Abstract
Alcohol is a hepatotoxin that is commonly consumed worldwide and is associated with a spectrum of liver injury including simple steatosis or fatty liver, alcoholic hepatitis, fibrosis, and cirrhosis. Alcoholic liver disease (ALD) is a general term used to refer to this spectrum of alcohol-related liver injuries. Excessive or harmful alcohol use is ranked as one of the top five risk factors for death and disability globally and results in 2.5 million deaths and 69.4 million annual disability adjusted life years. All patients who present with clinical features of hepatitis or chronic liver disease or who have elevated serum elevated transaminase levels should be screened for an alcohol use disorder. The diagnosis of ALD can generally be made based on history, clinical and laboratory findings. However, the diagnosis of ALD can be clinically challenging as there is no single diagnostic test that confirms the diagnosis and patients may not be forthcoming about their degree of alcohol consumption. In addition, clinical findings may be absent or minimal in early ALD characterized by hepatic steatosis. Typical laboratory findings in ALD include transaminase levels with aspartate aminotransferase greater than alanine aminotransferase as well as increased mean corpuscular volume, gamma-glutamyltranspeptidase, and IgA to IgG ratio. In unclear cases, the diagnosis can be supported by imaging and liver biopsy. The histological features of ALD can ultimately define the diagnosis according to the typical presence and distribution of hepatic steatosis, inflammation, and Mallory-Denk bodies. Because of the potential reversible nature of ALD with sobriety, regular screening of the general population and early diagnosis are essential.
Keywords: Alcoholic liver disease, Diagnosis, Alcohol screening, Histology, Mallory-Denk bodies, Prognosis
Core tip: The diagnosis of alcoholic liver disease (ALD) can be challenging and in most cases, the diagnosis will be established by thorough history, clinical and laboratory findings. However, in uncertain situations, it can be supported by imaging and liver biopsy results. Histological features of ALD can ultimately define the diagnosis according to the typical presence and distribution of hepatic steatosis, inflammation, and Mallory-Denk bodies. Clinical and laboratory parameters can help with establishing the prognosis of ALD in more advanced and severe cases and with determining the therapeutic approach.
INTRODUCTION
Alcohol is a hepatotoxin that is commonly consumed worldwide and is associated with a spectrum of liver injury including simple steatosis or fatty liver, alcoholic hepatitis, fibrosis, and cirrhosis. Alcoholic liver disease (ALD) is a general term used to refer to this spectrum of alcohol-related liver injuries[1,2].
Excessive alcohol consumption is a risk factor for a multitude of adverse health consequences and is indeed one of the leading causes of preventable morbidity and mortality worldwide[3] with a significant burden attributable to ALD[4,5]. Excessive or harmful alcohol use is ranked as one of the top five risk factors for death and disability globally[6] and results in 2.5 million deaths and 69.4 million annual disability adjusted life years[7]. In the United States, almost 9% of adults meet criteria for an alcohol-use disorder[8] with alcohol use disorders ranking in the top 20 leading diseases contributing to disability adjusted life years[9] and resulting in approximately $223.5 billion of societal costs annually[10].
There is a strong correlation between the prevalence of ALD, specifically cirrhosis, and a country’s annual per capita alcohol consumption. Levels of alcohol consumption vary geographically with Eastern European countries having the highest annual per capita consumption (15.7 L per person), while North Africa and the Middle East have the lowest annual per capita consumption (1.0 L per person)[11]. In the United States, the estimated annual per capita consumption of alcohol is 8.4 L per person[12].
Rates of ALD are highest in countries with the highest rates of alcohol consumption including Eastern Europe, Southern Europe and the United Kingdom. In 2010, ALD resulted in 493300 deaths worldwide and 14.5 million disability adjusted life years with alcoholic cirrhosis comprising 47.9% of all liver cirrhosis deaths[11]. In the United States, 31522 adults died from liver cirrhosis in 2009, with 48.2% of these deaths attributable to alcohol[13].
While alcohol is a well established hepatotoxin with higher levels of consumption associated with increased risk of development of ALD, no absolute threshold of alcohol consumption is necessary for the development of liver injury, and no direct linear correlation between level of alcohol consumption and severity of ALD has been established.
Approximately 60%-90% of individuals who drink more than 60 g of alcohol per day have been shown to have hepatic steatosis[14,15]. However, less than half of individuals with alcoholic steatosis, who continue to drink alcohol, will progress to fibrosis and only 10%-20% will eventually progress to cirrhosis[16,17]. Nonetheless, once steatohepatitis has developed, the risk of development of cirrhosis is increased compared with simple steatosis[18]. In addition, individuals who have demonstrated steatohepatitis who continue to drink alcohol or who develop symptomatic alcoholic hepatitis have higher rates of progression to cirrhosis compared with those who subsequently abstain from alcohol consumption or who have never had an episode of symptomatic alcoholic hepatitis. Alcoholic cirrhotics who abstain from alcohol consumption for at least 1.5 years have improved survival rates compared to those that continue to drink[19].
The underlying mechanisms which make some individuals more susceptible to severe forms of ALD are not entirely well understood and are likely multifactorial. Several risk factors have been identified that appear to be correlated with development and progression of ALD including amount and pattern of alcohol consumption, gender, ethnicity, age, obesity, co-existing chronic viral hepatitis, iron overload, smoking, and host genetic factors[20-27].
GENERAL DIAGNOSTIC APPROACH TO ALD
The diagnosis of ALD can generally be made based on clinical and laboratory features alone in patients with a history of significant alcohol consumption after other etiologies for chronic liver disease have been ruled out. However, the diagnosis of ALD can be clinically challenging as there is no single laboratory or imaging study that can confirm the diagnosis. Furthermore, patients may be completely asymptomatic, have no clinical signs of early ALD or early cirrhosis and may have normal liver enzymes. In addition, patients may have co-existing risk factors for non-alcoholic fatty liver disease such as obesity and diabetes and some may not be entirely forthcoming as to their degree of alcohol consumption.
In general, ALD should be suspected in patients with a significant history of alcohol use who present with abnormal serum transaminases, particularly if the level of aspartate aminotransferase (AST) is greater than that of alanine aminotransferase (ALT), hepatomegaly, clinical signs of chronic liver disease, radiographic evidence of hepatic steatosis or fibrosis/cirrhosis, or who have had a liver biopsy showing macrovesicular steatosis or cirrhosis.
Patients with ALD may or may not have elevated serum aminotransferase levels. The absolute level of liver enzyme elevation does not correlate well with the severity of ALD, however, the pattern of elevation in transaminases is helpful in making a diagnosis of liver injury due to alcohol as AST is typically two to three times greater than ALT in alcoholic liver injury[28]. They will also typically have an elevated serum gamma-glutamyltranspeptidase (GGT)[29]. However, it is important to rule out other etiologies for the patient’s liver disease before making a definitive diagnosis of ALD, including chronic viral hepatitis, autoimmune hepatitis, hemochromatosis and drug related hepatotoxicity. In some cases, when the diagnosis is unclear, a liver biopsy may be warranted.
SCREENING FOR ALCOHOL USE DISORDERS
To review, one standard alcoholic drink is considered any alcoholic beverage that contains 14 g of alcohol. Examples of a standard drink include 12 ounces of regular beer, 8-9 ounces of malt liquor, 5 ounces of wine and 1.5 ounces of distilled spirits. Men who consume more than 4 standard drinks in any single day (or more than 14 drinks per week) and women who consume more than 3 in any single day (or more than 7 drinks per week) are at increased risk for alcohol-related problems[30].
Worldwide, approximately 20%-30% of patients who present in primary care settings engage in hazardous or harmful drinking[31]. Hazardous drinking is defined as a pattern of drinking that increases the risk of physical or psychological problems[32] and harmful drinking is defined as a pattern of drinking that results in such problems[33]. Persistent drinking despite adverse health or psychological consequences constitutes an alcohol-use disorder which includes a spectrum of disease ranging from alcohol abuse to alcohol dependence[34]. At the severe end of the spectrum, individuals who are alcohol dependent suffer from a brain disorder characterized by loss of control over their drinking, alcohol craving, frequent drinking, continued drinking despite negative consequences, tolerance, withdrawal and disability[35]. It is recommended that health care providers screen for and counsel risk drinkers as part of routine medical and preventive care[35].
All patients who present with clinical features of hepatitis or chronic liver disease (Table 1) or who have elevated serum transaminase levels should be screened for an alcohol use disorder. Denial of alcohol abuse and underreporting of alcohol intake are common among alcoholics[41] and thus, clinicians should have a low threshold to screen their patients for alcohol abuse. In the United States, routine alcoholism screening is widely recommended and is now re-imbursed on an annual basis by Medicare[42]. The US Preventive Services Task Force (USPSTF) recommends routine screening of all adult primary care patients followed by a brief counseling intervention of persons who engage in risky or hazardous drinking (grade B recommendation: high certainty that the net benefits is moderate or there is moderate certainty that the net benefit is moderate to substantial)[43] and the National Institute on Alcohol Abuse and Alcoholism recommends annual screening of all adults with the use of a validated self-reporting tool[44].
Table 1.
| Spectrum of ALD | Clinical presentation |
| Alcoholic fatty liver | Asymptomatic |
| Alcoholic hepatitis | Jaundice |
| Anorexia | |
| Fever | |
| +/- RUQ/epigastric pain | |
| +/- Abdominal distention due to ascites | |
| +/- Proximal muscle weakness | |
| +/- Confusion due to HE | |
| Compensated cirrhosis | Asymptomatic |
| Anorexia | |
| Weight loss | |
| Weakness | |
| Fatigue | |
| Muscle cramps | |
| Amenorrhea or irregular menses | |
| Impotence, infertility, loss of sexual drive | |
| Decompensated cirrhosis | Jaundice |
| Pruritus | |
| GI bleeding | |
| Weight gain | |
| Abdominal distention due to ascites | |
| Lower extremity edema | |
| Easy bruising | |
| Sleep disturbances | |
| Confusion |
ALD: Alcoholic liver disease; GI: Gastrointestinal; RUQ: Right upper quadrant; HE: Hepatic encephalopathy.
Several validated screening tools that can easily be administered during a clinical visit are available to identify patients at risk for alcohol abuse. The USPSTF prefers the use of alcohol use disorders identification test (AUDIT), AUDIT-consumption (AUDIT-C) and single question screening in the primary care setting. Of the available screening instruments, the AUDIT is the most widely studied for detecting alcohol use disorders in the primary care setting[45]. The AUDIT comprises ten questions with a specific scoring system (Table 2) and requires approximately 2 to 5 min to administer. An optimal score for detecting unhealthy alcohol use in men is 5 for men (sensitivity 77%, specificity 76%) and 3 for women (sensitivity 86%, specificity 74%). A score of 6 or more for men (sensitivity 84%, specificity 76%) and 4 or more for women (sensitivity 88%, specificity 76%) is highly suggestive of alcohol dependence[47].
Table 2.
Alcohol use disorders identification test[46]
| 1 How often do you have on a drink containing alcohol? |
| (0) Never (skip to questions 9-10) |
| (1) Monthly or less |
| (2) 2 to 4 times a month |
| (3) 2 to 3 times a week |
| (4) 4 or more times a week |
| 2 How many drinks containing alcohol do you have on a typical day when you are drinking? |
| (0) 1 or 2 |
| (1) 3 or 4 |
| (2) 5 or 6 |
| (3) 7, 8, or 9 |
| (4) 10 or more |
| 3 How often do you have six or more drinks on one occasion? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 4 How often during the last year have you found that you were not able to stop drinking once you had started? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 5 How often during the last year have you failed to do what was normally expected from you because of drinking? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 6 How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 7 How often during the last year have you had a feeling of guilt or remorse after drinking? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 8 How often during the last year have you been unable to remember what happened the night before because you had been drinking? |
| (0) Never |
| (1) Less than monthly |
| (2) Monthly |
| (3) Weekly |
| (4) Daily or almost daily |
| 9 Have you or someone else been injured as a result of your drinking? |
| (0) No |
| (2) Yes, but not in the last year |
| (4) Yes, during the last year |
| 10 Has a relative or friend or a doctor or another health worker been concerned about your drinking or suggested you cut down? |
| (0) No |
| (2) Yes, but not in the last year |
| (4) Yes, during the last year |
Skip to questions 9 and 10 if total score for questions 2 and 3 = 0.
The AUDIT-C questionnaire, an abbreviated version of the AUDIT performs as well as the full 10 item AUDIT, and significantly better than self-reported risky drinking or the CAGE questionnaire[48]. The CAGE questionnaire, the name of which is an acronym of its four questions, is considered positive if a patient answers yes to two or more of the following questions: (1) Have you ever felt you needed to cut down on your drinking? (2) Have people annoyed you by criticizing your drinking? (3) Have you ever felt guilty about drinking? or (4) Have you ever felt you needed a drink first thing in the morning (eye-opener) to steady your nerves or to get rid of a hangover[49]?
The AUDIT-C is comprised of three questions with a specific scoring system (Table 3) ranging from 0 to 12 and takes approximately 1 to 2 min to complete. A positive screening result is a score of 3 or more for women and 4 or more for men. A score of 7 to 10 has been associated with increased risk of alcohol dependence[50]. The AUDIT-C screening tool has been shown to be 73% sensitive and 91% specific for an alcohol-use disorder and 85% sensitive, 89% specific for alcohol dependence[51]. The AUDIT-C score also serves as an excellent marker of alcohol misuse severity[52]. A positive result should prompt a more in-depth assessment of the patient’s alcohol use pattern and formal evaluation for an alcohol-use disorder.
Table 3.
| Test | Questions | Scoring | Positive result |
| AUDIT-C | Q1: How often did you have a drink containing alcohol in the past year? | For women ≥ 3 points; for men ≥ 4 points | |
| Never | 0 points | ||
| Monthly or less | 1 point | ||
| Two to four times a month | 2 points | ||
| Two to three times per week | 3 points | ||
| Four or more times a week | 4 points | ||
| Q2: How many drinks did you have on a typical day when you were drinking in the past year? | |||
| One or two | 0 points | ||
| Three or four | 1 point | ||
| Five or six | 2 points | ||
| Seven to nine | 3 points | ||
| Ten or more | 4 points | ||
| Q3: How often did you have six or more drinks on one occasion in the past year? | |||
| Never | 0 points | ||
| Less than monthly | 1 point | ||
| Monthly | 2 points | ||
| Weekly | 3 points | ||
| Daily or almost daily | 4 points | ||
| Single question screening test from NIAAA | How many times in the past year have you had five (four for women) or more drinks in a day? | One point per time | ≥ 1 time |
| Three question screening test from NIAA | Q1: On average, how many days per week do you drink alcohol? Q2: On a typical day when you drink, how many drinks do you have? Q3: What is the maximum number of drinks you had on any given occasion during the past month | For men, > 14 drinks per week or > 4 drinks per occasion; for women or person older than 65 years, > 7 drinks per week or > 3 drinks per occasion |
AUDIT-C: Alcohol use disorders identification test-consumption; NIAAA: National Institute on Alcohol Abuse and Alcoholism.
A second validated brief screening tool available with adequate sensitivity and specificity involves a single question asked of patients: “How many times in the past year have you had five (four for women) or more drinks in a day?” If a patient responds to this as one or more times, this is a positive screening result. This question has been demonstrated to be 82% sensitive and 79% specific for unhealthy use of alcohol[53].
The last brief screening tool available is a set of three questions that evaluates the typical quantity of drinks consumed on one occasion, frequency of drinking per week and maximum number of alcoholic beverages consumed on any given occasion in the past month (Table 3). A positive screen result is greater than 14 drinks consumed per week or more than 4 drinks consumed on one occasion for men and greater than 7 drinks consumed per week or more than three drinks consumed on one occasion for women or persons older than age 65. This tool has been shown to be 83% sensitive and 84% specific for alcohol abuse or dependence in the past year[54].
Validated self-report questionnaires have been shown to have both greater sensitivity and specificity for detecting alcohol abuse than blood tests for biochemical markers[55]. No single reliable diagnostic biomarker has been identified which has adequate sensitivity and specificity to be useful for general screening of alcohol consumption or abuse[56,57]. Nevertheless, biochemical markers may play a role in alcohol abuse screening when the clinician suspects heavy drinking in a patient who denies it (see laboratory studies section).
PHYSICAL EXAMINATION
A detailed physical examination should be performed to evaluate the patient for evidence of chronic liver disease (Table 4). The physical examination findings in patients with ALD will vary depending on the severity of disease and range from a completely normal examination to physical signs of cirrhosis with severe decompensation (Table 4). Physical findings may be normal and non-diagnostic particularly in patients with mild ALD, steatosis or early cirrhosis. Patients with cirrhosis and portal hypertension may exhibit stigmata of chronic liver disease and if concomitant hepatic decompensation exists, may also exhibit ascites, peripheral edema, asterixis and/or mental confusion. Patients with alcoholic hepatitis will have scleral icterus and jaundice as well as tender hepatomegaly with or without ascites and if their hepatitis is severe will have asterixis and exhibit mental confusion on examination. In addition, patients with ALD typically have co-morbidities due to the concomitant toxic effects of alcohol on other organ systems and may have signs of peripheral neuropathy, muscle wasting and heart failure[68,69].
Table 4.
| Spectrum of ALD | Physical examination findings |
| Fatty liver | Normal examination |
| +/- Hepatomegaly | |
| Alcoholic hepatitis | Jaundice |
| Tender hepatomegaly | |
| +/- Ascites | |
| +/- Hepatic bruit | |
| Proximal muscle wasting | |
| Decreased grip strength | |
| +/- Hepatic encephalopathy (confusion, asterixis, hippus) | |
| Cirrhosis | Spider angiomata (face, trunk, upper extremities) |
| Parotid gland enlargement | |
| +/- Fetor hepaticus | |
| Gynecomastia | |
| +/- Hepatomegaly | |
| Firm liver edge with nodular contour | |
| +/- Splenomegaly | |
| Caput medusa (abdominal wall collaterals) | |
| Cruveilhier-Baumgarten murmur | |
| Testicular atrophy | |
| Palmar erythema | |
| Digital clubbing | |
| Muehrcke nails (paired horizontal white bands) | |
| Terry nails (large white proximal nail bed) | |
| Hypertrophic osteoarthropathy | |
| Dupuytren’s contracture | |
| Decompensated cirrhosis | Cirrhotic physical finding plus: |
| Jaundice | |
| Ascites | |
| Peripheral edema | |
| Hepatic encephalopathy (confusion, asterixis, hippus) |
ALD: Alcoholic liver disease.
LABORATORY STUDIES
While no single laboratory test will confirm the diagnosis of ALD, common laboratory abnormalities in alcoholics have been identified and certain biomarkers are highly suggestive or indicative of ALD. Additional laboratory testing can aid in the identification of hepatic inflammation, portal hypertension, assess hepatic synthetic function and potentially aid in identifying chronic alcohol abuse.
As part of initial testing, all patients being evaluated for ALD should have a complete blood count, hepatic panel (transaminases, bilirubin, alkaline phosphatase, albumin), gamma-glutamyl transferase, and an international normalized ratio (INR) checked.
If a patient has evidence of hepatocellular injury as indicated by elevated serum transaminase levels, he or she should be screened for chronic viral hepatitis with measurements of hepatitis B surface antigen, hepatitis B core IgG and hepatitis C antibody; autoimmune hepatitis with anti-nuclear antibody, anti-smooth muscle antibody and IgG4 or gamma-globulin levels; hemochromatosis with serum ferritin, serum iron and transferrin with percent iron saturation; alpha one anti-trypsin deficiency with alpha one anti-trypsin level; and serum ceruloplasmin levels and 24 urinary copper for Wilson’s disease.
Common hematological findings in patients with ALD include thrombocytopenia, macrocytic anemia, lymphopenia, elevated erythrocyte sedimentation rate and an elevated INR[70,71]. Macrocytosis suggests chronic disease and may be secondary to toxicity of alcohol on bone marrow, folate or vitamin B12 deficiency, or increased lipid deposition in erythrocyte membranes. Thrombocytopenia is present in about a third of alcoholics admitted to hospitals and with abstinence will tend to normalize within 1-3 wk[72]. High density lipoprotein cholesterol, serum ferritin, and urate levels also increase as a consequence of alcohol consumption[73-76]. In addition to an elevated INR, patients with poor hepatic synthetic function will also have low serum albumin levels. Interestingly, patients who engage in chronic alcohol consumption but who do not have underlying ALD may have an elevated serum albumin level possibly secondary to effects of acetaldehyde[77].
Patients with ALD frequently demonstrate evidence of iron overload as reflected by elevated serum iron indices (ferritin and transferrin saturation) and hepatic iron concentration[75,78]. Nearly 30% of patients with ALD have increased hepatic iron stores[79] and serum transferrin saturation may approach or even exceed 60% in some cases[80]. The etiology of iron accumulation in alcoholics is unknown but may be due to alcohol suppression of liver transferrin synthesis or deregulation of hepcidin synthesis in the liver[81]. Regardless of the etiology, iron overload in ALD may be difficult to differentiate from hereditary hemochromatosis, and in fact, prior to the widespread availability of HH genetic testing, often led to misdiagnosis. In cases of significantly elevated ferritin or transferrin levels, additional testing, including a DNA analysis for HFE gene mutations, is warranted to rule out hereditary hemochromatosis.
The biochemical markers for chronic alcohol consumption that have been most commonly studied are serum GGT, AST, ALT, mean corpuscular volume (MCV) and carbohydrate-deficient transferrin (CDT)[82-84]. An AST to ALT ratio over 2 is highly suggestive of ALD[85,86]. Most patients with non-ALD have AST to ALT ratios below one. Specific IgA antibodies directed towards acetaldehyde-derived protein modifications are frequently seen alcoholics and thus IgA levels are increased in chronic ALD. An increased ratio of IgA to IgG is highly suggestive of ALD[87-89].
Chronic alcohol consumption is known to induce a rise in serum GGT and is a widely used index for excessive alcohol use[90,91]. However, elevated GGT alone has both low sensitivity and specificity for alcohol abuse[92,93]. GGT is not specific to alcoholism and is increased in many conditions such as obesity, advanced age, moderate alcohol consumption, all forms of liver disease including fatty liver and in particular intra and extrahepatic biliary obstruction, hepatocellular carcinoma and phenytoin use[94-97]. The sensitivity of GGT as a marker for alcohol consumption in young adults has been showed to be particularly poor even in cases of documented alcohol dependence[98].
Transferrins which have a low degree of bond with carbohydrates are collectively called CDT and are increased in the serum of alcoholics[99]. However, the mechanism in which the presence of ethanol in vivo causes this alteration in transferrin is largely unknown. CDT is a more sensitive marker of chronic alcohol consumption in men than women who may express higher levels of CDT under natural conditions and produce less CDT in response to heavy drinking[100,101]. In addition, some studies have shown elevated CDT levels in cirrhotic patients regardless of their alcohol consumption[102] while other studies have shown normal CDT levels in patients with chronic liver disease who abstain from alcohol[103].
No single biomarker has both adequate sensitivity and specificity for detecting chronic alcohol abuse. However, when certain biomarkers are combined, they may provide improved diagnostic yield[104]. For example, while CDT has the highest specificity for harmful or heavy alcohol consumption, combining this biomarker with GGT and/or MCV, improves sensitivity significantly (Table 5). In addition, combining CDT testing with screening questionnaires, particularly for patients in which alcohol abuse is strongly suspected but who have a negative screening questionnaire result, has also been shown to be cost effective[108].
Table 5.
Sensitivity and specificity of biomarkers in detecting harmful or heavy alcohol consumption[105-107]
| Biomarker | AST | ALT | MCV | CDT | CDT + GGT | CDT + GGT + MCV |
| Sensitivity | 47%-68% | 32%-50% | 45%-48% | 63%-84% | 83%-90% | 88% |
| Specificity | 80%-95% | 87%-92% | 52%-94% | 92%-98% | 95%-98% | 95% |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; MCV: Mean corpuscular volume; CDT: Carbohydrate-deficient transferring; GGT: Gamma-glutamyltranspeptidase.
Ethyl glucuronide (EtG), ethyl sulfate (EtS) and phosphatidylethanol (PEth) have been used with increasing frequency in the past decade to monitor abstinence from alcohol in outpatient and treatment settings[109,110]. In a study on forty patients, PEth was compared with CDT as a biomarker for active alcohol consumption and was found to be positive twice as often as CDT in patients who relapsed from abstinence while in a voluntary outpatient treatment program[111]. However, considerable inter-individual variability in PEth levels have been observed in clinical studies which may create problems with the interpretation of results and may limit the usefulness of PEth to identification of relapse from abstinence[112,113]. The utility of urinary EtG and EtS, similar to measurement of blood alcohol level, is limited to detecting recent intake of even small amounts of alcohol.
Patients with alcoholic hepatitis will typically have moderately elevated aminotransferases (less than 500 IU/mL), an AST:ALT ratio of two or greater and elevated serum bilirubin (greater than 5 mg/dL)[114,115]. Patients with severe alcoholic hepatitis may also have a leukocytosis and elevated C-reactive protein indicative of acute liver injury or concomitant infection[116].
While there are no ideal non-invasive biomarkers currently available to differentiate between simple steatosis and alcoholic steatohepatitis, newly discovered biomarkers for non-alcoholic steatohepatitis (NASH) may be potentially applied to ALD in the future. For example, serum cytokeratin-18, a marker of hepatocyte apoptosis, is a promising and accurate non-invasive test for the diagnosis of NASH [area under the receiver operating curve (AUROC): 0.83-0.91][117,118] particularly when used in combination with fibroblast growth factor-21[119]. However, additional research of the utility and accuracy of these biomarkers for use in the setting of alcoholic steatohepatitis (ASH) is necessary.
IMAGING
Current widely available imaging modalities for the liver include ultrasonography (US), computed tomography scan (CT) and magnetic resonance imaging (MRI). While each of these imaging studies are useful for determining the presence of underlying liver disease, they cannot confirm alcohol use as the etiology of a patient’s liver disease. Nonetheless, imaging studies can be useful for excluding other causes of abnormal liver tests in patients who abuse alcohol such as infiltrative disease, obstructive biliary pathology and neoplastic diseases of the liver[120]. Imaging can also aid in the diagnosis of cirrhosis and can be used to screen for and identify hepatocellular carcinoma.
US is a non-invasive technique that is routinely used in the initial evaluation of liver. The appearance of fat in the liver is highly variable on US, however, in general, a fatty liver will have a hyperechoic texture and macroscopic fat will appear as hyperechoic masses[121]. The sensitivity and specificity of a hyperechoic pattern on ultrasound for hepatic steatosis in patients with a liver replaced by at least thirty percent steatosis is 91% and 93% respectively. In patients who have less than thirty percent hepatic steatosis, the sensitivity is only approximately 64%[122].
Hepatic steatosis is more easily detected by a non-contrast CT scan which can be a particularly useful technique to detect macroscopic fat in the liver[123]. Measurement of attenuation differences between the liver and spleen is used to identify a fatty liver. A liver-to-spleen attenuation ratio greater than 10 hounsfield units is highly predictive of hepatic steatosis[124] and the liver attenuation index has been shown to closely predict the degree of hepatic steatosis in patients with living related liver transplantation[125]. MRI techniques in which water and fat are imaged in and out of phase may be the most sensitive and specific imaging modality for detecting hepatic steatosis (95% sensitivity, 98% specificity)[126]. However, in patients with hepatic iron overload, opposed phase MRI imaging may not be able to detect the presence of fat in the liver and MR spectroscopy may be a more useful imaging modality in these patients. As with CT imaging, MRI imaging can be prohibitively expensive as an initial study and may not provide additional diagnostic yield when compared to ultrasound in the setting of macroscopic steatosis. A newer imaging modality is currently under investigation that is controlled attenuation parameter used with transient elastography which shows promising performance for detection and quantification of steatosis but which is still not widely available[127,128].
On US, patients with fibrosis may have a coarsened echo pattern to their liver and patients with cirrhosis may have a nodular liver contour. The sensitivity of US for significant fibrosis is about 57% and 71% for patients with established cirrhosis. Overall, specificity is approximately 88%[129]. CT findings in patients with cirrhosis may include atrophy of the right lobe of the liver, hypertrophy of the caudate lobe, hypertrophy of the lateral segment of the left lobe, parenchymal nodularity, attenuation of hepatic vasculature, splenomegaly, venous collaterals and ascites[130]. Imaging features on ultrasound and MRI that may be suggestive of alcoholic cirrhosis include an enlarged caudate lobe, visualization of the right posterior hepatic notch and smaller size regenerative nodules[131,132].
Improved imaging modalities have been developed over the past decade in order to detect and quantify hepatic fibrosis and cirrhosis. These include transient elastography (FibroScan), acoustic radiation force impulse and magnetic resonance elastography. These imaging techniques measure liver “stiffness” by utilizing a transducer to transmit and measure vibration (elastic shear wave) as it propagates through the liver. The velocity of this wave as it passes through the liver correlates directly with tissue stiffness. These non-invasive radiologic studies may replace the more invasive liver biopsy in the future for accurate staging of hepatic fibrosis[133,134]. To our knowledge, however, the sensitivity and specificity of these new imaging modalities for diagnosing fibrosis and cirrhosis in patients with ALD have not yet been fully evaluated.
ROLE OF LIVER BIOPSY
A liver biopsy is not necessary for the diagnosis of ALD in most patients. Clinical findings in patients with chronically elevated characteristic liver enzymes together with a history of significant alcohol use have been found to be 91% sensitive and 97% specific for the diagnosis of ALD when compared to liver biopsy[135]. However, a liver biopsy may be useful for establishing the diagnosis in some patients if the diagnosis of ALD is not clear according to clinical presentation and laboratory studies and in patients in whom the clinician suspects more than one type of underlying liver disease. Approximately 20% of patients with a history of chronic alcohol abuse have a secondary or co-existing etiology for their liver disease[136]. A biopsy can also be useful in establishing the stage and severity of liver disease. A recent study of patients with acute deterioration of alcoholic cirrhosis suggests that early transjugular liver biopsy in these patients can also provide important diagnostic and prognostic information for the identification and treatment of a subset of patients with superimposed alcoholic steatohepatitis which can be difficult to differentiate from decompensated cirrhosis on the basis of clinical and laboratory evaluation alone[137].
Currently, liver biopsy is the gold standard for the diagnosis and assessment of severity of hepatic steatosis, staging of fibrosis and is the only modality available to differentiate between bland steatosis and steatohepatitis. Liver biopsy can facilitate the differentiation between simple steatosis and steatohepatitis based on distinct histological features as described in the next section. This differentiation is of clinical significance in that it provides important prognostic information for the patient. Clinical experience with large numbers of ALD patients has demonstrated that it can be difficult to clinically predict the stage of liver disease before the development of decompensated cirrhosis[138].
Nevertheless, the liver biopsy does have limitations. It is an invasive procedure to which patients may be adverse, can cause complications, is prone to sampling error and a firm etiology for underlying liver disease may not be achieved based on histology[139]. If no treatment of ALD is being considered other than alcohol abstinence and adequate nutrition, then a histologic diagnosis is usually not warranted. Furthermore, the role of liver biopsy in making the diagnosis of alcoholic hepatitis is controversial as it carries significant risk of bleeding in the setting of coagulopathy and thrombocytopenia when using a standard percutaneous approach.
HISTOLOGY OF ALD
The histologic features of ALD on liver biopsy vary based on the extent and stage of hepatic injury. Steatosis is the most common and earliest manifestation of ALD. Steatosis in ALD is typically macrovesicular in nature (Figure 1A) in which large lipid droplets occupy nearly the entire cytoplasm of hepatocytes and displace the nucleus and other organelles peripherally[140]. The pattern for macrovesicular steatosis in ALD is typically centrilobular but it can progress to include the entire lobule in severe cases. Other early changes seen in ALD include proliferation of smooth endoplasmic reticulum, distortion of mitochondria[141] and, if severe, can be associated with giant mitochondria[142]. Giant mitochondria have been associated with all types of ALD from fatty liver to cirrhosis, and although they are not specific to ALD, the presence of giant mitochondria favors alcohol-related disease and is a good indicator of recent heaving drinking[143,144].
Figure 1.
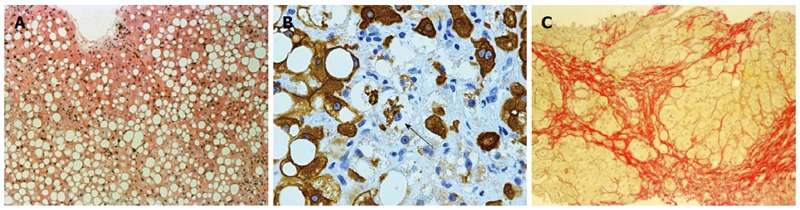
Histology of alcoholic fatty liver. A: Macrovesicular steatosis in alcoholic fatty liver (HE stain, × 218); B: Ballooned hepatocyte (arrow) containing a Mallory Denk body in alcoholic hepatitis (CAM5.2 stain for cytokeratins 8 and 18, × 218); C: Collagen surrounds nodules of hepatocytes in alcoholic cirrhosis (Serius red stain, × 872).
Steatosis may progress to steatohepatitis (ASH). ASH is characterized by liver cell damage, inflammation, and fibrosis. The typical histologic characteristics of ASH include centrilobular accentuated steatosis, hepatocyte ballooning eventually associated with Mallory-Denk bodies (MDB) (Figure 1B), a mixed inflammatory reaction of neutrophilic, lymphocytic and mononuclear cells, hepatocyte necrosis and perivenular fibrosis which can progress to spider-like pericellular fibrosis[145,146]. MDB are cytoplasmic accumulations of hepatocytic keratin intermediate filaments and are characteristic features of ASH and NASH[147,148]. In general, however, MDB are less prominent and more difficult to identify without immunohistochemistry in the non-alcoholic variant[149]. MDB can also be found in patients with amiodarone toxicity, primary biliary cirrhosis, chronic cholestasis syndromes, idiopathic copper toxicosis, Wilson’s disease, Indian childhood cirrhosis, alpha-1 antitrypsin deficiency, and hepatocellular carcinoma[150-152].
Steatosis and ASH are present in approximately one third of patients with alcoholic cirrhosis and their presence usually indicates persistent alcohol abuse. Histologically, a cirrhotic liver will have fibrous septae made of collagen surrounding hepatocytes resulting in pseudolobule formation (Figure 1C) which produces a nodular appearance to the liver and which may progress from micronodular to macronodular cirrhosis over time[153,154]. Bile duct proliferation may also be prominent in the cirrhotic stage of ALD[155].
ASSESSMENT OF PROGNOSIS
Several demographic, clinical, laboratory and histologic findings can provide prognostic information for patients diagnosed with ALD. While not all patients that drink heavily will develop ALD, continued alcohol use often leads to progressive liver disease once clinical and histological evidence for ALD has developed[156,157]. Other patient factors that are associated with increased risk of progression to cirrhosis included female sex, tobacco use, binge drinking, obesity and concomitant chronic viral hepatitis[23,158-162]. Demographic and clinical factors associated with increased mortality in patients with ALD include persistent alcohol use, increasing age, tobacco use, cirrhosis with a higher Child-Pugh score (based on bilirubin, albumin, INR, grade of encephalopathy and ascites), degree of malnutrition, severe deficiency in 25-hydroxyvitamin D, development of cirrhotic complications, and concomitant chronic viral hepatitis infection[163-168].
Overall, patients with alcoholic cirrhosis have a poor five year prognosis. However, alcoholic patients who develop complications from their cirrhosis do significantly worse than those with well compensated alcoholic cirrhosis. In a recent study, patients with well compensated alcoholic cirrhosis had an estimated 5-year mortality rate of approximately 58%. The presence of ascites only increased mortality by 1%, however, patients who developed both ascites and variceal bleeding had a significantly increased 5-year mortality of 80%. Patients who developed hepatic encephalopathy fared the worst with an estimated 5-year mortality of 85%[157]. Patients who develop hepatorenal syndrome (HRS) and who do not receive a liver transplantation, have a dismal prognosis. In a recent study of cirrhotic patients with type 1 HRS, patients with alcoholic cirrhosis had a median survival of only 8 d[169].
The degree of protein-calorie malnutrition (as measured by percent ideal body weight, tricep skin fold thickness, mid-arm muscle circumference, creatinine height index, albumin, transferrin, total lymphocyte count and delayed cutaneous hypersensitivity) in patients with ALD correlates closely with the development of serious complications from liver disease (ascites, encephalopathy, and hepatorenal syndrome), as well as overall mortality[166]. In patients with AH, those with moderate protein malnutrition had a significantly better 6-mo survival rate (75%) than those with severe malnutrition (55%)[166] as well as a significantly better 1-year survival rate (57%) than those with severe malnutrition (24%)[170]. In addition, cirrhotic patients with poor nutrition have a 3-fold greater probability of developing hepatorenal syndrome[171].
There is limited evidence that laboratory studies can have predictive prognostic significance for ALD. However, in a recent study of ALD patients, a decreased α-aminobutyrate/ cystathionine ratio predicted the presence of ALD on liver biopsy and cystathionine levels correlated with the stage of fibrosis in ALD patients[172].
The stage of liver disease is an important prognostic factor for patients with ALD. In a VA study, 281 alcoholic patients were followed prospectively over a 48 mo period and their ALD was staged with liver biopsy. Simple steatosis, an early stage of ALD, was associated with a 30% mortality at 4 years. Alcoholic hepatitis alone, the next stage along the ALD spectrum, carried an estimated 40% 4-year mortality rate. Stable cirrhosis without alcoholic hepatitis carried a 50% mortality rate, and, lastly, the combination of cirrhosis and alcoholic hepatitis was the most deadly and carried the highest mortality rate of approximately 65% at 4 years[173].
Several specific histological findings are important prognostic indices in ALD. For example, the presence of MDB is an important marker of alcoholic related liver injury in alcoholics. MDB have been found in 76% of patients with alcoholic hepatitis and in 95% of patients with concomitant alcoholic cirrhosis[174] and the presence of MDB is associated independently with progression of fibrosis[175]. Pericellular fibrosis, a progression of perivenular fibrosis[176,177], and the presence of ASH on biopsy[178] are also independent predictors of progression to fibrosis and development of cirrhosis in patients with ALD.
In a recent study of Danish men and women with biopsy verified alcoholic steatosis or steatohepatitis, patients with alcoholic fatty liver disease had markedly increased 5 year risk of cirrhosis (6.9%) and mortality (16.7%) compared with a matched reference cohort from the general population (0.3% and 4.3% respectively). In addition, the cirrhosis risk was more than twice as high for patients with steatohepatitis than those with pure steatosis and was higher for women than for men[179]. In another European study of patients with histologically documented ASH, a liver biopsy with the presence of marked intraparenchymal cholestasis was an independent predictor of poor short term outcome in addition to the patient’s age and Maddrey’s discriminant function score[180].
Several scoring systems have been developed and validated to assess the severity and prognosis of patients with alcoholic hepatitis. Maddrey’s discriminant function (MDF), a calculation based on prothrombin time and total bilirubin level (MDF = 4.6 × prothrombin time - control prothrombin time + serum bilirubin), has been used in clinical practice for over three decades to identify patients with severe alcoholic hepatitis who might benefit from corticosteroid therapy[181]. Patients with a MDF score of 32 or greater have been shown to have a high short-term mortality with improved clinical outcomes after receiving corticoidsteroids[182].
The model for end stage liver disease (MELD) score (based on serum bilirubin, creatinine, and INR) was initially developed to predict survival in patients with cirrhosis and was later found to accurately predict short-term survival in patients hospitalized for alcoholic hepatitis with some evidence that it is a better prognostic model for alcoholic hepatitis than the MDF score or Child-Pugh (CP) score and classification[183], which is based on bilirubin, albumin, prothrombin time prolongation, degree of ascites and degree of hepatic encephalopathy. The sensitivity and specificity of the MELD score (12 or greater) for predicting 30-d mortality in ASH has been shown to be 86% and 81% as compared to the MDF score (32 or greater) which has a sensitivity of 86% and specificity of 48%[184]. A higher MELD score cut off value (21 or greater) has been shown to have improved sensitivity (75%) and specificity of (75%) for predicting 90-d mortality in AH[185].
The Glasgow alcoholic hepatitis (GAH) score identifies a subgroup of patients with a MDF score of 32 or greater who will recover without corticosteroid therapy[186]. The GAH is a multivariable model that includes age, serum bilirubin, blood urea nitrogen, prothrombin time, and peripheral white blood cell count. In a study of 225 patients with AH and a MDF score of 32 or higher, patients with a GAH score of 9 or greater who received corticosteroids had improved survival rates when compared with those who did not receive therapy (78% vs 52% survival at 28 d; 59% vs 38% survival at 84 d)[187]. No survival benefit was observed in patients with a GAH score of 8 or less who received early corticosteroid treatment.
The Lille score evaluates a patient’s serum bilirubin response to corticosteroid treatment after 7 d and can aid the clinician in determining whether or not to continue corticosteroid therapy for a full 28 d course. The model includes age, albumin, change in bilirubin over 7 d, prothrombin time and creatinine. A score > 0.45 suggests that a patient is not responding to therapy. Interestingly, the Lille model outperformed CP, MDF, GAH and MELD scores in predicting survival at six months[188].
The age, serum bilirubin, INR and serum creatinine (ABIC) score was developed to stratify patients with AH based on their prognosis. Patients were categorized into low, moderate and high risk groups based on their risk of death at 90 d and one year (25%, 70% and 100% respectively). This model could potentially be used in order to identify patients who may benefit from clinical trials. The ABIC score performed equally well as compared to MDF, MELD and GAH in predicting 90-d survival (AUROC 0.80-0.81) in a confirmatory cohort[189].
A recent study evaluated the utility of CP, MELD, MDF, GAH and AIBC scores in predicting short-term and long term survival in 44 patients with histologic confirmation of AH and found that all scores, with the exception of CP, had similar accuracy in predicting short-term prognosis. All models were poor predictors of survival beyond six months with none of the model’s AUROC exceeding 0.74[190]. The only factor that was significantly associated with survival after one year was abstinence from alcohol within 3-6 mo of diagnosis of AH (AUROC of 0.83).
CONCLUSION
ALD is a condition that affects only a small percentage of heavy drinkers. The diagnosis of ALD can be challenging and is based on a combination of clinical and laboratory findings in addition to the essential role of communication with the patient to assess the amount and duration of alcohol intake. Clinical findings may be minimal or absent in early ALD characterized only by hepatic steatosis, whereas in cirrhosis there will be typical signs and symptoms of cirrhosis and portal hypertension. Laboratory studies characteristic of ALD include elevated transaminase levels with AST greater than ALT but also increased MCV, GGT, and IgA to IgG ratio.
In most patients, the diagnosis will be established by thorough history, clinical and laboratory findings. However, in uncertain situations, it can be supported by imaging and liver biopsy results. In most cases, the histological features of ALD can ultimately define the diagnosis according to the typical presence and distribution of hepatic steatosis, inflammation, and Mallory-Denk bodies. Consideration should be given to non-invasive methods, including FibroScan and magnetic resonance elastography, which have the potential to diagnose early ALD but they have not been evaluated yet in this condition.
In addition, clinical and laboratory parameters are important for predicting the prognosis of ALD in more advanced and severe cases and for determining the therapeutic approach. Because of the potential reversible nature of ALD with sobriety, regular screening of the general population and early diagnosis are essential.
Currently, there are no clear, uniform definitions available for ASH and alcoholic hepatitis, particularly in the presence of chronic liver disease or cirrhosis. It is unclear if they represent the same entity or if they are different conditions along the spectrum of ALD. The status of ASH and alcoholic hepatitis in the spectrum of ALD represents a gap in current research and an area of needed further investigation.
Footnotes
P- Reviewer: Kaymakoglu S S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
References
- 1.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Warren KR, Murray MM. Alcoholic liver disease and pancreatitis: global health problems being addressed by the US National Institute on Alcohol Abuse and Alcoholism. J Gastroenterol Hepatol. 2013;28 Suppl 1:4–6. doi: 10.1111/jgh.12246. [DOI] [PubMed] [Google Scholar]
- 4.Trimble G, Zheng L, Mishra A, Kalwaney S, Mir HM, Younossi ZM. Mortality associated with alcohol-related liver disease. Aliment Pharmacol Ther. 2013;38:596–602. doi: 10.1111/apt.12432. [DOI] [PubMed] [Google Scholar]
- 5.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global status report on alcohol and health 2011. Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/en.
- 8.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmann A, Fuhr D, Poznyak V, Rekve D. World Health Organization Global Status Report on Alcohol and Health 2011. Available from: http://www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf.
- 13.Yoon YH, Yi HY. Liver cirrhosis mortality in the United States, 1970-2009. Surveillance Report 93. Division of Epidemiology and Prevention Research, National Institute on Alcohol Abuse and Alcoholism (NIAAA), Arlington, VA. August 2012. Available from: http://www.pubs.niaaa.nih.gov/publications/Surveillance93/Cirr09.htm.
- 14.Crabb DW. Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med. 1999;48:184–188. doi: 10.2302/kjm.48.184. [DOI] [PubMed] [Google Scholar]
- 15.Becker U, Deis A, Sørensen TI, Grønbaek M, Borch-Johnsen K, Müller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 16.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 17.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 18.Deleuran T, Grønbaek H, Vilstrup H, Jepsen P. Cirrhosis and mortality risks of biopsy-verified alcoholic pure steatosis and steatohepatitis: a nationwide registry-based study. Aliment Pharmacol Ther. 2012;35:1336–1342. doi: 10.1111/j.1365-2036.2012.05091.x. [DOI] [PubMed] [Google Scholar]
- 19.Xie YD, Feng B, Gao Y, Wei L. Effect of abstinence from alcohol on survival of patients with alcoholic cirrhosis: A systematic review and meta-analysis. Hepatol Res. 2013:Epub ahead of print. doi: 10.1111/hepr.12131. [DOI] [PubMed] [Google Scholar]
- 20.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstee QM, Daly AK, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128–146. doi: 10.1055/s-0031-1276643. [DOI] [PubMed] [Google Scholar]
- 22.Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159–1162. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]
- 23.Hatton J, Burton A, Nash H, Munn E, Burgoyne L, Sheron N. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–592. doi: 10.1111/j.1360-0443.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 24.Clouston AD, Jonsson JR, Powell EE. Steatosis as a cofactor in other liver diseases: hepatitis C virus, alcohol, hemochromatosis, and others. Clin Liver Dis. 2007;11:173–189, x. doi: 10.1016/j.cld.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 26.Stewart SH. Racial and ethnic differences in alcohol-associated aspartate aminotransferase and gamma-glutamyltransferase elevation. Arch Intern Med. 2002;162:2236–2239. doi: 10.1001/archinte.162.19.2236. [DOI] [PubMed] [Google Scholar]
- 27.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 28.Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 29.Moussavian SN, Becker RC, Piepmeyer JL, Mezey E, Bozian RC. Serum gamma-glutamyl transpeptidase and chronic alcoholism. Influence of alcohol ingestion and liver disease. Dig Dis Sci. 1985;30:211–214. doi: 10.1007/BF01347885. [DOI] [PubMed] [Google Scholar]
- 30.Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin Exp Res. 2005;29:902–908. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- 31.Funk M, Wutzke S, Kaner E, Anderson P, Pas L, McCormick R, Gual A, Barfod S, Saunders J. A multicountry controlled trial of strategies to promote dissemination and implementation of brief alcohol intervention in primary health care: findings of a World Health Organization collaborative study. J Stud Alcohol. 2005;66:379–388. doi: 10.15288/jsa.2005.66.379. [DOI] [PubMed] [Google Scholar]
- 32.Saunders JB, Lee NK. Hazardous alcohol use: its delineation as a subthreshold disorder, and approaches to its diagnosis and management. Compr Psychiatry. 2000;41:95–103. doi: 10.1016/s0010-440x(00)80015-2. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. International classification of diseases. 10th revision. WHO, 1992. Available from: http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf.
- 34.O’brien CP, Crowley TJ. Substance-Related and Addictive Disorders. In: Diagnostic and statistical manual of mental disorders, fifth edition., editors. Arlington, VA, United States: American Psychiatric Association; 2013. pp. 481–589. [Google Scholar]
- 35.Friedmann PD. Alcohol use in adults. N Engl J Med. 2013;368:1655–1656. doi: 10.1056/NEJMc1302445. [DOI] [PubMed] [Google Scholar]
- 36.Stickel F, Seitz HK. Update on the management of alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2013;22:189–197. [PubMed] [Google Scholar]
- 37.Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56 Suppl 1:S39–S45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 38.Hamberg KJ, Carstensen B, Sørensen TI, Eghøje K. Accuracy of clinical diagnosis of cirrhosis among alcohol-abusing men. J Clin Epidemiol. 1996;49:1295–1301. doi: 10.1016/0895-4356(95)00517-x. [DOI] [PubMed] [Google Scholar]
- 39.Angeli P, Albino G, Carraro P, Dalla Pria M, Merkel C, Caregaro L, De Bei E, Bortoluzzi A, Plebani M, Gatta A. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology. 1996;23:264–273. doi: 10.1002/hep.510230211. [DOI] [PubMed] [Google Scholar]
- 40.Burra P, Germani G, Masier A, De Martin E, Gambato M, Salonia A, Bo P, Vitale A, Cillo U, Russo FP, et al. Sexual dysfunction in chronic liver disease: is liver transplantation an effective cure? Transplantation. 2010;89:1425–1429. doi: 10.1097/TP.0b013e3181e1f1f6. [DOI] [PubMed] [Google Scholar]
- 41.Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol. 1997;58:365–371. doi: 10.15288/jsa.1997.58.365. [DOI] [PubMed] [Google Scholar]
- 42.Lapham GT, Rubinsky AD, Heagerty PJ, Williams EC, Hawkins EJ, Maynard C, Kivlahan DR, Bradley KA. Annual rescreening for alcohol misuse: diminishing returns for some patient subgroups. Med Care. 2013;51:914–921. doi: 10.1097/MLR.0b013e3182a3e549. [DOI] [PubMed] [Google Scholar]
- 43.US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554–556. doi: 10.7326/0003-4819-140-7-200404060-00016. [DOI] [PubMed] [Google Scholar]
- 44.National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide. Rockville, MD: Department of health and Human Services, National Institutes of Health; 2007. Available from: http://pubs.niaaa.nih.gov/publications/Practitioner/ClinicianGuide2005/clinicians_guide.htm. [Google Scholar]
- 45.Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159:210–218. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- 46.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The alcohol use disorders identification test: guidelines for use in primary care. 2nd Ed. World Health Organization: Switzerland; 2001. Available from: http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. [Google Scholar]
- 47.Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37 Suppl 1:E253–E259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 48.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 49.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 50.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108:29–36. doi: 10.1016/j.drugalcdep.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, Maynard C, Burman ML, Kivlahan DR. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- 52.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37:1380–1390. doi: 10.1111/acer.12092. [DOI] [PubMed] [Google Scholar]
- 53.Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedmann PD, Saitz R, Gogineni A, Zhang JX, Stein MD. Validation of the screening strategy in the NIAAA “Physicians’ Guide to Helping Patients with Alcohol Problems”. J Stud Alcohol. 2001;62:234–238. doi: 10.15288/jsa.2001.62.234. [DOI] [PubMed] [Google Scholar]
- 55.Hoeksema HL, de Bock GH. The value of laboratory tests for the screening and recognition of alcohol abuse in primary care patients. J Fam Pract. 1993;37:268–276. [PubMed] [Google Scholar]
- 56.Alte D, Luedemann J, Rose HJ, John U. Laboratory markers carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume are not useful as screening tools for high-risk drinking in the general population: results from the Study of Health in Pomerania (SHIP) Alcohol Clin Exp Res. 2004;28:931–940. doi: 10.1097/01.alc.0000128383.34605.16. [DOI] [PubMed] [Google Scholar]
- 57.Center for Substance Abuse Treatment. The role of biomarkers in the treatment of alcohol use disorders. Substance abuse treatment advisory 2006; 5: 1. Available from: http://store.samhsa.gov/shin/content//SMA12-4686/SMA12-4686.pdf.
- 58.Baraona E, Leo MA, Borowsky SA, Lieber CS. Alcoholic hepatomegaly: accumulation of protein in the liver. Science. 1975;190:794–795. doi: 10.1126/science.1198096. [DOI] [PubMed] [Google Scholar]
- 59.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 60.Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. 1984;76:211–222. doi: 10.1016/0002-9343(84)90776-9. [DOI] [PubMed] [Google Scholar]
- 61.Pirovino M, Linder R, Boss C, Köchli HP, Mahler F. Cutaneous spider nevi in liver cirrhosis: capillary microscopical and hormonal investigations. Klin Wochenschr. 1988;66:298–302. doi: 10.1007/BF01727516. [DOI] [PubMed] [Google Scholar]
- 62.Dutta SK, Dukehart M, Narang A, Latham PS. Functional and structural changes in parotid glands of alcoholic cirrhotic patients. Gastroenterology. 1989;96:510–518. doi: 10.1016/0016-5085(89)91578-3. [DOI] [PubMed] [Google Scholar]
- 63.Van Thiel DH, Gavaler JS, Schade RR. Liver disease and the hypothalamic pituitary gonadal axis. Semin Liver Dis. 1985;5:35–45. doi: 10.1055/s-2008-1041756. [DOI] [PubMed] [Google Scholar]
- 64.Erlinger S, Benhamou J. Cirrhosis: clinical aspects. In: Mcintyre N, Benhamou J, Rizzetto M, editors. Oxford textbook of clinical hepatology. Oxford: University Press; 1991. p. 380. [Google Scholar]
- 65.Groszman R, Franchis R. Portal hypertension. In: Schiff E, Sorrell M, Maddrey W, editors. Diseases of the liver. Philadelphia: Lippincot Williams & Wilkens; 1999. p. 415. [Google Scholar]
- 66.Epstein O, Dick R, Sherlock S. Prospective study of periostitis and finger clubbing in primary biliary cirrhosis and other forms of chronic liver disease. Gut. 1981;22:203–206. doi: 10.1136/gut.22.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Attali P, Ink O, Pelletier G, Vernier C, Jean F, Moulton L, Etienne JP. Dupuytren’s contracture, alcohol consumption, and chronic liver disease. Arch Intern Med. 1987;147:1065–1067. [PubMed] [Google Scholar]
- 68.Lieber CS. ALCOHOL: its metabolism and interaction with nutrients. Annu Rev Nutr. 2000;20:395–430. doi: 10.1146/annurev.nutr.20.1.395. [DOI] [PubMed] [Google Scholar]
- 69.Klatsky AL, Chartier D, Udaltsova N, Gronningen S, Brar S, Friedman GD, Lundstrom RJ. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am J Cardiol. 2005;96:346–351. doi: 10.1016/j.amjcard.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 70.Kazemi-Shirazi L, Veloso MP, Frommlet F, Steindl-Munda P, Wrba F, Zehetmayer S, Marsik C, Ferenci P. Differentiation of nonalcoholic from alcoholic steatohepatitis: are routine laboratory markers useful? Wien Klin Wochenschr. 2008;120:25–30. doi: 10.1007/s00508-007-0921-1. [DOI] [PubMed] [Google Scholar]
- 71.Das SK, Mukherjee S, Vasudevan DM, Balakrishnan V. Comparison of haematological parameters in patients with non-alcoholic fatty liver disease and alcoholic liver disease. Singapore Med J. 2011;52:175–181. [PubMed] [Google Scholar]
- 72.Niemelä O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39–49. doi: 10.1016/j.cca.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg DM, Hahn SE, Parkes JG. Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chim Acta. 1995;237:155–187. doi: 10.1016/0009-8981(95)06069-p. [DOI] [PubMed] [Google Scholar]
- 74.Lucas DL, Brown RA, Wassef M, Giles TD. Alcohol and the cardiovascular system: research challenges and opportunities. J Am Coll Cardiol. 2005;45:1916–1924. doi: 10.1016/j.jacc.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 75.Whitfield JB, Zhu G, Heath AC, Powell And LW, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25:1037–1045. [PubMed] [Google Scholar]
- 76.Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–1281. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 77.Tyulina OV, Prokopieva VD, Boldyrev AA, Johnson P. Erythrocyte and plasma protein modification in alcoholism: a possible role of acetaldehyde. Biochim Biophys Acta. 2006;1762:558–563. doi: 10.1016/j.bbadis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Cylwik B, Chrostek L, Szmitkowski M. [The effect of alcohol on iron metabolism] Pol Merkur Lekarski. 2008;24:561–564. [PubMed] [Google Scholar]
- 79.Chapman RW, Morgan MY, Laulicht M, Hoffbrand AV, Sherlock S. Hepatic iron stores and markers of iron overload in alcoholics and patients with idiopathic hemochromatosis. Dig Dis Sci. 1982;27:909–916. doi: 10.1007/BF01316575. [DOI] [PubMed] [Google Scholar]
- 80.Fletcher LM, Halliday JW, Powell LW. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J Gastroenterol Hepatol. 1999;14:202–214. doi: 10.1046/j.1440-1746.1999.01836.x. [DOI] [PubMed] [Google Scholar]
- 81.Harrison-Findik DD. Role of alcohol in the regulation of iron metabolism. World J Gastroenterol. 2007;13:4925–4930. doi: 10.3748/wjg.v13.i37.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yersin B, Nicolet JF, Dercrey H, Burnier M, van Melle G, Pécoud A. Screening for excessive alcohol drinking. Comparative value of carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume. Arch Intern Med. 1995;155:1907–1911. doi: 10.1001/archinte.155.17.1907. [DOI] [PubMed] [Google Scholar]
- 83.Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, Helander A, Tabakoff B. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26:332–339. [PubMed] [Google Scholar]
- 84.Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96–109. doi: 10.1016/j.jchromb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Rosman AS, Lieber CS. Diagnostic utility of laboratory tests in alcoholic liver disease. Clin Chem. 1994;40:1641–1651. [PubMed] [Google Scholar]
- 86.Salaspuro M. Conventional and coming laboratory markers of alcoholism and heavy drinking. Alcohol Clin Exp Res. 1986;10:5S–12S. doi: 10.1111/j.1530-0277.1986.tb05174.x. [DOI] [PubMed] [Google Scholar]
- 87.Latvala J, Hietala J, Koivisto H, Järvi K, Anttila P, Niemelä O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am J Gastroenterol. 2005;100:1303–1310. doi: 10.1111/j.1572-0241.2005.41509.x. [DOI] [PubMed] [Google Scholar]
- 88.Hietala J, Koivisto H, Latvala J, Anttila P, Niemelä O. IgAs against acetaldehyde-modified red cell protein as a marker of ethanol consumption in male alcoholic subjects, moderate drinkers, and abstainers. Alcohol Clin Exp Res. 2006;30:1693–1698. doi: 10.1111/j.1530-0277.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 89.Worrall S, de Jersey J, Wilce PA, Seppä K, Hurme L, Sillanaukee P. Relationship between alcohol intake and immunoglobulin a immunoreactivity with acetaldehyde-modified bovine serum albumin. Alcohol Clin Exp Res. 1996;20:836–840. doi: 10.1111/j.1530-0277.1996.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 90.Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98 Suppl 2:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- 91.Hietala J, Puukka K, Koivisto H, Anttila P, Niemelä O. Serum gamma-glutamyl transferase in alcoholics, moderate drinkers and abstainers: effect on gt reference intervals at population level. Alcohol Alcohol. 2005;40:511–514. doi: 10.1093/alcalc/agh201. [DOI] [PubMed] [Google Scholar]
- 92.Sillanaukee P, Massot N, Jousilahti P, Vartiainen E, Sundvall J, Olsson U, Poikolainen K, Pönniö M, Allen JP, Alho H. Dose response of laboratory markers to alcohol consumption in a general population. Am J Epidemiol. 2000;152:747–751. doi: 10.1093/aje/152.8.747. [DOI] [PubMed] [Google Scholar]
- 93.Reynaud M, Schellenberg F, Loisequx-Meunier MN, Schwan R, Maradeix B, Planche F, Gillet C. Objective diagnosis of alcohol abuse: compared values of carbohydrate-deficient transferrin (CDT), gamma-glutamyl transferase (GGT), and mean corpuscular volume (MCV) Alcohol Clin Exp Res. 2000;24:1414–1419. [PubMed] [Google Scholar]
- 94.Daeppen JB, Smith TL, Schuckit MA. Influence of age and body mass index on gamma-glutamyltransferase activity: a 15-year follow-up evaluation in a community sample. Alcohol Clin Exp Res. 1998;22:941–944. [PubMed] [Google Scholar]
- 95.Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemelä O. Age-related changes on serum ggt activity and the assessment of ethanol intake. Alcohol Alcohol. 2006;41:522–527. doi: 10.1093/alcalc/agl052. [DOI] [PubMed] [Google Scholar]
- 96.Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemelä O. Additive effects of moderate drinking and obesity on serum gamma-glutamyl transferase activity. Am J Clin Nutr. 2006;83:1351–1354; quiz 1448-1449. doi: 10.1093/ajcn/83.6.1351. [DOI] [PubMed] [Google Scholar]
- 97.Helander A. Biological markers in alcoholism. J Neural Transm Suppl. 2003;(66):15–32. doi: 10.1007/978-3-7091-0541-2_2. [DOI] [PubMed] [Google Scholar]
- 98.Bisson JI, Milford-Ward A. A comparison of carbohydrate deficient transferrin with other markers of alcohol misuse in male soldiers under the age of thirty. Alcohol Alcohol. 1994;29:315–321. [PubMed] [Google Scholar]
- 99.Stibler H. Carbohydrate-deficient transferrin in serum: a new marker of potentially harmful alcohol consumption reviewed. Clin Chem. 1991;37:2029–2037. [PubMed] [Google Scholar]
- 100.Mundle G, Munkes J, Ackermann K, Mann K. Sex differences of carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean corpuscular volume in alcohol-dependent patients. Alcohol Clin Exp Res. 2000;24:1400–1405. [PubMed] [Google Scholar]
- 101.Anton RF, Moak DH. Carbohydrate-deficient transferrin and gamma-glutamyltransferase as markers of heavy alcohol consumption: gender differences. Alcohol Clin Exp Res. 1994;18:747–754. doi: 10.1111/j.1530-0277.1994.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 102.Berlakovich GA, Soliman T, Freundorfer E, Windhager T, Bodingbauer M, Wamser P, Hetz H, Peck-Radosavljevic M, Muehlbacher F. Pretransplant screening of sobriety with carbohydrate-deficient transferrin in patients suffering from alcoholic cirrhosis. Transpl Int. 2004;17:617–621. doi: 10.1007/s00147-004-0765-9. [DOI] [PubMed] [Google Scholar]
- 103.Kapur A, Wild G, Milford-Ward A, Triger DR. Carbohydrate deficient transferrin: a marker for alcohol abuse. BMJ. 1989;299:427–431. doi: 10.1136/bmj.299.6696.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J, Conigrave KM, Macaskill P, Whitfield JB, Irwig L. Combining carbohydrate-deficient transferrin and gamma-glutamyltransferase to increase diagnostic accuracy for problem drinking. Alcohol Alcohol. 2003;38:574–582. doi: 10.1093/alcalc/agg113. [DOI] [PubMed] [Google Scholar]
- 105.Madhubala V, Subhashree AR, Shanthi B. Serum carbohydrate deficient transferrin as a sensitive marker in diagnosing alcohol abuse: a case - control study. J Clin Diagn Res. 2013;7:197–200. doi: 10.7860/JCDR/2013/5137.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hock B, Schwarz M, Domke I, Grunert VP, Wuertemberger M, Schiemann U, Horster S, Limmer C, Stecker G, Soyka M. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100:1477–1486. doi: 10.1111/j.1360-0443.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 107.Hietala J, Koivisto H, Anttila P, Niemelä O. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2006;41:528–533. doi: 10.1093/alcalc/agl050. [DOI] [PubMed] [Google Scholar]
- 108.Kapoor A, Kraemer KL, Smith KJ, Roberts MS, Saitz R. Cost-effectiveness of screening for unhealthy alcohol use with % carbohydrate deficient transferrin: results from a literature-based decision analytic computer model. Alcohol Clin Exp Res. 2009;33:1440–1449. doi: 10.1111/j.1530-0277.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walsham NE, Sherwood RA. Ethyl glucuronide. Ann Clin Biochem. 2012;49:110–117. doi: 10.1258/acb.2011.011115. [DOI] [PubMed] [Google Scholar]
- 110.Skipper GE, Thon N, Dupont RL, Baxter L, Wurst FM. Phosphatidylethanol: the potential role in further evaluating low positive urinary ethyl glucuronide and ethyl sulfate results. Alcohol Clin Exp Res. 2013;37:1582–1586. doi: 10.1111/acer.12121. [DOI] [PubMed] [Google Scholar]
- 111.Helander A, Péter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552–557. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- 112.Nalesso A, Viel G, Cecchetto G, Mioni D, Pessa G, Favretto D, Ferrara SD. Quantitative profiling of phosphatidylethanol molecular species in human blood by liquid chromatography high resolution mass spectrometry. J Chromatogr A. 2011;1218:8423–8431. doi: 10.1016/j.chroma.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 113.Stewart SH, Reuben A, Brzezinski WA, Koch DG, Basile J, Randall PK, Miller PM. Preliminary evaluation of phosphatidylethanol and alcohol consumption in patients with liver disease and hypertension. Alcohol Alcohol. 2009;44:464–467. doi: 10.1093/alcalc/agp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277–283. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 115.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 116.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 117.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, Rafiq N, Goodman Z, Chandhoke V, Baranova A. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18:1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 119.Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, Chim AM, Yeung DK, Chan FK, Woo J, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363–1370. doi: 10.1016/j.jhep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 120.Vilgrain V. Ultrasound of diffuse liver disease and portal hypertension. Eur Radiol. 2001;11:1563–1577. doi: 10.1007/s003300101050. [DOI] [PubMed] [Google Scholar]
- 121.Valls C, Iannacconne R, Alba E, Murakami T, Hori M, Passariello R, Vilgrain V. Fat in the liver: diagnosis and characterization. Eur Radiol. 2006;16:2292–2308. doi: 10.1007/s00330-006-0146-0. [DOI] [PubMed] [Google Scholar]
- 122.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, Torella R, Persico M. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 123.Mortele KJ, Ros PR. Imaging of diffuse liver disease. Semin Liver Dis. 2001;21:195–212. doi: 10.1055/s-2001-15496. [DOI] [PubMed] [Google Scholar]
- 124.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 125.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, Saab S, Lu DS. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 126.Borra RJ, Salo S, Dean K, Lautamäki R, Nuutila P, Komu M, Parkkola R. Nonalcoholic fatty liver disease: rapid evaluation of liver fat content with in-phase and out-of-phase MR imaging. Radiology. 2009;250:130–136. doi: 10.1148/radiol.2501071934. [DOI] [PubMed] [Google Scholar]
- 127.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 128.de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918. doi: 10.1111/j.1478-3231.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 129.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rofsky NM, Fleishaker H. CT and MRI of diffuse liver disease. Semin Ultrasound CT MR. 1995;16:16–33. doi: 10.1016/0887-2171(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 131.Okazaki H, Ito K, Fujita T, Koike S, Takano K, Matsunaga N. Discrimination of alcoholic from virus-induced cirrhosis on MR imaging. AJR Am J Roentgenol. 2000;175:1677–1681. doi: 10.2214/ajr.175.6.1751677. [DOI] [PubMed] [Google Scholar]
- 132.Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology. 2002;224:769–774. doi: 10.1148/radiol.2243011495. [DOI] [PubMed] [Google Scholar]
- 133.Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450–455. doi: 10.1016/j.ejrad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 134.Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, Martínez SM, Gilabert R, Forns X, Jiménez W, Navasa M. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57:281–287. doi: 10.1016/j.jhep.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Van Ness MM, Diehl AM. Is liver biopsy useful in the evaluation of patients with chronically elevated liver enzymes? Ann Intern Med. 1989;111:473–478. doi: 10.7326/0003-4819-111-6-473. [DOI] [PubMed] [Google Scholar]
- 136.Levin DM, Baker AL, Riddell RH, Rochman H, Boyer JL. Nonalcoholic liver disease. Overlooked causes of liver injury in patients with heavy alcohol consumption. Am J Med. 1979;66:429–434. doi: 10.1016/0002-9343(79)91064-7. [DOI] [PubMed] [Google Scholar]
- 137.Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, Jalan R. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103–1111. doi: 10.1016/j.jhep.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 138.Phillips MG, Preedy VR, Hughes RD. Assessment of prognosis in alcoholic liver disease: can serum hyaluronate replace liver biopsy? Eur J Gastroenterol Hepatol. 2003;15:941–944. doi: 10.1097/00042737-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 139.Bianchi L. Liver biopsy in elevated liver functions tests? An old question revisited. J Hepatol. 2001;35:290–294. doi: 10.1016/s0168-8278(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 140.Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis. 2005;9:37–53. doi: 10.1016/j.cld.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Rubin E, Lieber CS. Alcohol-induced hepatic injury in nonalcoholic volunteers. N Engl J Med. 1968;278:869–876. doi: 10.1056/NEJM196804182781602. [DOI] [PubMed] [Google Scholar]
- 142.Fromenty B, Grimbert S, Mansouri A, Beaugrand M, Erlinger S, Rötig A, Pessayre D. Hepatic mitochondrial DNA deletion in alcoholics: association with microvesicular steatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 143.Chedid A, Mendenhall CL, Tosch T, Chen T, Rabin L, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorrell M. Significance of megamitochondria in alcoholic liver disease. Gastroenterology. 1986;90:1858–1864. doi: 10.1016/0016-5085(86)90253-2. [DOI] [PubMed] [Google Scholar]
- 144.Uchida T, Kronborg I, Peters RL. Giant mitochondria in the alcoholic liver diseases--their identification, frequency and pathologic significance. Liver. 1984;4:29–38. doi: 10.1111/j.1600-0676.1984.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 145.Sohail U, Satapathy SK. Diagnosis and management of alcoholic hepatitis. Clin Liver Dis. 2012;16:717–736. doi: 10.1016/j.cld.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 146.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stumptner C, Fuchsbichler A, Zatloukal K, Denk H. In vitro production of Mallory bodies and intracellular hyaline bodies: the central role of sequestosome 1/p62. Hepatology. 2007;46:851–860. doi: 10.1002/hep.21744. [DOI] [PubMed] [Google Scholar]
- 148.Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 149.Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol. 1998;15:246–258. [PubMed] [Google Scholar]
- 150.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 151.Zatloukal K, Stumptner C, Fuchsbichler A, Janig E, Denk H. Intermediate filament protein inclusions. Methods Cell Biol. 2004;78:205–228. doi: 10.1016/s0091-679x(04)78008-5. [DOI] [PubMed] [Google Scholar]
- 152.Zatloukal K, Stumptner C, Fuchsbichler A, Fickert P, Lackner C, Trauner M, Denk H. The keratin cytoskeleton in liver diseases. J Pathol. 2004;204:367–376. doi: 10.1002/path.1649. [DOI] [PubMed] [Google Scholar]
- 153.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fauerholdt L, Schlichting P, Christensen E, Poulsen H, Tygstrup N, Juhl E. Conversion of micronodular cirrhosis into macronodular cirrhosis. Hepatology. 1983;3:928–931. doi: 10.1002/hep.1840030607. [DOI] [PubMed] [Google Scholar]
- 155.Van Eyken P, Sciot R, Desmet VJ. A cytokeratin immunohistochemical study of alcoholic liver disease: evidence that hepatocytes can express ‘bile duct-type’ cytokeratins. Histopathology. 1988;13:605–617. doi: 10.1111/j.1365-2559.1988.tb02092.x. [DOI] [PubMed] [Google Scholar]
- 156.Parés A, Caballería J, Bruguera M, Torres M, Rodés J. Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J Hepatol. 1986;2:33–42. doi: 10.1016/s0168-8278(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 157.Borowsky SA, Strome S, Lott E. Continued heavy drinking and survival in alcoholic cirrhotics. Gastroenterology. 1981;80:1405–1409. [PubMed] [Google Scholar]
- 158.Saunders JB, Davis M, Williams R. Do women develop alcoholic liver disease more readily than men? Br Med J (Clin Res Ed) 1981;282:1140–1143. doi: 10.1136/bmj.282.6270.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barrio E, Tomé S, Rodríguez I, Gude F, Sánchez-Leira J, Pérez-Becerra E, González-Quintela A. Liver disease in heavy drinkers with and without alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2004;28:131–136. doi: 10.1097/01.ALC.0000106301.39746.EB. [DOI] [PubMed] [Google Scholar]
- 160.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Crocè L, Sasso F, Pozzato G, Cristianini G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–850. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 162.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 163.Pessione F, Ramond MJ, Peters L, Pham BN, Batel P, Rueff B, Valla DC. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 164.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 165.Orrego H, Israel Y, Blake JE, Medline A. Assessment of prognostic factors in alcoholic liver disease: toward a global quantitative expression of severity. Hepatology. 1983;3:896–905. doi: 10.1002/hep.1840030602. [DOI] [PubMed] [Google Scholar]
- 166.Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19:635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 167.Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, Gustot T, Degré D, Vercruysse V, Deltenre P, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344–350. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 168.Alvarez MA, Cirera I, Solà R, Bargalló A, Morillas RM, Planas R. Long-term clinical course of decompensated alcoholic cirrhosis: a prospective study of 165 patients. J Clin Gastroenterol. 2011;45:906–911. doi: 10.1097/MCG.0b013e3182284e13. [DOI] [PubMed] [Google Scholar]
- 169.Olivera-Martinez M, Sayles H, Vivekanandan R, D’ Souza S, Florescu MC. Hepatorenal syndrome: are we missing some prognostic factors? Dig Dis Sci. 2012;57:210–214. doi: 10.1007/s10620-011-1861-1. [DOI] [PubMed] [Google Scholar]
- 170.Mendenhall CL, Tosch T, Weesner RE, Garcia-Pont P, Goldberg SJ, Kiernan T, Seeff LB, Sorell M, Tamburro C, Zetterman R. VA cooperative study on alcoholic hepatitis. II: Prognostic significance of protein-calorie malnutrition. Am J Clin Nutr. 1986;43:213–218. doi: 10.1093/ajcn/43.2.213. [DOI] [PubMed] [Google Scholar]
- 171.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 172.Medici V, Peerson JM, Stabler SP, French SW, Gregory JF, Virata MC, Albanese A, Bowlus CL, Devaraj S, Panacek EA, et al. Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J Hepatol. 2010;53:551–557. doi: 10.1016/j.jhep.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 174.French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, Mendenhall CL. Pathology of alcoholic liver disease. VA Cooperative Study Group 119. Semin Liver Dis. 1993;13:154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 175.Rakoski MO, Brown MB, Fontana RJ, Bonkovsky HL, Brunt EM, Goodman ZD, Lok AS, Omary MB. Mallory-Denk bodies are associated with outcomes and histologic features in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:902–909.e1. doi: 10.1016/j.cgh.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maher JJ. Hepatic fibrosis caused by alcohol. Semin Liver Dis. 1990;10:66–74. doi: 10.1055/s-2008-1040458. [DOI] [PubMed] [Google Scholar]
- 177.Nasrallah SM, Nassar VH, Galambos JT. Importance of terminal hepatic venule thickening. Arch Pathol Lab Med. 1980;104:84–86. [PubMed] [Google Scholar]
- 178.Mathurin P, Beuzin F, Louvet A, Carrié-Ganne N, Balian A, Trinchet JC, Dalsoglio D, Prevot S, Naveau S. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047–1054. doi: 10.1111/j.1365-2036.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 179.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999-2008: a nationwide population based cohort study. J Hepatol. 2011;54:760–764. doi: 10.1016/j.jhep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 180.Spahr L, Rubbia-Brandt L, Genevay M, Hadengue A, Giostra E. Early liver biopsy, intraparenchymal cholestasis, and prognosis in patients with alcoholic steatohepatitis. BMC Gastroenterol. 2011;11:115. doi: 10.1186/1471-230X-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 182.Carithers RL, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 183.Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 184.Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002;2:2. doi: 10.1186/1471-230X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, Malinchoc M, Kamath PS, Shah V. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 186.Forrest EH, Evans CD, Stewart S, Phillips M, Oo YH, McAvoy NC, Fisher NC, Singhal S, Brind A, Haydon G, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Forrest EH, Morris AJ, Stewart S, Phillips M, Oo YH, Fisher NC, Haydon G, O’Grady J, Day CP. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut. 2007;56:1743–1746. doi: 10.1136/gut.2006.099226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 189.Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, García-Pagán JC, Fernández R, Moreno M, Bañares R, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 190.Palaniyappan N, Subramanian V, Ramappa V, Ryder SD, Kaye P, Aithal GP. The utility of scoring systems in predicting early and late mortality in alcoholic hepatitis: whose score is it anyway? Int J Hepatol. 2012;2012:624675. doi: 10.1155/2012/624675. [DOI] [PMC free article] [PubMed] [Google Scholar]


