Abstract
AIM: To explore the mechanism of abnormal Connexin (Cx) 32 and Cx43 expression in the gastric mucosa after Helicobacter pylori (H. pylori) infection.
METHODS: Biopsy specimens of gastric mucosa in different gastric carcinogenesis stages with H. pylori infection, that is, non-atrophic gastritis (NAG; n = 24), chronic atrophic gastritis (CAG; n = 25), intestinal metaplasia (IM; n = 28), dysplasia (DYS; n = 24), and gastric cancer (GC; n = 30), as well as specimens of normal gastric mucosa without H. pylori infection (NGM; n = 25), were confirmed by endoscopy and pathological examination. Cx32 and Cx43 mRNA expression was detected by real-time polymerase chain reaction (PCR). Cx32 and Cx43 promoter CpG island methylation status was determined by methylation-specific PCR (MSP), bisulfite PCR sequencing (BSP) and MassArray methods.
RESULTS: The relative mRNA expression levels in the gastric mucosa of patients with NGM, NAG, CAG, IM, DYS and GC were 0.146 ± 0.011, 0.133 ± 0.026, 0.107 ± 0.035, 0.039 ± 0.032, 0.037 ± 0.01 and 0.03 ± 0.011 for Cx32; and 0.667 ± 0.057, 0.644 ± 0.051, 0.624 ± 0.049, 0.555 ± 0.067, 0.536 ± 0.058 and 0.245 ± 0.121 for Cx43, respectively, which were gradually decreasing and significantly different (GC vs NGM: P < 0.001 for Cx32, P < 0.001 for Cx43). The promoter methylation levels in the gastric mucosa from NGM to GC stages by MSP were 38.8% ± 9.0%, 43.1% ± 9.4%, 56.5% ± 3.1%, 64.4% ± 9.7%, 72.5% ± 4.2% and 79.6% ± 6.8% for Cx32; and 49.0% ± 3.9%, 58.1% ± 5.0%, 66.5% ± 7.9%, 74.0% ± 8.8%, 78.3% ± 3.6% and 88.7% ± 6.2% for Cx43, respectively, which were gradually increasing and significantly different (P = 0.039, P = 0.019). The promoter methylation levels by BSP and MassArray exhibited similar trends. Cx32 and Cx43 mRNA expression was negatively correlated with promoter methylation status and gastric carcinogenesis stages (P < 0.001, P = 0.016).
CONCLUSION: Cx32 and Cx43 mRNA expression decreased gradually during H. pylori infection-associated gastric carcinogenesis, and it is associated with hypermethylation of these genes’ promoter.
Keywords: Gastric cancer, Helicobacter pylori, Cx32, Cx43, DNA methylation
Core tip: The relationship between Connexin (Cx) 32 and Cx43 mRNA expression and gene promoter methylation at different gastric carcinogenesis stages with H. pylori infection, that is, non-atrophic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and gastric cancer, is not clear. Here, gastric mucosa biopsy specimens from these carcinogenic stages, as well as normal gastric mucosa without H. pylori infection, were examined for Cx32 and Cx43 mRNA expression and promoter methylation by real-time polymerase chain reaction and methylation detection. Cx32 and Cx43 mRNA expression decreased gradually during gastric carcinogenesis, and it is associated with hypermethylation of these genes’ promoter.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is an important risk factor for gastric cancer (GC)[1], with its carcinogenic mechanisms not yet fully understood[2,3]. Connexin (Cx) 32 and Cx43 are key members of gap junctions between gastric epithelial cells, showing a gradual down-regulation trend from normal mucosa to precancerous lesions and GC[4]. We have found that the decrease in Cx32 and Cx43 expression in precancerous lesions and GC is associated with H. pylori infection[5], coculture of gastric epithelial cells with H. pylori reduces expression of Cx43[6], and eradication of H. pylori upregulates Cx32 and Cx43 expression in precancerous lesions[7]. However, the mechanisms by which H. pylori infection decreases Cx32 and Cx43 expression are unclear. Inactivation of gastric tumor suppressor genes, such as CDX2, RASSF1A and P16(INK4A), is induced by promoter hypermethylation[8-10]. In this study, we observed Cx32 and Cx43 mRNA expression and its relationship with the promoter methylation status in different stages of GC, and from the Cx32 and Cx43 gene methylation perspective, to explore the mechanism of abnormal Cx32 and Cx43 expression after H. pylori infection and its role in the occurrence and development of GC.
MATERIALS AND METHODS
Patients and tissues
A total of 1550 patients underwent endoscopic and pathological examinations because of upper gastrointestinal symptoms between September 2011 and April 2012 in the Third Xiangya Hospital, Central South University, Changsha, China. Fifty cases at each stage of gastric carcinogenesis with H. pylori infection, i.e. non-atrophic gastritis (NAG), chronic atrophic gastritis (CAG), intestinal metaplasia (IM), atypical hyperplasia (dysplasia, DYS) and GC, were screened; and 50 cases of normal gastric mucosa from age-matched subjects without H. pylori infection (NGM) in the same period were chosen as controls. Patients with gastric surgery or those taking antibiotics, nonsteroidal anti-inflammatory drugs, proton-pump inhibitors (PPIs), or histamine receptor (H2) antagonists within 1 mo before endoscopy were excluded. Signed informed consent was obtained from all patients and controls, and the study was approved by the hospital medical ethics committee. Endoscopic and pathological diagnosis was made according to the 8th edition of the Cecil Essentials of Medicine[11], Chinese consensus on chronic gastritis[12] and Chinese guidelines for diagnosis and treatment of gastric cancer (2011 edition)[13]. Generally, the endoscopic findings of NAG included mucosal congestion and edema, accompanied with little hemorrhage and erosion, and those of CAG included a thinning mucous layer, shallowing or disappearing folds, visibility of the submucosal vascularity, and fine granules on the surface. The pathological findings of NAG included necrosis of the superficial mucosal epithelium and infiltration of lymphocytes and plasma cells in the lamina propria, and those of CAG included shrinking gastric glands with a reduced number and shallowing of gastric pits. IM was identified by replacement of gastric epithelium by intestinal epithelium, accompanied with goblet cells secreting acidic mucus, absorptive epithelial cells with striated edge, and Paneth cells. DYS was identified by proliferation of atypical cells, but it was not sufficient to be diagnosed as cancer. GC was identified as cancerous tissues infiltrating the mucosal, submucosal or entire layers, taking on polypoid, ulcerous, and diffuse infiltrative types.
Any two positives of rapid urease test, 14C-urea breath test and histological examination, or positive H. pylori by culture were identified as H. pylori infection, and if these four tests were all negative, the patient was identified as being without H. pylori infection. Four pieces of lesioned or normal gastric mucosa biopsies were taken by gastroscopy, and mRNA and DNA were extracted for Cx32 and Cx43 mRNA expression and methylation detection. According to the quantity of mRNA and DNA, as well as no significant difference in sex, age and disease duration, there were 25 cases of NGM, 24 of NAG, 25 of CAG, 28 of IM, 24 of DYS, and 30 of GC, which were screened for Cx32 and Cx43 expression and methylation. Table 1 shows the clinical characteristics of the study population.
Table 1.
Sex, age and disease duration of cases of each stage (mean ± SD)
| Stage | n | M | F | Age, yr | Disease duration (yr) |
| NGM without H. pylori infection | 25 | 14 | 11 | 54.12 ± 8.21 (45-60) | 0.85 ± 0.64 (0.12-1.23) |
| NAG with H. pylori infection | 24 | 13 | 11 | 56.44 ± 11.29 (47-65) | 0.85 ± 0.35 (0.37-1.03) |
| CAG with H. pylori infection | 25 | 11 | 14 | 55.90 ± 7.80 (45-66) | 0.73 ± 0.24 (0.13-0.90) |
| IM with H. pylori infection | 28 | 12 | 16 | 52.16 ± 8.59 (48-68) | 0.90 ± 0.65 (0.47-1.04) |
| DYS with H. pylori infection | 24 | 12 | 12 | 54.05 ± 7.36 (48-69) | 0.73 ± 0.57 (0.56-1.15) |
| GC with H. pylori infection | 30 | 17 | 13 | 55.43 ± 10.33 (46-73) | 0.60 ± 0.46 (0.10-1.04) |
NGM: Normal gastric mucosa; NAG: Non-atrophic gastritis; CAG: Chronic atrophic gastritis; IM: Intestinal metaplasia; DYS: Dysplasia; GC: Gastric cancer.
Reagents
Total RNA extraction and reverse transcription reagents were purchased from Toyobo (Osaka, Japan); Wizard Genomic DNA purification kit was purchased from Promega (Madison, WI, United States); the EpiTect Bisulfite Kit was purchased from Qiagen (Germany); and methylase (M.SssI) was purchased from New England Biotech (United States).
Primers
The primers for detection of Cx32 and Cx43 expression and promoter methylation were designed with Primer5 [for real-time reverse transcription (RT)-polymerase chain reaction (PCR), including internal reference β-actin], MethPrimer [for methylation-specific PCR (MSP) and bisulfite sequencing PCR (BSP)], and EpiDesigner (for MassArray). The primers were synthesized by Shanghai Sangon Biotech (China) (Figure 1, Table 2).
Figure 1.
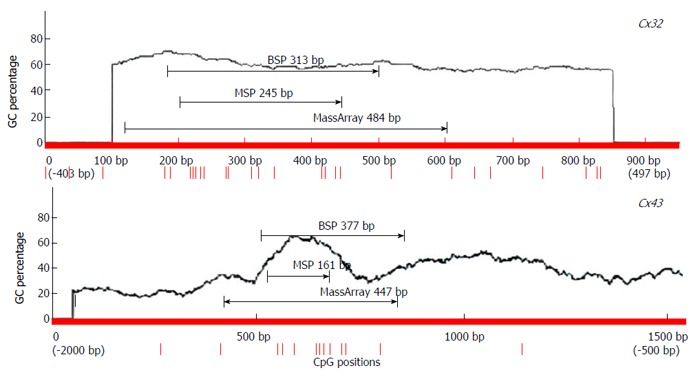
Location of primers for polymerase chain reaction for methylation detection.
Table 2.
Cx32 and Cx43 primer sequences, amplified fragment size and annealing temperature
| Method | Gene | Primer sequence (5’→3’) | Amplified fragment size (bp) | Annealing temperature (°C) | ||
| Real-time RT-PCR | Cx32 | F: | ATGAACTGGACAGGTTTGTAC | 302 | 56 | |
| R: | ATGTGTTGCTGGTGCAGCCA | |||||
| Cx43 | F: | TGCAGCAGTCTGCCTTTCGTTG | 219 | 56 | ||
| R: | CCATCAGTTTGGGCAACCTTG | |||||
| β-actin | F: | TGGACTTCGCAGCACAGCAGATGG | 289 | 56 | ||
| R: | ATCTCCTTCTGCATCCTGTCG | |||||
| MSP | Cx32 | M | F: | GGGGCGGGTGCGGCGAT | 245 | 64 |
| R: | CTCCGCGCCTACGTCCC | |||||
| U | F: | GGGGTGGGTGTGGTGAT | 245 | 64 | ||
| R: | CTCCACACCTACATCCCAA | |||||
| Cx43 | M | F: | AAATTGTAATATTTGGGTTTCAGCGC | 156 | 58 | |
| R: | AATAACGCCATCTCTACTCACCG | |||||
| U | F: | TTTTAAAATTGTAATATTTGGGTTTCAGTGT | 161 | 56 | ||
| R: | AATAACACCATCTCTACTCACCACA | |||||
| BSP | Cx32 | F: | GGTTATTTTTTTGGTGGGGTTATG | 313 | 58 | |
| R: | ACCCAAACAAATCCCCTATAATCTC | |||||
| Cx43 | F: | TGTTTTTTAAAATTGTAATATTTGGGTTTA | 377 | 56 | ||
| R: | AAAAACAAACTCATCTAACCTTCCTATTC | |||||
| MassArray | Cx32 | F: | CAGTTT CAGCAGTTTTTGGGTTTTTTGG | 484 | 60 | |
| R: | TAACTCCCTATCCCCTAACTCCTTA | |||||
| Cx43 | F: | ATGTTTTTGCAGGTTGGATCAGGAAAT | 447 | 60 | ||
| R: | ACCAACAAATAAAAACAAAATTATTCC |
Detection of Cx32 and Cx43 mRNA expression by real-time RT-PCR
The biopsy tissues of gastric mucosa were ground in liquid nitrogen, TRIzol was added to extract total RNA, and 1 μg total RNA was reversely transcribed to cDNA. With 2 μL cDNA as a template, real-time RT-PCR was carried out using SYBR qPCR Mix under the following conditions: 94 °C 4 min; 94 °C for 30 s, 56 °C for 45 s, 72 °C for 45 s, for 45 cycles; and 72 °C for 5 min. β-Actin was used as an internal reference, and nuclease-free water as a negative control. The relative mRNA expression was calculated according to the 2−ΔCt formula.
Detection of Cx32 and Cx43 promoter methylation
DNA was extracted from the tissue with Wizard DNA purification kit, and then bisulfite-modified according to the steps in EpiTect Bisulfite Kit. DNA of normal human peripheral blood lymphocytes was methylated by M.SssI methylation enzyme (thus all the GC-sites were methylated), bisulfite-modified and acted as an all-site methylation positive control. The following three methods were used to detect Cx32 and Cx43 promoter methylation.
MSP method: After the tissue DNA was bisulfite modified, PCR was carried out using MSP primers under the following conditions: 95 °C for 10 min; 94 °C for 15 s, annealing temperature for 30 s, 72 °C for 30 s, for 38 cycles; and 72 °C for 10 min. The PCR products were electrophoresed on 2% agarose gel and imaged. From the gray values of methylation and unmethylation bands, the methylation level was calculated by the formula [M/(M + U) × 100%].
BSP sequencing method: The PCR reaction mixture (25 μL) contained 2 μL bisulfite-modified DNA template, 12.5 μL TaKaRa Premix Taq HS, 1 μL each 10 μmol/L forward and reverse primers, and 8.5 μL deionized distilled water. The PCR products were identified by electrophoresis, and sent to Huada Biotechnology (Shenzhen, China) for sequencing. The peak height ratio of the sulfonated methyl-CpG site still as C to the sum of C and the sulfonated non-methylated CpG site changed as T was calculated as the degree of methylation [i.e., C/(C+T) × 100%].
MassArray method: Genomic DNA after bisulfite modification was amplified with MassArray primers. The PCR products were introduced with T7 promoter sequence in the Beijing Bio-Miao Biotech Company, then in vitro transcribed to RNA products, processed by T-base-specific cleavage, and small RNA fragments were obtained. Flight mass spectrometry (MALDI-TOF) was used to detect the molecular weight of each fragment, and the methylation data were outputted with EpiTyper software.
Statistical analysis
All data were processed with SPSS 16.0 software and shown as mean ± SD. Averages of multiple samples were compared using univariate analysis of variance, and correlation analysis of ranked data was tested by the Spearman rank correlation method. P < 0.05 was considered statistically significant.
RESULTS
Cx32 and Cx43 mRNA expression profiles at different gastric carcinogenesis stages with H. pylori infection
For cases with endoscopic and pathological confirmation and high mRNA quality (25 NGM, 24 NAG, 25 CAG, 28 IM, 24 DYS and 30 GC), the relative mRNA expression in the gastric mucosa was 0.146 ± 0.011, 0.133 ± 0.026, 0.107 ± 0.035, 0.039 ± 0.032, 0.037 ± 0.01 and 0.03 ± 0.011 for Cx32; and 0.667 ± 0.057, 0.644 ± 0.051, 0.624 ± 0.049, 0.555 ± 0.067, 0.536 ± 0.058 and 0.245 ± 0.121 for Cx43 (Figure 2). Cx32 and Cx43 mRNA expression decreased from NAG to GC stages with H. pylori infection (P < 0.001), and that at CAG, IM, DYS and GC stages was lower than that at NGM (P < 0.008; the largest in these comparisons), and that at IM, DYS and GC stages was lower than that at NAG and CAG stages (P < 0.036). Specially, Cx43 mRNA expression at GC stage was lower than that at IM and DYS stages (P < 0.001).
Figure 2.
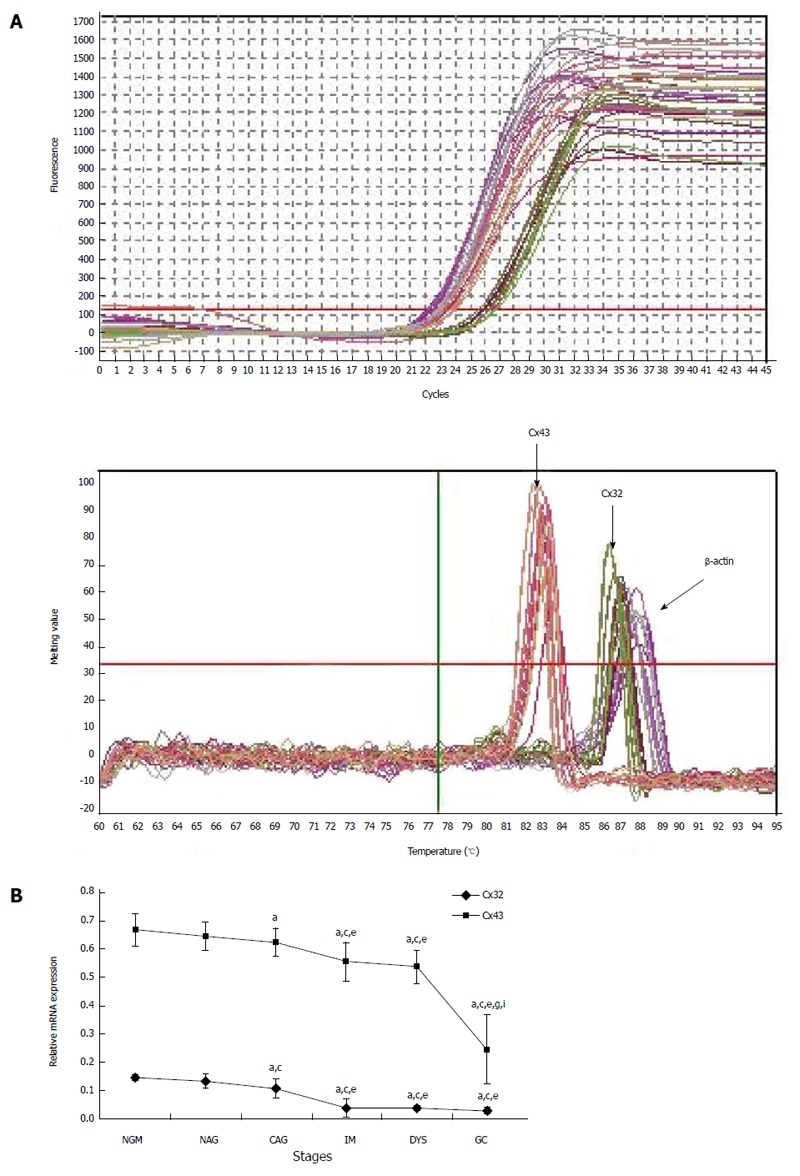
Real-time polymerase chain reaction results for Cx32 and Cx43 mRNAs. A: The amplification curve and melting curve of the real-time polymerase chain reaction; B: The relative expression of Cx32 and Cx43 mRNAs at different stages. aP < 0.05 vs NGM; cP < 0.05 vs NAG; eP < 0.05 vs CAG; gP < 0.05 vs IM; iP < 0.05 vs DYS. NGM: Normal gastric mucosa; NAG: Non-atrophic gastritis; CAG: Chronic atrophic gastritis; IM: Intestinal metaplasia; DYS: Dysplasia; GC: Gastric cancer.
Cx32 and Cx43 promoter methylation at different gastric carcinogenesis stages with H. pylori infection
MSP: Eight DNA samples of good quality were selected from 24 cases in each group for MSP detection, and the actual number of samples whose methylated and unmethylated bands were significant was six for NGM, six for NAG, seven for CAG, six for IM, seven for DYS and eight for GC. Their promoter methylation levels were 38.8% ± 9.0%, 43.1% ± 9.4%, 56.5% ± 3.1%, 64.4% ± 9.7%, 72.5% ± 4.2% and 79.6% ± 6.8% for Cx32; and 49.0% ± 3.9%, 58.1% ± 5.0%, 66.5% ± 7.9%, 74.0% ± 8.8%, 78.3% ± 3.6% and 88.7% ± 6.2% for Cx43 (Figure 3). Cx32 and Cx43 promoter methylation levels gradually increased from NAG to GC stages with H. pylori infection (P = 0.039, P = 0.019), and those at CAG, IM, DYS and GC stages were higher than that at NGM (P = 0.018, P = 0.013), with the highest at GC stage. Cx32 methylation level at GC stage was higher than those at NAG, CAG and IM stages (P < 0.031), and Cx43 methylation level at GC stage was higher than those at NAG and CAG stages (P < 0.027).
Figure 3.
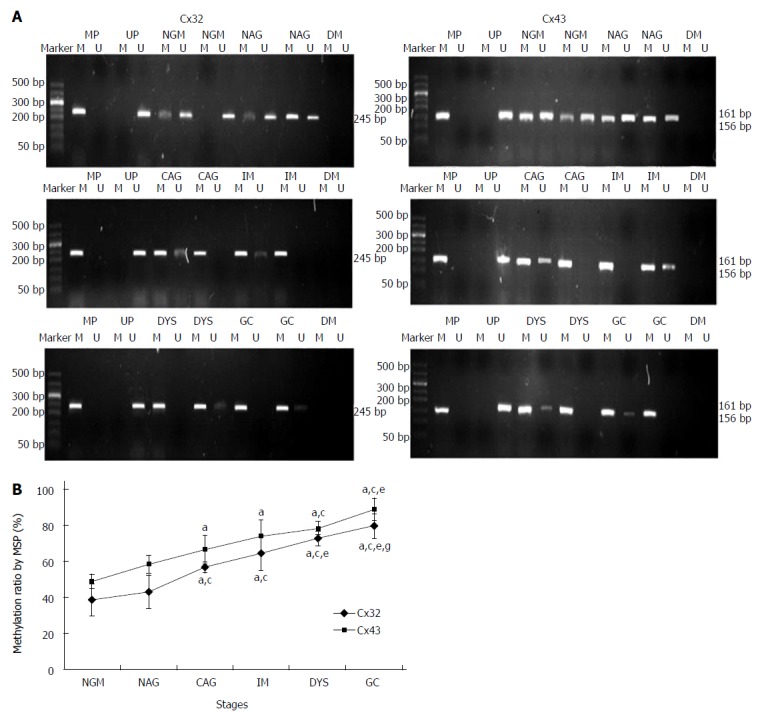
Methylation-specific polymerase chain reaction results for Cx32 and Cx43 promoters at different gastric carcinogenesis stages with Helicobacter pylori infection. A: The agarose gel electrophoresis of the methylation-specific polymerase chain reaction (MSP) bands. Marker: 50bp ladder; M: Methylated; U: Unmethylated; MP: Methylation positive control; UP: Unmethylated positive control; DW: Negative control. B: The methylation levels of Cx32 and Cx43 promoters at different stages by MSP method. aP < 0.05 vs NGM; cP < 0.05 vs NAG; eP < 0.05 vs CAG; gP < 0.05 vs IM; iP < 0.05 vs DYS. NGM: Normal gastric mucosa; NAG: Non-atrophic gastritis; CAG: Chronic atrophic gastritis; IM: Intestinal metaplasia; DYS: Dysplasia; GC: Gastric cancer.
BSP sequencing: The samples with good MSP bands were selected for BSP reaction and sequencing. The BSP-amplified fragment from the Cx32 gene CpG island had a length of 313 bp, containing 15 CpG sites, and the 5-15th CpG sites were detected by sequencing. The BSP amplification fragment from the Cx43 gene CpG island had a length of 377 bp, containing 12 CpG sites, and the 5-12th CpG sites were detected by sequencing (Table 3). The peak height ratio of the sulfonated methyl-CpG site still as C to the sum of this C and the sulfonated non-methylated CpG site changed as T was calculated as the degree of methylation. Cx32 and Cx43 methylation levels showed an increasing trend from NAG to GC stages with H. pylori infection (P = 0.031, P = 0.040), and those at CAG, IM, DYS and GC stages were higher than that at NGM (P < 0.029, P < 0.03), with the highest at GC. Cx32 methylation level at GC stage was higher than those at NAG and CAG stages (P < 0.018), and Cx43 methylation level at GC stage was higher than that at NAG stage (P = 0.032) (Figure 4, Table 4).
Table 3.
Cx32 and Cx43 CpG loci detected by bisulfite polymerase chain reaction sequencing
| Cx32 | CpG | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| bp | 28 | 34 | 37 | 45 | 49 | 83 | 86 | 120 | 132 | 155 | 227 | 231 | 247 | 253 | 255 | |
| ○ | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Cx43 | CpG | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| bp | 32 | 34 | 37 | 44 | 71 | 127 | 134 | 143 | 159 | 190 | 198 | 280 | ||||
| ○ | ○ | ○ | ○ | ● | ● | ● | ● | ● | ● | ● | ● |
“●” denotes the detected CpG site; “○”denotes the undetected CpG site.
Figure 4.
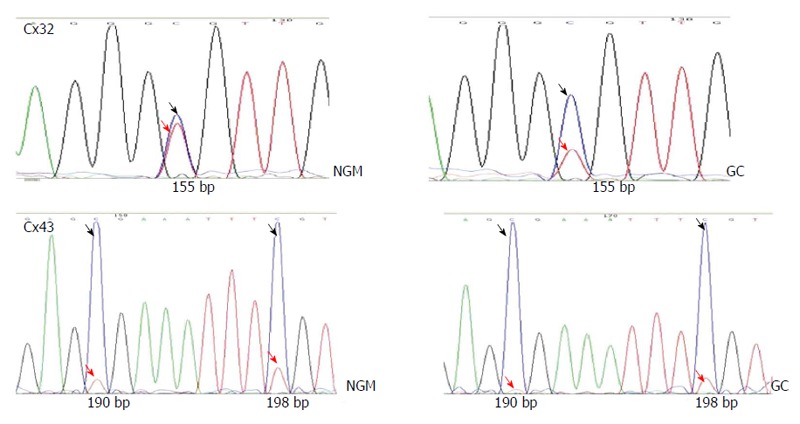
Screenshots of bisulfite polymerase chain reaction sequencing of the Cx32 and Cx43 promoter CpG islands. The upper two show the 10th Cx32 CpG site (155bp), with the C/(C+T) ratio of 60% in NM and 80% at GC stage. The lower two shows the 10th and 11th Cx43 CpG sites (190bp, 198bp). Dark arrow indicates the peak of the sulfonated methyl-CpG site still as C; red arrow indicates the sulfonated non-methylated CpG site changed as T.
Table 4.
Cx32 and Cx43 promoter methylation status at different gastric carcinogenesis stages with Helicobacter pylori infection by bisulfite polymerase chain reaction sequencing method
| n | Cx32 (%) | Cx43 (%) | |
| NGM without H. pylori infection | 7 | 60.1 ± 5.9 | 75.5 ± 4.3 |
| NAG with H. pylori infection | 6 | 67.3 ± 4.9 | 82.9 ± 6.3 |
| CAG with H. pylori infection | 7 | 74.5 ± 7.5a | 87.1 ± 5.4a |
| IM with H. pylori infection | 7 | 84.2 ± 6.8ace | 90.5 ± 9.3ac |
| DYS with H. pylori infection | 6 | 82.3 ± 6.0ac | 91.6 ± 8.3ac |
| GC with H. pylori infection | 9 | 85.3 ± 9.7ace | 92.0 ± 7.1ac |
P < 0.05 vs NM;
P < 0.05 vs NAG;
P < 0.05 vs CAG. NGM: Normal gastric mucosa; NAG: Non-atrophic gastritis; CAG: Chronic atrophic gastritis; IM: Intestinal metaplasia; DYS: Dysplasia; GC: Gastric cancer.
MassArray detection: The methylation of the CpG island was validated by MassArray method using one sample from each group. As shown in Figure 5, the amplified fragment with MassArray Cx32 primers contained 18 CpG sites, the first, second and 15th CpG sites were not detected by the MassArray method, and the 3-4-5, 6-7, 8-9, 13-14, and 16-17 loci were in close proximity and only measured on average, so a total of nine data were obtained. For the 12 CpG sites in the Cx43 amplified fragment, the second site was not detected, and the 6-7 and 10-11-12 loci were in close proximity and only measured on average, so a total of eight data were obtained. The average of the methylation levels of these loci is shown in Table 5. The average of the methylation levels at all Cx32 loci showed an increasing trend from NAG to GC stages with H. pylori infection (P = 0.037), and that at IM, DYS and GC stages was higher than that at NGM (P < 0.028); the methylation level at the 10-12th loci had an increasing trend from NAG to GC stages, while the methylation level of the remaining CpG sites did not change significantly. The average methylation levels at all Cx43 loci showed an increasing trend from NAG to GC stages with H. pylori infection (P = 0.045), and those at DYS and GC stages were higher than that at NGM (P < 0.041). The methylation levels at the 3-5th loci tended to increase from NAG to GC stages, while the methylation levels of the remaining CpG sites did not change significantly.
Figure 5.
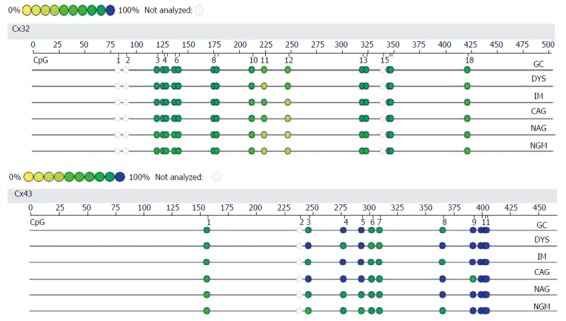
Methylation levels of Cx32 and Cx43 promoters at different gastric carcinogenesis stages with Helicobacter pylori infection by MassArray method. The validated length for Cx32 gene was 484 bp, containing a total of 18 CpG sites (15 detected); the validated length for Cx43 gene was 447 bp, containing a total of 12 CpG sites (11 detected).
Table 5.
Methylation levels (%) of Cx32 and Cx43 CpG islands at gastric different stages with Helicobacter pylori infection detected by MassArray method
| Cx32 (9 loci) | Cx43 (8 loci) | |
| NGM without H. pylori infection | 55.0 ± 14.9 | 77.9 ± 16.8 |
| NAG with H. pylori infection | 61.2 ± 17.9 | 86.0 ± 12.5 |
| CAG with H. pylori infection | 69.9 ± 16.2 | 88.6 ± 9.9 |
| IM with H. pylori infection | 72.4 ± 19.2a | 89.1 ± 9.6 |
| DYS with H. pylori infection | 72.6 ± 19.2a | 90.0 ± 9.3a |
| GC with H. pylori infection | 76.0 ± 20.1a | 91.6 ± 8.2a |
P < 0.05 vs NGM. NGM: Normal gastric mucosa; NAG: Non-atrophic gastritis; CAG: Chronic atrophic gastritis; IM: Intestinal metaplasia; DYS: Dysplasia; GC: Gastric cancer.
Relationship between GC stages and Cx32 and Cx43 expression and methylation levels
Spearman rank correlation analysis showed that Cx32 and Cx43 mRNA expression at different stages of gastric carcinogenesis with H. pylori infection was negatively correlated with the methylation level of their promoters (r = -0.653, P < 0.001; r = -0.367, P = 0.016, respectively), and negatively correlated with gastric carcinogenesis stage (r = -0.796, -0.852, respectively, P < 0.001 for both).
DISCUSSION
Many studies have shown that expression of Cx32 and Cx43 shows a gradual downward trend from normal gastric mucosa to precancerous lesions and GC. Cx32 and Cx43 expression progresses from a high to a low level or no expression in the development of GC, and Cx32 and Cx43 abnormalities are an important molecular mechanism in the inhibition of gastric gap junction intercellular communication (GJIC)[4,14-16].
The relationship between Cx32 and Cx43 expression and H. pylori infection is less reported. The results of our previous clinical studies[5-7] have suggested that eradication of H. pylori infection may improve Cx32 and Cx43 expression, promote recovery of cell GJIC function, and delay or prevent development of precancerous lesions. However, the pattern and mechanism by which H. pylori infection causes the change of Cx32 and Cx43 expression in gastric epithelial cells in the development of the inflammation-carcinoma chain are unclear.
In this study, we found that gastric Cx32 and Cx43 mRNA expression was downregulated from the initial CAG stage of H. pylori infection to latter carcinogenesis stages, which may have caused the decline in GJIC function, leading to the development of GC. H. pylori is deemed to be the first category of carcinogen for gastric cancer[17], and current studies suggest that eradication therapy of H. pylori should be carried out at early stages of GC. Treatment before the occurrence of precancerous lesions can reduce the risk of GC, and treatment at the precancerous stage significantly decreases its role in prevention of GC[18-20]. From the profiles of Cx32 and Cx43 mRNA expression, we provide a rationale that H. pylori eradication therapy should be carried out before the occurrence of CAG, that is, before the decline in Cx32 and Cx43 expression and GJIC function (NAG stage), thus the effect of preventing the occurrence and development of precancerous lesions and GC may be improved.
H. pylori infection can cause promoter CpG island hypermethylation of a variety of genes, such as CDH1, p14, p16, APC and COX2, and the methylation can be reversed after H. pylori eradication[21,22], suggesting that gene hypermethylation is associated with H. pylori infection[23-25], or H. pylori infection may be an inducer for some gene hypermethylation[26,27]. However, it has not been reported whether H. pylori infection causes Cx32 and Cx43 methylation.
Our research showed that Cx32 and Cx43 promoter methylation exhibited an increasing trend from NAG to GC stages with H. pylori infection, with the highest at the GC stage. Data from the MSP method were more significant than from those BSP or MassArray methods, mainly because MSP used direct PCR of several CpG sites in the primer regions, while different fluorescence or mass spectrum quantification and many CpG sites were considered in BSP sequencing or MassArray method. Cx32 and Cx43 mRNA expression was negatively correlated with methylation and gastric carcinogenesis stage, suggesting that Cx32 and Cx43 promoter hypermethylation may be an important mechanism for the reduction of Cx32 and Cx43 expression and occurrence of GC. Hypermethylated promoter binds specific chromosome remodeling proteins and suppresses the transcription of genes[8,28-30].
Currently, many molecular technologies are being developed and applied for cancer epigenetics[31]. In GC patients, TGF-β1 promoter is methylated[32], and in colorectal cancer, underexpression of LATS1 is associated with promoter hypermethylation[33]. Intervention with demethylation drugs has been reported to reactivate the expression of gastric tumor suppressor genes such as CDX2, RASSF1A and P16 (INK4A), and restore their functions[8-10]. H. pylori infection may cause Cx32 and Cx43 promoter hypermethylation to decrease their expression, then inhibit the GJIC function, and induce GC. Promoter methylation can be reversed, thus, it is expected that we can treat against promoter methylation to restore GJIC function, providing new therapies for H. pylori infection-related GC. Based on our research, the treatment of GC may include eradiation of H. pylori, adding DNA-demethylation agents (e.g., 5-azacytidine), and overexpression of Cx32 and Cx43 to compensate the decrease of Cx32 and Cx43 in the carcinogenesis.
In summary, Cx32 and Cx43 expression at the CAG stage of H. pylori infection began to decrease, suggesting that H. pylori eradication therapy before the CAG stage could effectively prevent the occurrence of precancerous lesions and GC. Cx32 and Cx43 promoter hypermethylation may be an important mechanism of the decrease of Cx32 and Cx43 expression after H. pylori infection, and provides a new target for the demethylation treatment of GC.
COMMENTS
Background
Helicobacter pylori (H. pylori) infection is an important risk factor for gastric cancer (GC), yet its carcinogenic mechanism is not yet fully understood. Connexin (Cx) 32 and Cx 43 are key members of gap junctions between gastric epithelial cells. The authors have found that the decrease of Cx32 and Cx43 expression in precancerous lesions and GC is associated with H. pylori infection, but the mechanisms by which H. pylori infection leads to the decrease of Cx32 and Cx43 expression are unclear.
Research frontiers
It is important to understand the epigenetic mechanism of occurrence of GC. Many studies have demonstrated that the expression of Cx32 and Cx43 shows a gradual downward trend from normal gastric mucosa to precancerous lesions and GC. Cx32 and Cx43 abnormalities are the important molecular mechanism of the inhibition of gastric gap junction intercellular communication (GJIC) which may then lead to gastric carcinogenesis. H. pylori infection can cause promoter CpG island hypermethylation of a variety of genes, such as CDH1, p14, p16, APC and COX2, and methylation can be reversed after H. pylori eradication.
Innovations and breakthroughs
Following previous work showing that the decrease of Cx32 and Cx43 expression in precancerous lesions and GC was associated with H. pylori infection, the present study found that Cx32 and Cx43 mRNA expression was negatively correlated with promoter methylation and gastric carcinogenesis stage. This suggests that Cx32 and Cx43 promoter hypermethylation may be an important mechanism in the reduction of Cx32 and Cx43 expression and occurrence of GC. This can help find new targets for applying appropriate means to control the incidence of GC.
Applications
This study shows that Cx32 and Cx43 promoter CpG islands are gradually methylated at gastric carcinogenesis stages with H. pylori infection. Promoter methylation can be reversed, therefore, it is expected that we can treat against promoter methylation to restore GJIC function, providing new therapies for H. pylori infection-related GC.
Terminology
CpG islands: CpG rich areas located in the promoter regions of many genes; CpG island methylation: the addition of a methyl group to a cytosine residue that lies next to guanine within CpG dinucleotides. Cx32 and 43: key members of gap junctions between gastric epithelial cells. Gastric carcinogenesis stages: graded according to the diagnoses by endoscopy and pathology, including non-atrophic gastritis, chronic atrophic gastritis, intestinal metaplasia, dysplasia, and GC.
Peer review
The hypothesis was sound, the experiments were well designed and the results supported the conclusions. This is a well written manuscript about new therapeutic targets for treatment of GC and its different carcinogenesis stages with H. pylori infection. The analyses were performed well and are clearly described in the manuscript.
Footnotes
Supported by The National Natural Science Foundation of China, No. 81172301; and Changsha Municipal Science and Technology Project, No. K1106036-31
P- Reviewer: Fujita T, Liu JY, Reeh M S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upham BL. Role of integrative signaling through gap junctions in toxicology. Curr Protoc Toxicol. 2011;Chapter 2:Unit2.18. doi: 10.1002/0471140856.tx0218s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012;2:1981–2035. doi: 10.1002/cphy.c110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Zhou HF, Wang CH, Zhang B, Liu D, Wang W, Sui GJ. [Decreased expression of Cx32 and Cx43 and their function of gap junction intercellular communication in gastric cancer] Zhonghua Zhongliu Zazhi. 2007;29:742–747. [PubMed] [Google Scholar]
- 5.Xu CX, Jia Y, Yang WB, Wang F, Shen SR. [Relationship between Helicobacter pylori infection and expression of connexin (Cx) 32 and Cx43 genes in gastric cancer and gastric precancerous lesions] Zhonghua Yixue Zazhi. 2008;88:1523–1527. [PubMed] [Google Scholar]
- 6.Xu CX, Qi YM, Yang WB, Wang F, Zhou JD, Shen SR. [Effect of CagA(+) helicobacter pylori strain on the expression of connexin 43 and cell proliferation in BGC-823 cells] Zhongnan Daxue Xuebao Yixueban. 2007;32:288–294. [PubMed] [Google Scholar]
- 7.Jia Y, Xu CX, Yang WB. [Expressions of connexin 32 and connexin 43 in patients with gastric precancerous lesion after eradication of Helicobacter pylori] Zhongnan Daxue Xuebao Yixueban. 2008;33:628–633. [PubMed] [Google Scholar]
- 8.Zhang JF, Zhang JG, Kuai XL, Zhang H, Jiang W, Ding WF, Li ZL, Zhu HJ, Mao ZB. Reactivation of the homeotic tumor suppressor gene CDX2 by 5-aza-2'-deoxycytidine-induced demethylation inhibits cell proliferation and induces caspase-independent apoptosis in gastric cancer cells. Exp Ther Med. 2013;5:735–741. doi: 10.3892/etm.2013.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen WJ, Dai DQ, Teng Y, Liu HB. Regulation of demethylation and re-expression of RASSF1A gene in gastric cancer cell lines by combined treatment of 5-Aza-CdR and NaB. World J Gastroenterol. 2008;14:595–600. doi: 10.3748/wjg.14.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Xie YS, Wang FL, Zhang LJ, Zhang Y, Luo HS. Cytotoxicity of 5-Aza-2’-deoxycytidine against gastric cancer involves DNA damage in an ATM-P53 dependent signaling pathway and demethylation of P16(INK4A) Biomed Pharmacother. 2013;67:78–87. doi: 10.1016/j.biopha.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Andreoli TE, Benjamin IJ, Griggs RC, Wing EJ. Andreoli and Carpenter’s Cecil Essentials of Medicine. 8th ed. Saunders: Elsevier; 2010. [Google Scholar]
- 12.Chinese Medical Association Gastroenterology Branch. Chinese consensus on chronic gastritis [in Chinese] Weichangbingxue. 2013;18:24–29. [Google Scholar]
- 13.Gastric Cancer Diagnosis, Treatment Expert Panel of the Chinese Ministry of Health. Chinese guidelines for diagnosis and treatment of gastric cancer (2011 edition) Transl Gastrointest Cancer. 2012;1:103–114. [Google Scholar]
- 14.Huang Y, Chen LY, Gao MQ. Expression and significance of connexin 32 in gastric cancer and precancerous lesions [in Chinese] Fujian Yike Daxue Xuebao. 2002;36:257–259. [Google Scholar]
- 15.Wu CL, Zhou Z, Qian JX, Pan J, Liu K, Yu GZ, Wang JJ. Expression of Cx43 in gastric cancer and its clinical significance [in Chinese] Linchuang Zhongliuxue Zazhi. 2011;16:421–424. [Google Scholar]
- 16.Li L, Liu J, Qian W, Wang HZ, Song H. Expression of C-erbB-2 and Cx43 protein in gastric carcinoma [in Chinese] Weichangbingxue He Gangzangxue Zazhi. 2007;16:132–135. [Google Scholar]
- 17.Ghoshal UC, Chaturvedi R, Correa P. The enigma of Helicobacter pylori infection and gastric cancer. Indian J Gastroenterol. 2010;29:95–100. doi: 10.1007/s12664-010-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roesler BM, Costa SC, Zeitune JM. Eradication Treatment of Helicobacter pylori Infection: Its Importance and Possible Relationship in Preventing the Development of Gastric Cancer. ISRN Gastroenterol. 2012;2012:935410. doi: 10.5402/2012/935410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaguchi K, Ogawa K, Katsube T, Konno S, Aiba M. Does eradication of Helicobacter pylori reduce the risk of carcinogenesis in the residual stomach after gastrectomy for early gastric cancer? Comparison of mucosal lesions in the residual stomach before and after Helicobacter pylori eradication. Langenbecks Arch Surg. 2004;389:83–91. doi: 10.1007/s00423-003-0451-x. [DOI] [PubMed] [Google Scholar]
- 20.Sachs G, Scott DR. Helicobacter pylori: Eradication or Preservation. F1000 Med Rep. 2012;4:7. doi: 10.3410/M4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perri F, Cotugno R, Piepoli A, Merla A, Quitadamo M, Gentile A, Pilotto A, Annese V, Andriulli A. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. Am J Gastroenterol. 2007;102:1361–1371. doi: 10.1111/j.1572-0241.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- 22.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 23.Shin CM, Kim N, Jung Y, Park JH, Kang GH, Park WY, Kim JS, Jung HC, Song IS. Genome-wide DNA methylation profiles in noncancerous gastric mucosae with regard to Helicobacter pylori infection and the presence of gastric cancer. Helicobacter. 2011;16:179–188. doi: 10.1111/j.1523-5378.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 24.Compare D, Rocco A, Liguori E, D’Armiento FP, Persico G, Masone S, Coppola-Bottazzi E, Suriani R, Romano M, Nardone G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J Clin Pathol. 2011;64:677–682. doi: 10.1136/jcp.2010.087858. [DOI] [PubMed] [Google Scholar]
- 25.Alves MK, Ferrasi AC, Lima VP, Ferreira MV, de Moura Campos Pardini MI, Rabenhorst SH. Inactivation of COX-2, HMLH1 and CDKN2A gene by promoter methylation in gastric cancer: relationship with histological subtype, tumor location and Helicobacter pylori genotype. Pathobiology. 2011;78:266–276. doi: 10.1159/000329475. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T. The presence of a methylation fingerprint of Helicobacter pylori infection in human gastric mucosae. Int J Cancer. 2009;124:905–910. doi: 10.1002/ijc.24018. [DOI] [PubMed] [Google Scholar]
- 27.Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, Ichinose M, Tatematsu M, Ushijima T. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 28.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison A, Parle-McDermott A. DNA methylation: a timeline of methods and applications. Front Genet. 2011;2:74. doi: 10.3389/fgene.2011.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang H, Shin H. Current trends in the development and application of molecular technologies for cancer epigenetics. World J Gastroenterol. 2013;19:1030–1039. doi: 10.3748/wjg.v19.i7.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YQ, Li YM, Li X, Liu T, Liu XK, Zhang JQ, Guo JW, Guo LY, Qiao L. Hypermethylation of TGF-β1 gene promoter in gastric cancer. World J Gastroenterol. 2013;19:5557–5564. doi: 10.3748/wjg.v19.i33.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I, Celinski K, Gach T, Kulig J, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013;19:4363–4373. doi: 10.3748/wjg.v19.i27.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]


