Abstract
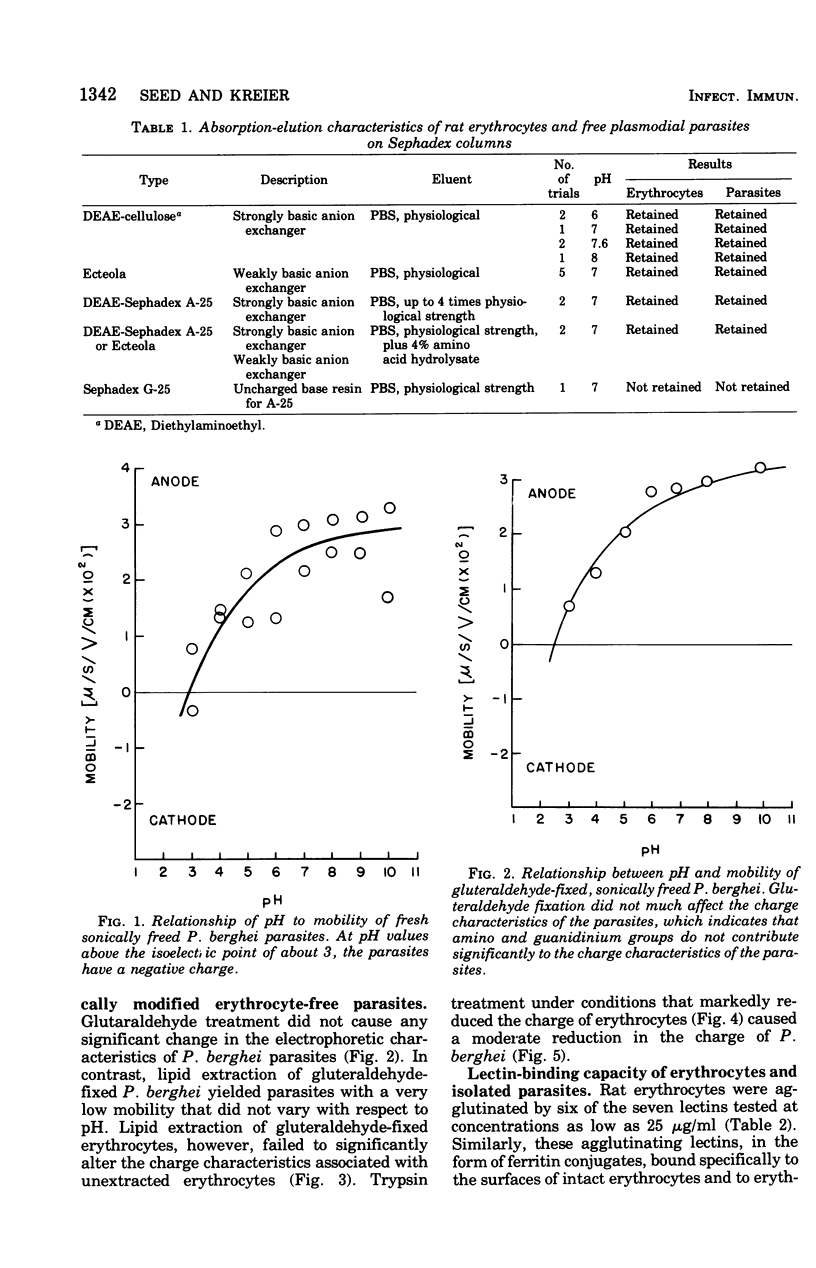
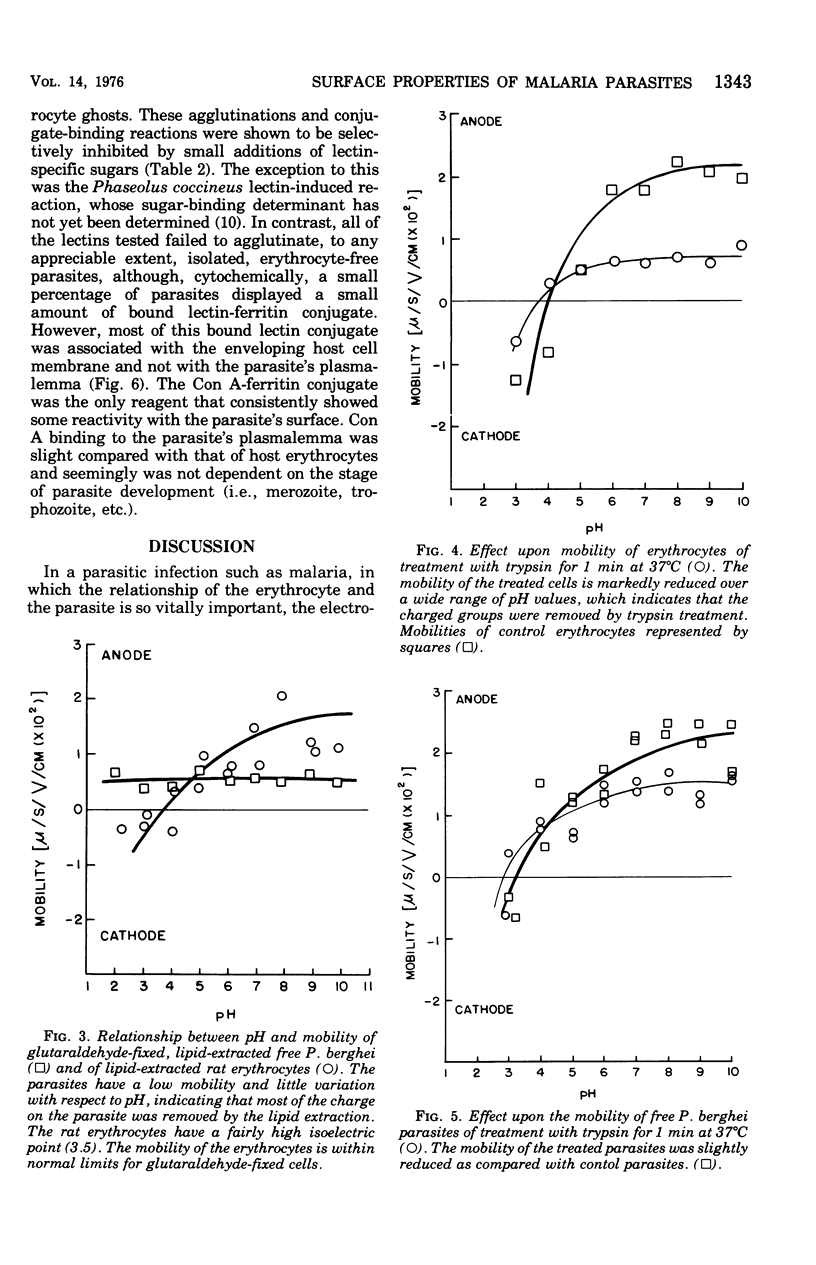
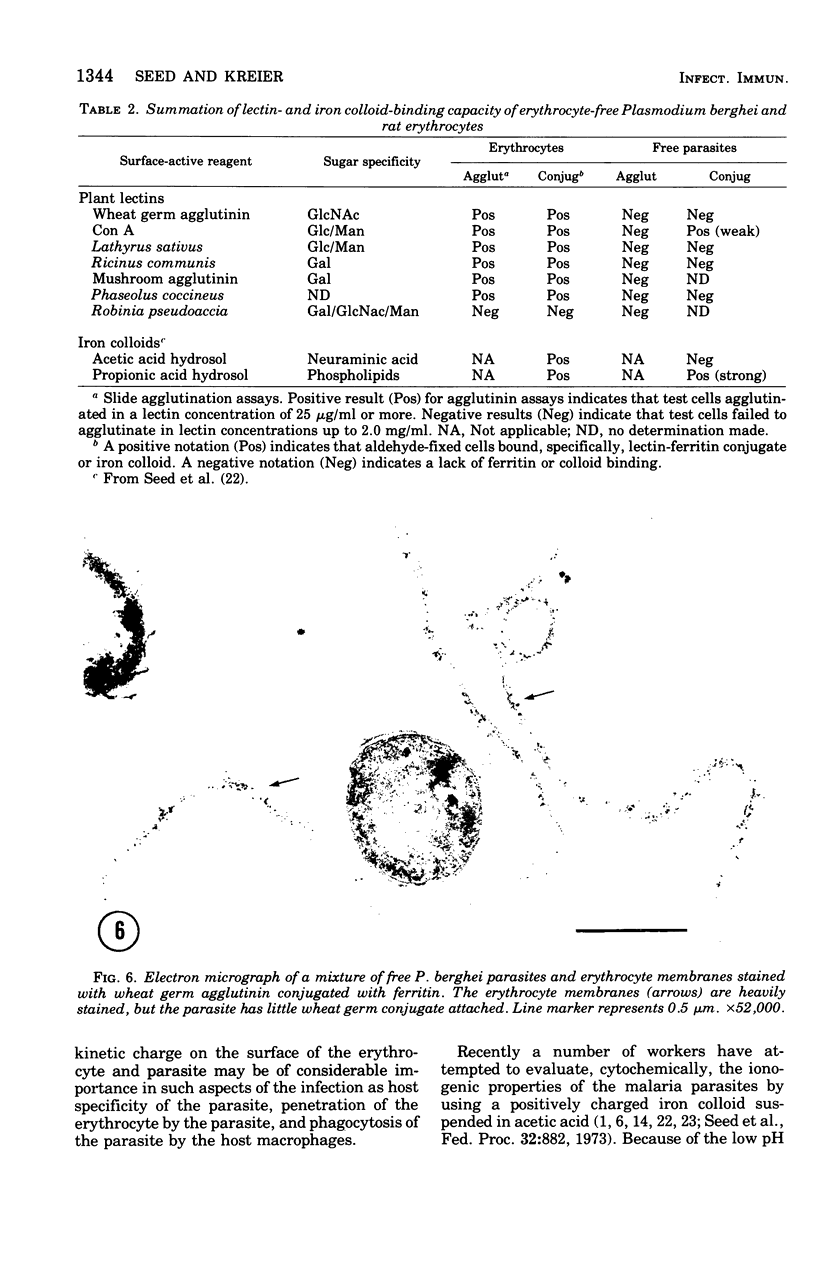
The surface charge and lectin-binding capacity of isolated malaria parasites and host erythrocytes were analyzed and compared by chromatographic, electrophoretic, and cytochemical methods. Results indicated that at physiological pH values both freshly prepared and glutaraldehyde-fixed parasites and erythrocytes possess a net negative surface charge. Both cell types were strongly bound to cation-exchange resins and underwent cathode-directed electrophoretic migration. The isoelectric points for erythrocyte-free parasites and uninfected erythrocytes were approximately 3.0 and 4.0, respectively. The different effects of selective enzymatic digestion and solvent extraction on the electrophoretic mobilities of free parasites and erythrocytes suggested that the chemical constituents responsibile for the net negative surface charges on each type of cell are different. The surface charge of the free parasites seemed mainly to be a function of ionized phospholipids rather than of the ionogenic sialic acid moieties, which are the major contributors to the negative charge on erythrocytes. Results of lectin-binding studies indicated that specific glycosidimoieties (i.e., glucose, galactose, mannose, and n-acetyglucosamine), common to the erythrocyte surface, were either absent or in low concentration at the parasite's surface. These observations suggest that the normally intracellular malaria parasites have surface characteristics, differing from those of the host cell, characterized by a scarcity of lectin-binding receptors and sialic acid residues and by the major contribution of lipids to their surface charge.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972 Nov 28;71(3):523–528. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- Cooper G. W., Miller L. H. Propanoic acid-ferric oxide hydrosols. Differential cell surface binding and its relation to membrane lipid. J Histochem Cytochem. 1974 Sep;22(9):856–867. doi: 10.1177/22.9.856. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., LINDSAY D. B. The phospholipids of the erythrocyte 'ghosts' of various species. Biochem J. 1960 Nov;77:226–230. doi: 10.1042/bj0770226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Mel H. C. Microelectrophoretic and enzymic studies concerning the carbohydrate at the surface of rat erythrocytes. Arch Biochem Biophys. 1966 Jan;113(1):77–82. doi: 10.1016/0003-9861(66)90158-5. [DOI] [PubMed] [Google Scholar]
- Greenberg J. H., Jamieson G. A. The effects of various lectins on platelet aggregation and release. Biochim Biophys Acta. 1974 Apr 29;345(2):231–242. doi: 10.1016/0005-2736(74)90261-2. [DOI] [PubMed] [Google Scholar]
- HEARD D. H., SEAMAN G. V. The action of lower aldehydes on the human erythrocyte. Biochim Biophys Acta. 1961 Oct 28;53:366–374. doi: 10.1016/0006-3002(61)90448-6. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Powers K. G., Finerty J., Vanderberg J. P. Difference in surface charge between host cells and malarial parasites. J Parasitol. 1973 Oct;59(5):925–927. [PubMed] [Google Scholar]
- Nelson G. J. Lipid composition of erythrocytes in various mammalian species. Biochim Biophys Acta. 1967 Oct 2;144(2):221–232. doi: 10.1016/0005-2760(67)90152-x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. The distribution and asymmetry of mammalian cell surface saccharides utilizing ferritin-conjugated plant agglutinins as specific saccharide stains. J Cell Biol. 1974 Jan;60(1):236–248. doi: 10.1083/jcb.60.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R. B., Kreier J. P. Plasmodium berghei freed from host erythrocytes by a continuous-flow ultrasonic system. Exp Parasitol. 1972 Oct;32(2):239–243. doi: 10.1016/0014-4894(72)90030-6. [DOI] [PubMed] [Google Scholar]
- SEAMAN G. V., HEARD D. H. The surface of the washed human erythrocyte as a polyanion. J Gen Physiol. 1960 Nov;44:251–268. doi: 10.1085/jgp.44.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed T. M., Aikawa M., Sterling C. R. An electron microscope-cytochemical method for differentiating membranes of host red cells and malaria parasites. J Protozool. 1973 Nov;20(5):603–605. doi: 10.1111/j.1550-7408.1973.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Seed T. M., Aikawa M., Sterling C., Rabbege J. Surface properties of extracellular malaria parasites: morphological and cytochemical study. Infect Immun. 1974 Apr;9(4):750–761. doi: 10.1128/iai.9.4.750-761.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed T. M., Seed J. R., Brindley D. Surface properties of bloodstream Trypanosomes (Trypanosoma brucei). Tropenmed Parasitol. 1976 Jun;27(2):202–212. [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Tanigoshi L. Incorporation of 14C-amino acids by malarial plasmodia (Plasmodium iophurae). VI. Changes in the kinetic constants of amino acid transport during infection. Exp Parasitol. 1974 Jun;35(3):369–373. doi: 10.1016/0014-4894(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K. The pattern of mammalian brain gangliosides. II. Evaluation of the extraction procedures, postmortem changes and the effect of formalin preservation. J Neurochem. 1965 Jul;12(7):629–638. doi: 10.1111/j.1471-4159.1965.tb04256.x. [DOI] [PubMed] [Google Scholar]
- al-Abbassy S. N., Seed T. M., Kreier J. P. Isolation of the trypomastigote form of Trypanosoma cruzi from a mixture of the trypomastigote and epimastigote forms of the parasite by use of a DEAE-cellulose column. J Parasitol. 1972 Jun;58(3):631–632. [PubMed] [Google Scholar]




