter, lateral kidney position was achieved with usual precautions. Maintenance of anaesthesia was achieved with isoflurane (1 MAC), oxygen in nitrous oxide with the ratio of 40:60 and vecuronium as a muscle relaxant as and when required. Isoflurane and nitrous oxide were tapered before the anticipated end of surgery and stopped during the completion of skin closure. An intravenous (IV) infusion of diclofenac sodium (75 mg) was given just before the conclusion of surgery for post-operative analgesia. The residual muscle blockade was antagonised with neostigmine and glycopyrrolate. The patients were extubated after adequate recovery and thereafter kept in the recovery room for 4 h and vital parameters as well as side-effects were observed for and treated as and when required. Post-operative analgesia was maintained with supplemental doses of tramadol 50 mg in addition to 8 hourly doses of diclofenac sodium.
In Group E, with patient in a sitting position epidural space was identified with 18G Touhy needle in L2-L3 or L3-L4 intervertebral space with the loss of resistance to air technique. Epidural catheter was threaded, directed cephalad and secured. After confirming negativity of test dose, 3 mg/kg of ropivacaine up to a maximum of 150 mg (20 ml of 0.75%) admixed with 1 μg/kg of dexmedetomidine was injected through the catheter into the epidural space. Sensory levels were checked with bilateral pin-prick method while motor blockade was assessed with modified Bromage scale (0 = no block, 1 = inability to raise extended leg, 2 = inability to flex the knee and 3 = inability to flex ankle and foot). Abdominal muscle relaxation was assessed by using the rectus abdominis muscle (RAM) score 10, 20, and 30 min after the injection. RAM score ranged from 0 to 5; 0, full motor activity and 5, full abdominal muscle relaxation.[9] A minimum score of 3 was required for the surgery. The RAM-test was performed as follows: The patient was made to lie in the supine position with no pillow and legs extended. To test the abdominal muscle blockade, the patient was asked to come up slowly and with a curled trunk from the supine to a sitting position and the block was graded accordingly [Table 1]. Patients were turned to kidney (lateral) position after complete establishment of sensory and motor block. No IV sedation was administered to patients throughout the study period. The post block parameters observed included: Initial period of onset of analgesia (from administration of the drug to the establishment of sensory analgesia at T-10 dermatome level); the highest dermatomal level of sensory analgesia; the complete establishment of motor blockade (from administration of the drug to time to achieve Bromage scale IV), patient comfort during surgery, surgical conditions as assessed by the surgeon, regression of analgesic level to S1 dermatome and time to complete recovery (from the onset of motor block to mean time to return to Bromage degree 1 block). The sedation level in Group E was assessed using observer's assessment of alertness scale (OAA/S).[10] Sedation scores were recorded just before the initiation of surgery and thereafter every 20 min during the surgical procedure. Post-operative analgesia was maintained with epidural top-ups with 0.2% ropivacaine.
Table 1.
RAM test of abdominal muscles

The criteria for surgeon's satisfaction included the surgical field bleeding, immobility of the patient, degree of muscle relaxation and the quality of post-operative analgesia in the ward. Patient satisfaction criteria included any pain or discomfort during surgery and in the post-operative period. These scores were measured by the questionnaires prepared during the planning stage of the study. Hypotension was defined as fall in systolic blood pressure ≥25% of the baseline and was treated with IV fluids and injection mephenteramine in aliquots of 3 mg. Bradycardia, a decrease of heart rate ≥25% of the baseline was treated with 0.3 mg bolus dose of atropine.
Side-effects such as nausea and vomiting, headache, respiratory depression, shivering and dry mouth were noted during the post-operative period in both groups as well as in the intra-operative period in Group E.
At the end of the study period, all the data were compiled and subjected to statistical evaluation by a bio-statistician with Statistical Package for Social Sciences (SPSS®) version 17 for Windows®. Data were expressed as mean and standard deviation. The parametric and normally distributed data in the groups were compared with ANOVA for repeated measurements so as to identify the differences between the groups. Non-parametric data in both groups were compared with Mann–Whitney U-test and Wilcoxon test. P < 0.05 was considered statistically significant while P < 0.001 as highly significant.
RESULTS
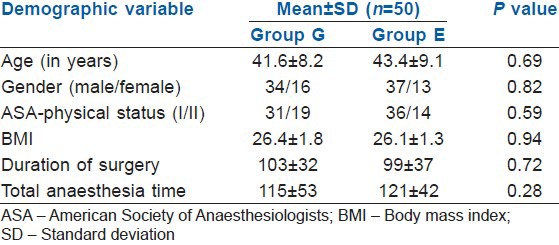
A total of 100 patients were enrolled in the present study and were randomly divided into two groups. Various demographic characteristics such as age, gender distribution, ASA physical status, body mass index, duration of surgery and total anaesthesia time were comparable in both groups and no significant difference was observed [Table 2].
Table 2.
The demographic variables in the Group G and E
The surgical conditions were excellent to fair in the majority of the patients in both groups. In few patients, 10% in Group G and 4% in Group E, adequate muscle relaxation was not achieved and the surgeon was not satisfied [Table 3].
Table 3.
Surgical satisfaction scores and overall patient's satisfaction
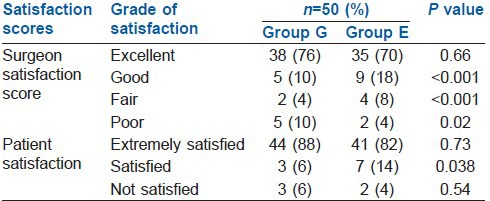
Besides intra-operative evaluation exclusively in Group E, post-operative satisfaction scores were also recorded in both groups. Majority of patients were satisfied with the type of anaesthesia administered. However, the patient satisfactory scores were significantly higher in Group E as compared with Group G on overall statistical evaluation (P = 0.038).
Patient in Group E had good intra-operative sedation without the addition of any IV sedation. Majority of the patients in Group E had a score of 3 or 4 on OAAS/S. It could not be compared with Group G as the latter were administered GA.
Table 4 shows the various block characteristics in Group E patients which however cannot be compared with Group G.
Table 4.
Block characteristics in Group E patients

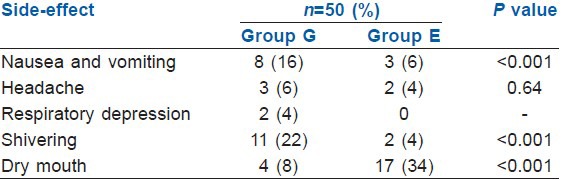
Fewer side-effects were observed in Group E as compared with Group G [Table 5]. The incidence of headache in the post-operative period was comparable in both the groups (P = 0.64). The other side-effects such as, nausea and vomiting, respiratory depression and shivering were observed more frequently in Group G patients. However, the incidence of dry mouth was much higher in Group E patients (34%) as compared with Group G patients (8%) which were highly significant on statistical analysis (P < 0.001).
Table 5.
Side-effect profile of patients in both groups
DISCUSSION
While choosing an anaesthetic technique for any surgical procedure, desirable characteristics include stable haemodynamic parameters, minimal blood loss intra-operatively, early ambulation, good post-operative analgesia and lower incidence of various side-effects such as nausea and vomiting, shivering, cough, headache, respiratory depression and so on.[11] GA has remained the most popular technique for renal surgeries because of the discomfited body position during prolonged renal procedures. RA supplemented with good sedation has been advocated recently.[1]
General anaesthesia carries its own risks and complications such as stress response and cardiac complications during induction of anaesthesia, airway difficulties during intubation, awareness during surgical procedures, need for supplementing analgesia in the post-operative period, additive contribution from comorbidities, difficult extubation, post-operative restlessness, over-sedation and agitation. A higher incidence of side-effects like nausea and vomiting can make GA a very unpleasant experience.[12] In one of the studies, it was observed that epidural anaesthesia was safer than GA in patients with deranged renal functions.[13] Similar concerns can be of huge significance in patients undergoing surgery for renal trauma.[14]
Previous studies have compared combined spinal-epidural anaesthesia and GA for donor nephrectomies and renal transplantation.[1,2] These studies reported that RA can be safely and effectively used for these procedures. The only disadvantage with the combined approach is the haemodynamic instability and unpredictable sensory blockade levels. Taking advantage of the good haemodynamic profile characteristics of ropivacaine and excellent sedative properties of dexmedetomidine, we planned our study to compare GA and epidural anaesthesia in patients undergoing various renal surgeries.
The demographic profile of patients in both groups was similar which provided a very neutral ground for comparing the efficacy of two entirely different techniques. Haemodynamic parameters were also comparable in both groups. There were no statistically significant change in the heart rate, blood pressure, respiratory rate and oxygen saturation during the surgery as compared to baseline except during two stressful periods in GA, intubation and extubation. Addition of dexmedetomidine to ropivacaine in the present study helped in achieving the objective of effective neuraxial anaesthesia with good operating conditions and patient comfort during the surgical procedure. Surgical conditions and patient satisfaction scores were comparable in both groups. Previous studies comparing neuraxial and GA for donor nephrectomies did not observe any significant difference in the levels of surgeon's satisfaction during the peri-operative period.[15]
Addition of dexmedetomidine to local anaesthetics is associated with a rapid onset and establishment of action of local anaesthetics, enhanced post-operative analgesia and dose sparing of local anaesthetics.[6,7] Dexmedetomidine provided good intra-operative sedation and most of the patients were sleeping comfortably during the surgical procedure. Majority of the patients had a score of 3-4 on OAAS/S. The side-effect profile in both groups was strikingly different as a significant number of patients in Group G suffered from pain (38%), nausea and vomiting (16%) and shivering (22%) in the post-operative period. In spite of administration of butorphanol, the incidence of shivering was surprisingly high in the Group G. The lower incidence of shivering in Group E patients can probably be explained on the basis of anti-shivering properties of dexmedetomidine. Peri-operative administration of dexmedetomidine markedly diminishes the incidence of shivering in patients undergoing laparoscopic surgeries.[16] Though no typical headache was observed in both groups, any discomfort in the cranial region (6% in Group G vs. 4% in Group E) was included in the side-effect profile and was clubbed under headache. The only statistically and clinically significant side-effect observed in Group E patients as compared to Group G patients was a higher incidence of dry mouth (34%) during later part of intra-operative and early part of the post-operative period. Drying up of secretions resulting in dry mouth is a typical side-effect of α-2 agonists, which has been observed by various authors after administration of dexmedetomidine and clonidine. Until date, there is no solution for this side-effect and patients were administered 5 ml of distilled water by wetting the lips so as to get relief from this discomfort. One of the remarkable properties of dexmedetomidine includes complete elimination by metabolism with hepatic extraction accounting for 70% of the metabolic pathway. Renal blood flow and renal clearance has no role to play in metabolism or elimination of dexmedetomidine as literary reports confirmed no traces of unchanged dexmedetomidine in urine. These properties will provide another added advantage of dexmedetomidine to be used in RA in patients with deranged renal functions as compared with GA, but not in hepatic dysfunction.[17]
CONCLUSION
Epidural anaesthesia with ropivacaine and dexmedetomidine can be safely and effectively used in patients undergoing renal surgeries as compared with conventionally used GA technique. Surgical conditions and patient satisfaction scores show only marginal difference in favour of epidural anaesthesia, but sedation scores are better with epidurally administered ropivacaine and dexmedetomidine.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sener M, Torgay A, Akpek E, Colak T, Karakayali H, Arslan G, et al. Regional versus general anesthesia for donor nephrectomy: Effects on graft function. Transplant Proc. 2004;36:2954–8. doi: 10.1016/j.transproceed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Hadimioglu N, Ertug Z, Bigat Z, Yilmaz M, Yegin A. A randomized study comparing combined spinal epidural or general anesthesia for renal transplant surgery. Transplant Proc. 2005;37:2020–2. doi: 10.1016/j.transproceed.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Akpek E, Kayhan Z, Kaya H, Candan S, Haberal M. Epidural anesthesia for renal transplantation: A preliminary report. Transplant Proc. 1999;31:3149–50. doi: 10.1016/s0041-1345(99)00761-7. [DOI] [PubMed] [Google Scholar]
- 4.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth. 2012;56:123–8. doi: 10.4103/0019-5049.96303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5:365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–83. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopacz DJ, Allen HW, Thompson GE. A comparison of epidural levobupivacaine 0.75% with racemic bupivacaine for lower abdominal surgery. Anesth Analg. 2000;90:642–8. doi: 10.1097/00000539-200003000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the observer's assessment of alertness/sedation scale: Study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–51. [PubMed] [Google Scholar]
- 11.Gulur P, Nishimori M, Ballantyne JC. Regional anaesthesia versus general anaesthesia, morbidity and mortality. Best Pract Res Clin Anaesthesiol. 2006;20:249–63. doi: 10.1016/j.bpa.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, Arslan G. Comparison of local anaesthesia with dexmedetomidine sedation and general anaesthesia during septoplasty. Eur J Anaesthesiol. 2010;27:960–4. doi: 10.1097/EJA.0b013e32833a45c4. [DOI] [PubMed] [Google Scholar]
- 13.Kazimirov VG, Pisariuk SN, Perlin DV. Advantages of epidural anesthesia in patients with end-stage chronic renal failure. Anesteziol Reanimatol. 1995;4:63–6. [PubMed] [Google Scholar]
- 14.Bajwa SJ, Kulshrestha A. Renal endocrine manifestations during polytrauma: A cause of concern for the anesthesiologist. Indian J Endocrinol Metab. 2012;16:252–7. doi: 10.4103/2230-8210.93744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberal M, Emiroğlu R, Arslan G, Apek E, Karakayali H, Bilgin N. Living-donor nephrectomy under combined spinal-epidural anesthesia. Transplant Proc. 2002;34:2448–9. doi: 10.1016/s0041-1345(02)03173-1. [DOI] [PubMed] [Google Scholar]
- 16.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karol MD, Maze M. Pharmacokinetics and interaction pharmacodynamics of dexmedetomidine in humans. Balliere's Clin Anaesthesiol. 2000;14:261–9. [Google Scholar]


