Abstract
Background and Aims:
Gag reflex is unwanted during upper gastrointestinal endoscopy (UGIE). Experimental studies have demonstrated that N-methyl-D-aspartate receptor antagonism prevents gag reflex. We conducted a study to determine if sub-anaesthetic doses of ketamine, added to propofol, reduce the incidence of gag reflex.
Methods:
This prospective, randomised, double-blind and placebo-controlled study was done in a tertiary care hospital. A total of 270 patients undergoing UGIE, were randomised to propofol (P) group (n = 135) or propofol plus ketamine (PK) group (n = 135). All patients received propofol boluses titrated to Ramsay sedation score of not <4. Patients in PK group in addition received ketamine, 0.15 mg/kg immediately before the first-propofol dose. Top-up doses of propofol were given as required. Stata 11 software (StataCorp.) was used to calculate the proportion of patients with gag reflex and the corresponding relative risk. Propofol consumed and time to recovery in the two groups was compared using Student's t-test and Cox proportional hazards regression respectively.
Results:
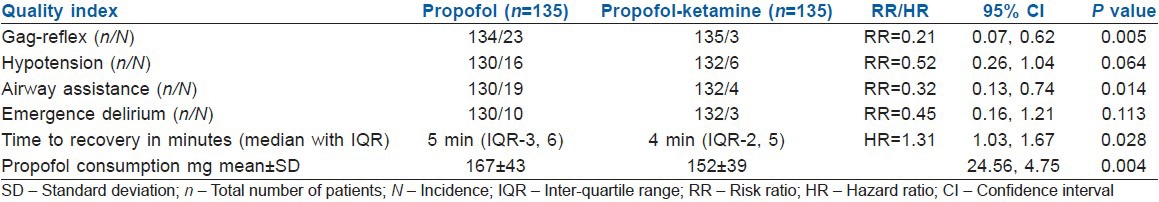
Significantly, fewer patients in the PK group had gag reflex compared to the P group (3 vs. 23, risk ratio = 0.214, 95% confidence interval [CI], 0.07-0.62; P = 0.005). The incidence of hypotension (6 vs. 16, risk ratio = 0.519, 95% CI = 0.25-1.038; P = 0.06), number of required airway manoeuvres (4 vs. 19, risk ratio = 0.32, 95% CI = 0.13-0.74; P = 0.014), median time to recovery (4 min vs. 5 min, hazard ratio = 1.311, 95% CI = 1.029-1.671; P = 0.028) and propofol dose administered (152 mg vs. 167 mg, 95% CI = 4.74-24.55; P = 0.004) was also less in the PK group compared to the P group.
Conclusion:
Ketamine in sub-anaesthetic dose decreases gag reflex during UGIE.
Keywords: Endoscopy, gag reflex, ketamine
INTRODUCTION
Upper gastrointestinal endoscopy (UGIE) is increasingly being performed under propofol sedation. Even under propofol sedation, UGIE is associated with the gag reflex and retching in approximately 29% of patients.[1,2] Any further deepening of sedation to minimise gagging may cause respiratory depression and compromise haemodynamics, while continued gag reflex could affect the safety of the procedure. In a laboratory study, N-methyl-D-aspartate (NMDA) receptor antagonism has been shown to prevent gag reflex.[3] NMDA receptor antagonism has also been shown in a separate laboratory study to abolish the coupling between loss-of-consciousness and upper-airway dilator muscle dysfunction in a wide dose-range.[4]
Propofol is a preferred drug for sedation during UGIE[5] while ketamine, a phencyclidine derivative and NMDA receptor antagonist, is commonly used in sub-anaesthetic doses as an adjunct for anaesthesia technique.[6,7]
We evaluated the effects of pre-treatment with sub-anaesthetic dose of ketamine, an NMDA receptor antagonist on propofol based sedation for UGIE. The primary endpoint of the study was to determine the effect of ketamine pre-treatment on the incidence of gag reflex during propofol based sedation, while the secondary end-points were to study the quality of sedation and the recovery profile between the study groups.
METHODS
After Institutional Ethical Committee approval and following written informed consent from patients, 270 adult American Society of Anaesthesiologists class I and II patients, including patients with well compensated cirrhosis of the liver, scheduled for UGIE, from May 2012 to January 2013 were enrolled in this prospective double-blind study. Patients with clinically significant cardiovascular and respiratory disease (including history of obstructive sleep apnoea), history of epilepsy, and patients allergic to the study drugs were excluded.
The reported incidence of patients having a gag reflex during propofol based sedation for UGIE is around 29%.[1,2] Assuming a 50% reduction in the incidence with a type I error of 5% and power of 80% to determine the difference, the study required 126 patients in each group. We prepared a list of 290 random numbers to take care of any exclusion and allocated the treatment, propofol (P) or propofol with ketamine (PK) to each random number. The random numbers with allotted treatment were written on separate paper slips, and these were then put in a box. Patients were confirmed nil oral and were evaluated during pre-anaesthetic check-up for eligibility for inclusion in the study. Those found eligible were given information booklet about the study and were asked if they would agree to be included in the study. Those consented were allocated to either of the study groups on the basis of the random number slip which was taken out of the box at that moment. Depending upon the group allocation, syringe was loaded with either ketamine 0.15 mg/kg in 5-ml saline or equivalent volume of only saline, and it was labelled with the random number. The paper slip bearing the used random number was then put in another box which was opened at the end of the study to enable analysis of the results.
Anaesthesiologist aware of the group allocation and drawing the drug was not involved with further patient care. Two anaesthesiologists, one administering the drug and the other recording the observations were unaware of the group allocation. All observations were recorded and submitted with that number written on the syringe. Baseline heart rate and blood pressure using automated non-invasive blood pressure device was recorded. An intravenous access was established. Patients were not pre-medicated. No anti-sialagogue or topical anaesthetic was used. Two ml of 2% preservative-free lignocaine was injected intravenously with an upper-arm tourniquet for 1 min to prevent propofol-induced pain. This was followed by the administration of content of the test syringe (either saline or ketamine). Immediately after this injection a bolus of 50-mg propofol (10 mg/ml) was given slowly over 1 min, following which sedation was assessed and if needed further top up doses of propofol were given in 10 mg increments. Sedation was always maintained at Ramsay score[8] of not <4. Sedation levels were checked every 2-3 min by a light glabellar tap. Top up doses of propofol were administered in 10-mg increments if sedation was assessed to be inadequate or if the patient was visibly uncomfortable. Supplemental oxygen was given to all using nasal prongs. Heart rate and peripheral oxygen saturation was continuously monitored, and blood pressure was recorded every 2 min. Hypotension was defined as 20% decrease in mean blood pressure from the baseline value or mean blood pressure <70 mmHg whichever was lower. Hypotension was treated with a bolus dose of ephedrine 6 mg. Gag reflex was recorded as “present” when a vomiting like response was elicited upon insertion of the endoscope. A separate anaesthesiologist who was blinded to the group allocation assessed the depth of sedation using Ramsay sedation scale, recorded the incidence of hypotension, gag reflex, need for airway support like neck extension and jaw thrust, duration of the procedure, and the total dose of propofol administered in each patient. If the banding of the varices were deemed necessary, endoscope was removed and reinserted. Propofol was not given during the period the endoscope was out and loaded with variceal bands allowing patient to recover from the effect of the first dose. Before the reinsertion of the endoscope, (several minutes after the first dose of the propofol) all patients received half of the original loading dose of propofol slowly over 1 min. The time to recovery (time to recovery to Ramsay sedation score 2 from the point of time when the endoscope was pulled out) was also recorded. Patient talking irrelevant or disoriented upon recovery was labelled as having “emergence delirium”. Immediately before discharge, patients were explicitly asked for any recall of the procedure. They were asked “do you remember anything about the endoscopy procedure performed on you?”
The continuous data was summarised using number of patients (N), mean, standard deviation, median, minimum, maximum, and interquartile range (IQR). The categorical data was summarized using frequencies (n) and percentages (%). For intention-to-treat analyses, we used Stata 11 (StataCorp) software to calculate the proportion with gag in the two-trial arms and the corresponding relative risk. We compared the dose of propofol consumed between the two arms with the Student's t-test. We used Cox proportional hazards regression to compare the time to recovery between the trial arms. The hazards ratio (HR) >1 indicated a beneficial effect. P < 0.05 was considered as statistically significant.
RESULTS
A total of 282 patients were found eligible for inclusion in the study. Twelve patients declined to participate in the study. A total of 270 patients were randomised to either study groups [Table 1]. In 8 patients, food was found in the stomach (5 in the P group and 3 in the PK group), hence procedure was abandoned. Data of these patients about the primary end-point was however included for analysis with the intention to treat. There were thus 135 patients in each group. Seventy-seven patients in P group and 86 patients in PK group were follow-up cases for cirrhosis of the liver. Outcome data from one patient in the P group was missing. The primary outcome could, therefore, be assessed in 269 patients [Figure 1]. Of the 269 patients, 26 patients exhibited gag reflex upon insertion of the endoscope. Out of these 26 patients 23 were from the propofol group (Group P) and 3 were from the treatment group (Group PK) (risk ratio [RR] =0.214, 95% CI = 0.07-0.62; P = 0.05). Ten patients in the P group were labelled having “emergence delirium” upon recovery from sedation compared to only three patients in the PK group (RR = 0.445, 95% CI = 0.16-1.21; P = 0.113). Twenty-two patients had hypotension necessitating intervention; of these 16 were from P group, while 6 were from PK group (RR = 0.519, 95% CI = 0.25-1.038; P = 0.06). Nineteen patients in P group and 4 in PK group required airway assistance (RR = 0.32, 95% CI = 0.13-0.74; P = 0.014). Mean dose of propofol consumed during the procedure was 167 ± 43 mg in P group and was 152 ± 39 mg in PK group (95% CI = 4.74-24.55; P = 0.004). Median time to recovery in P group was 5 min with IQR of 3-6 min and was 4 min with IQR of 2-5 min in PK group (HR = 1.311, 95% CI = 1.029-1.671; P = 0.028). No patients in either group had any recall of the procedure [Table 2]. Oxygen saturation in all the patients at all points of time was >95%.
Table 1.
The demographic characteristics of the study groups
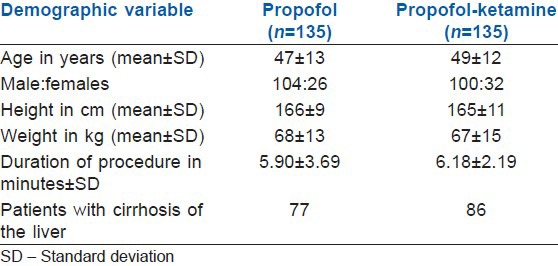
Figure 1.
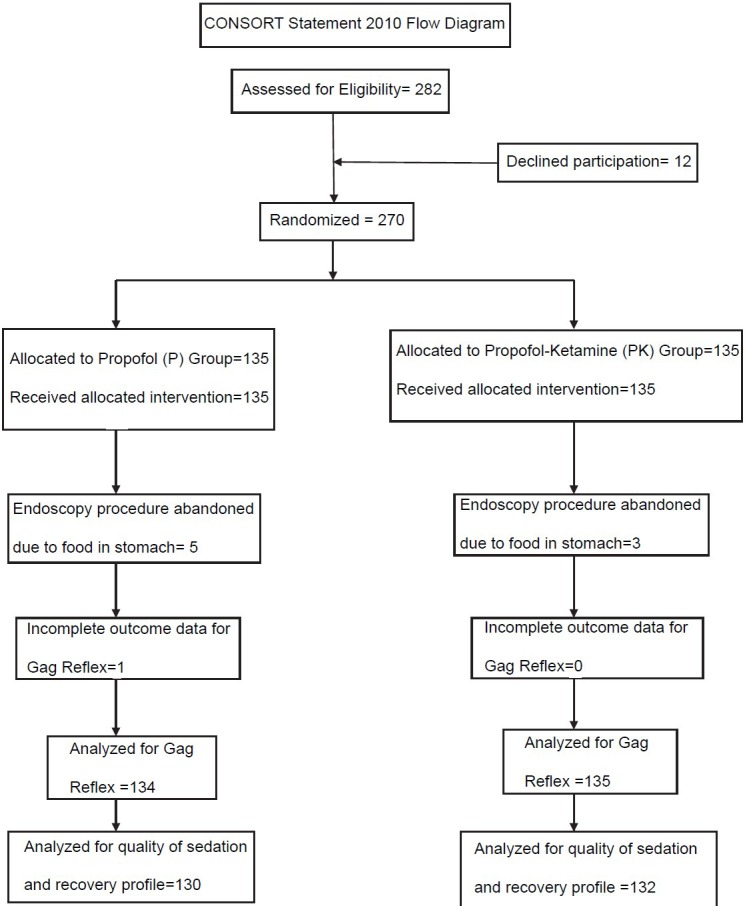
CONSORT flow diagram
Table 2.
Indices of quality of sedation and recovery in the study groups
DISCUSSION
Upper gastrointestinal endoscopy is a day care procedure, wherein patient comfort and safety can be compromised by gag reflex while the endoscope is still inside the oesophagus. Gag reflex is elicited due to the stimulation of the oropharynx by the endoscope. The reported incidence of gag reflex while UGIE is around 29%.[1,2] Gag reflex during UGIE, decreases the ease of the procedure and may even cause trauma to the patient. Soweid et al. tried to address the problem of gag reflex during UGIE by applying lignocaine gel at the base of the tongue and pretonsillar area and compared it with lignocaine spray.[1] They evaluated the incidence of gag reflex and need for rescue sedation and concluded that application of lidocaine gel significantly decreased the need for rescue sedation and also had fewer incidences of gag reflex and retching during the procedure. In another randomized trial Heuss et al. compared propofol alone with a combination of propofol with pharyngeal lidocaine anaesthesia for routine UGIE.[2] They reported that topical pharyngeal anaesthesia reduces the gag reflex in patients sedated with propofol. However, the ease of the procedure and the patient discomfort were similar in both the groups.
The sub-anaesthetic doses of ketamine as an adjunct to anaesthesia technique has been reported from as small as 0.15 mg/kg to 0.25 mg/kg and higher.[7,9,10] Increased incidence of sedation however has been reported with 0.25 mg/kg dose.[10] We, therefore, decided upon 0.15 mg/kg dose for our study.
The incidence of gag reflex during UGIE in our study group P was 17% (23 out of 134). In an experimental study on decerebrate rats, Yamagata and Koga identified neuronal receptors involved in severe gag reflex elicited by superior laryngeal nerve stimulation.[3] Investigators concluded that both Tachykinin NK1 and NMDA receptors are involved in the neural circuit in the development of severe gag reflex. They demonstrated that the pretreatment with NMDA receptor antagonist, MK-801 significantly diminished the induction of severe gag reflex. In our study, we observed 79% reduction in the incidence of gag reflex in the treatment group (PK) compared to the placebo group [Table 2]. Addition of ketamine in sub-anaesthetic doses, therefore, appears to be protective against gag reflex upon insertion of the endoscope.
Eikermann et al.[4] in their experimental study on rats concluded that ketamine is a respiratory stimulant that abolishes the coupling between loss-of-consciousness and upper-airway dilator muscle dysfunction in a wide dose-range. Ketamine, therefore, might help stabilize airway patency during sedation and anaesthesia. Thus, loss of oropharyngeal muscle tone during propofol sedation[11,12] which may necessitate jaw thrust and chin lift manoeuvre to establish a patent airway[13] may be prevented by ketamine because it maintains oropharyngeal muscle tone and does not cause upper-airway collapse.[4] In our study, we observed significant reduction in the number of patients in PK group who required manoeuvres like neck extension and jaw thrust to maintain upper-airway patency in comparison to the P group [Table 2]. Preservation of the pharyngeal muscle tone and maintenance of respiration could have possibly contributed to the suppression of the gag reflex upon insertion of the endoscope.
Ketamine has additive hypnotic and anaesthetic effects when used with propofol.[14] In our study, the mean dose of propofol consumed in P group was significantly more than that in PK group [Table 2]. The median time of recovery was 5 min (IQR 3, 6) in P group while it was 4 min (IQR 2, 5) in PK group [Table 2] possibly due to decreased consumption of propofol, despite addition of sub-anaesthetic dose of ketamine.
Propofol decreases mean arterial pressure due to peripheral vasodilatation and the negative inotropic effects, whereas ketamine tends to stabilise the blood pressure by virtue of its sympathomimetic properties. We observed 48% reduction in the incidence of hypotension in PK group compared to P group however this reduction failed to reach statistical levels of significance [Table 2].
There are concerns with regard to the adverse effects associated with the use of ketamine. But a Cochrane review of 55 randomized control trials concluded that ketamine in sub-anaesthetic dose (that is a dose which is below that required to produce anaesthesia) reduces postoperative nausea and vomiting, and the adverse effects were either mild or absent.[15] We found decreased incidence, though statistically nonsignificant, of emergence delirium in PK group compared with P group [Table 2]. Emergence delirium in either group could have been due to the element of underlying anxiety[16] especially because none of our patients received any antianxiety premedication, and the protective effect of ketamine on emergence delirium[17] could have possibly contributed to the observed difference in the incidence amongst the two groups. No patient in either group in our study had recall of the endoscopy procedure.
CONCLUSION
Our study demonstrated that 0.15 mg/kg ketamine when used along with propofol significantly decreased the incidence of gag reflex during UGIE. Addition of ketamine decreased propofol requirements, the incidence of hypotension and respiratory manoeuvres. We suggest that ketamine 0.15 mg/kg added to propofol can minimize the incidence of gag reflex during UGIE without significant side effects as compared to use of propofol alone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Soweid AM, Yaghi SR, Jamali FR, Kobeissy AA, Mallat ME, Hussein R, et al. Posterior lingual lidocaine: A novel method to improve tolerance in upper gastrointestinal endoscopy. World J Gastroenterol. 2011;17:5191–6. doi: 10.3748/wjg.v17.i47.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heuss LT, Hanhart A, Dell-Kuster S, Zdrnja K, Ortmann M, Beglinger C, et al. Propofol sedation alone or in combination with pharyngeal lidocaine anesthesia for routine upper GI endoscopy: A randomized, double-blind, placebo-controlled, non-inferiority trial. Gastrointest Endosc. 2011;74:1207–14. doi: 10.1016/j.gie.2011.07.072. [DOI] [PubMed] [Google Scholar]
- 3.Yamagata R, Koga T. Effects of NK1 and NMDA receptor antagonists on severe gagging induced by superior laryngeal nerve stimulation in rats. Kawasaki J Med Welf. 2010;16:1–8. [Google Scholar]
- 4.Eikermann M, Grosse-Sundrup M, Zaremba S, Henry ME, Bittner EA, Hoffmann U, et al. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne MF, Chiba N, Singh H, Sadowski DC. Clinical Affairs Committee of the Canadian Association of Gastroenterology. Propofol use for sedation during endoscopy in adults: A Canadian Association of Gastroenterology position statement. Can J Gastroenterol. 2008;22:457–9. doi: 10.1155/2008/268320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wason R, Jain N, Gupta P, Gogia AR. Randomized double-blind comparison of prophylactic ketamine, clonidine and tramadol for the control of shivering under neuraxial anaesthesia. Indian J Anaesth. 2012;56:370–5. doi: 10.4103/0019-5049.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khutia SK, Mandal MC, Das S, Basu SR. Intravenous infusion of ketamine-propofol can be an alternative to intravenous infusion of fentanyl-propofol for deep sedation and analgesia in paediatric patients undergoing emergency short surgical procedures. Indian J Anaesth. 2012;56:145–50. doi: 10.4103/0019-5049.96313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubrun F, Gaillat C, Rosenthal D, Dupuis M, Mottet P, Marchetti F, et al. Effect of a low-dose ketamine regimen on pain, mood, cognitive function and memory after major gynaecological surgery: A randomized, double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2008;25:97–105. doi: 10.1017/S0265021507002566. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Gupta D, Kumar M, Dhiraaj S, Tandon M, Singh PK. Ketamine for treatment of catheter related bladder discomfort: A prospective, randomized, placebo controlled and double blind study. Br J Anaesth. 2006;96:587–9. doi: 10.1093/bja/ael048. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MW, Rohan D, Macgowan CK, Yoo SJ, Macpherson BA. Effect of propofol anesthesia and continuous positive airway pressure on upper airway size and configuration in infants. Anesthesiology. 2006;105:45–50. doi: 10.1097/00000542-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Sundman E, Witt H, Sandin R, Kuylenstierna R, Bodén K, Ekberg O, et al. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: Volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anesthesiology. 2001;95:1125–32. doi: 10.1097/00000542-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Cheng KI, Yun MK, Chang MC, Lee KW, Huang SC, Tang CS, et al. Fiberoptic bronchoscopic view change of laryngopharyngeal tissues by different airway supporting techniques: Comparison of patients with and without open mouth limitation. J Clin Anesth. 2008;20:573–9. doi: 10.1016/j.jclinane.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickx JF, Eger EI, 2nd, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506. doi: 10.1213/ane.0b013e31817b859e. [DOI] [PubMed] [Google Scholar]
- 15.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;1:CD004603. doi: 10.1002/14651858.CD004603.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–54. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 17.Hirota K, Lambert DG. Ketamine: New uses for an old drug? Br J Anaesth. 2011;107:123–6. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]


