Abstract
AIM: To evaluate the impact of medical therapy on Crohn’s disease patients undergoing their first surgical resection.
METHODS: We retrospectively evaluated all patients with Crohn’s disease undergoing their first surgical resection between years 1995 to 2000 and 2005 to 2010 at a tertiary academic hospital (St. Paul’s Hospital, Vancouver, Canada). Patients were identified from hospital administrative database using the International Classification of Diseases 9 codes. Patients’ hospital and available outpatient clinic records were independently reviewed and pertinent data were extracted. We explored relationships among time from disease diagnosis to surgery, patient phenotypes, medication usage, length of small bowel resected, surgical complications, and duration of hospital stay.
RESULTS: Total of 199 patients were included; 85 from years 1995 to 2000 (cohort A) and 114 from years 2005 to 2010 (cohort B). Compared to cohort A, cohort B had more patients on immunomodulators (cohort A vs cohort B: 21.4% vs 56.1%, P < 0.0001) and less patients on 5-aminosalysilic acid (53.6% vs 29.8%, P = 0.001). There was a shift from inflammatory to stricturing and penetrating phenotypes (B1/B2/B3 38.8% vs 12.3%, 31.8% vs 45.6%, 29.4% vs 42.1%, P < 0.0001). Both groups had similar median time to surgery. Within cohort B, 38 patients (33.3%) received anti-tumor necrosis factor (TNF) agent. No patient in cohort A was exposed to anti-TNF agent. Compared to patients not on anti-TNF agent, ones exposed were younger at diagnosis (anti-TNF vs without anti-TNF: A1/A2/A3 39.5% vs 11.8%, 50% vs 73.7%, 10.5% vs 14.5%, P = 0.003) and had longer median time to surgery (90 mo vs 48 mo, P = 0.02). Combination therapy further extended median time to surgery. Using time-dependent multivariate Cox proportional hazard model, patients who were treated with anti-TNF agents had a significantly higher risk to surgery (adjusted hazard ratio 3.57, 95%CI: 1.98-6.44, P < 0.0001) compared to those without while controlling for gender, disease phenotype, smoking status, and immunomodulator use.
CONCLUSION: Significant changes in patient phenotypes and medication exposures were observed between the two surgical cohorts separated by a decade.
Keywords: Crohn’s disease, Surgery, Medication, Phenotype, Biologics, Anti-tumor necrosis factor, Immunomodulators, Inflammatory bowel disease
Core tip: Comparing two cohorts separated by a decade of Crohn’s disease patients who required surgical resections, this study showed significant changes in patient phenotypes and medication usage. Those that required surgery shifted from more inflammatory to stricturing and penetrating phenotypes, and had more immunomodulators but less 5-aminosalysilic acid exposures. Patients treated with biologics had significantly longer time from Crohn’s disease diagnosis to surgery. However, they were at increased risk for surgery, suggesting that biologics were often used too late in the patients’ treatment courses.
INTRODUCTION
Crohn’s disease (CD) is a chronic idiopathic inflammatory condition affecting the gastrointestinal tract. It often takes a relapsing and remitting course. Despite advances in medical management, many patients eventually develop complications requiring surgical interventions[1-3]. Up to 60% of CD patients require surgery within ten years of their disease diagnosis[3,4]. Although surgical resection can be associated with long-lasting remission, it has many inherent complications[5]. Therefore, it is usually reserved for cases where medical management fails. To avoid or delay surgery while keeping patients in remission is a desirable goal in CD management.
Medical management of CD traditionally involved a step-up approach[6,7]. Once in remission, patients are maintained with an immunomodulator (IM) or anti-tumor necrosis factor (TNF) agent. Infliximab was approved by Health Canada for use in CD treatment in 2001 and adalimumab was approved in 2008. They have demonstrated efficacy in inducing and maintaining CD remission in patients who have previously failed conventional therapy[8-13]. However, due to costs and reimbursement restrictions, early use of anti-TNF agents is limited in Canada. The usual treatment approach remains in a step-up fashion. This study evaluated the impact of medical treatments on CD patients undergoing their first surgical resection at a tertiary academic hospital. In particular, we assessed time from disease diagnosis to their first surgical resection.
MATERIALS AND METHODS
We retrospectively evaluated all patients with Crohn’s disease who had their first surgical resection in years 1995 to 2000 (cohort A) and years 2005 to 2010 (cohort B) at a tertiary academic hospital (St. Paul’s Hospital, Vancouver, Canada). These two time cohorts were selected based on the availability of anti-TNF agents in Canada. We intended to further delineate the impact of anti-TNF agents on surgical resections. Only patients with excisional intestinal surgery were included in this study. Stricturoplasty, bypass, and elective surgical treatment of abscess were not considered. The surgical approach over the study time period did not change at this hospital. Patients were initially identified using an electronic search of the hospital’s medical records using the International Classification of Diseases 9 codes. Trained abstractors (Fu YTN, Hong T, Round A) conducted standardized chart reviews on all patients. Both hospital and available outpatient clinic charts were reviewed. The diagnosis of Crohn’s disease was accepted if standard radiographic, endoscopic, or histological criteria were documented in the medical record. All diagnosis was confirmed with surgical pathology specimens. Crohn’s disease phenotypes at the time of surgery were recorded. Pertinent information regarding the patients’ baseline demographics, disease phenotypes, medication exposure, time from disease diagnosis to surgery, and details surrounding the first surgery specifically amount of small intestine resected, post-operative complications and length of hospital stay were retrieved from their charts. Azathioprine, 6-mercaptopurine and methotrexate were designated as IM. The Montreal classification was used to denote disease phenotypes[14]. This study received full institutional ethics approval.
Statistical analysis
We performed χ2 or Fisher’s exact tests for categorical variables and t-tests or Wilcoxon rank-sum test for continuous variables. Kruskal-Wallis test was used to compare the time from disease diagnosis to surgery among three patient groups; patients in the 1995-2000 cohort (cohort A), patients exposed to, and not exposed to anti-TNF agent in the 2005-2010 cohort (cohort B). Time-dependent adjusted multivariate Cox proportional hazard model was used to assess risk to surgical resection. We performed all statistical analyses using SAS Version 9.2 (SAS Institute, NC, United States). All statistical tests were two-sided with a 0.05 significance level.
RESULTS
Eighty five patients with Crohn’s disease had their first surgical resection between the years 1995 to 2000 (cohort A) and 114 patients had first surgery between years 2005 to 2010 (cohort B).
Comparing the two time cohorts, the patients had similar median age at the time of surgery but there were more males in 2005-2010 (cohort B) (Table 1). Significantly different disease phenotypes were identified. There was a shift from inflammatory to stricturing and penetrating diseases as well as a change from ileal and colonic to ileocolonic diseases (Table 1; cohort A vs cohort B: B1/B2/B3 38.8%/31.8%/29.4% vs 12.3%/45.6%/42.1%, P < 0.0001; L1/L2/L3 32.9%/28.2%/38.8% vs 26.3%/14.9%/58.8%, P = 0.01). The patients also had significantly different medication exposure. The later cohort B had significantly less 5-aminosalysilic acid (5-ASA) but more IM exposure (Table 1; cohort A vs cohort B: 5-ASA 53.6% vs 29.8%, P = 0.01; IM 21.4% vs 56.1%, P < 0.0001). There was no difference in corticosteroid exposure, surgical details, median length of hospital stay, post-operative complication rate, and median time from disease diagnosis to first surgical resection. The median time from disease diagnosis to surgery was 72 mo.
Table 1.
Demographic and surgical details for Crohn’s disease patients with first resections in years 1995-2000 (cohort A) and years 2005-2010 (cohort B)
| Cohort A 1995-2000 (n = 85) | Cohort B 2005-2010 (n = 114) | P value | |
| Age | 33 (± 12.1) | 31.5 (±13.9) | 0.440 |
| Gender (M/F) | 30.6 (26)/69.4 (59) | 54.4 (62)/ 45.6 (52) | 0.001 |
| Montreal classification | |||
| Age at diagnosis (A1/A2/A3) | 9.8%/74.4%/15.9% | 21.1%/65.8%/13.2% | 0.110 |
| Disease behavior (B1/B2/B3) | 38.8%/31.8%/29.4% | 12.3%/45.6%/42.1% | < 0.0001 |
| Disease location (L1/L2/L3) | 32.9%/28.2%/38.8% | 26.3%/14.9%/58.8% | 0.010 |
| Medication exposure | |||
| 5-ASA | 53.6 (45) | 29.8 (34) | 0.001 |
| CS | 69.1 (58) | 75.4 (86) | 0.320 |
| IM | 21.4 (18) | 56.1 (64) | < 0.0001 |
| Surgical details | |||
| Time from diagnosis to surgery (mo) | 72 ± 83.8 | 72 ± 89.5 | 0.710 |
| Amount of small bowel resected (cm) | 21 ± 12.6 | 23 ± 17.5 | 0.820 |
| Length of hospital stay (d) | 10 ± 16.7 | 9 ± 8.0 | 0.050 |
Data are expressed as absolute numbers (percentage) or mean ± SD. 5-ASA: 5-aminosalysilic acid; CS: Corticosteroid; IM: Immunomodulator; M: Male; F: Female.
Within the 2005-2010 cohort (cohort B), 38 patients (33.3%) received anti-TNF therapy; 18 treated with infliximab, five with adalimumab, and 15 with both agents sequentially. Only eight subjects were treated with anti-TNF agent alone, and all others were treated concomitantly with IM. No patient was exposed to anti-TNF in the earlier cohort A. Patients treated with and without anti-TNF agent had comparable median age at the time of surgery and gender distribution (Table 2). However, those received anti-TNF agent were younger at disease diagnosis (Table 2; anti-TNF vs without anti-TNF; 39.5% vs 11.8%, 50% vs 73.7%, 10.5% vs 14.5%, P = 0.003), had more colonic diseases (23.7% vs 27.6%, 29% vs 7.9%, 47.4% vs 64.5%, P = 0.01), and higher IM usage (79% vs 44.7%, P = 0.001). No difference was seen in corticosteroid and 5-ASA exposure, median length of hospital stay, and post-operative complication rate.
Table 2.
Demographic and Surgical details for patients treated with and without anti-tumor necrosis factoragents in years 2005-2010 (cohort B)
| Anti-TNF (n = 38) | Without Anti-TNF (n = 76) | P value | |
| Age | 29.5 (± 13.14) | 33.5 (± 14.05) | 0.10 |
| Gender (M/F) | 60.5 (23)/39.5 (15) | 51.3 (39)/48.7 (37) | 0.35 |
| Smoking status (yes) | 26.3 (10) | 23.7 (18) | 0.82 |
| Montreal classification | |||
| Age at diagnosis (A1/A2/A3) | 39.5%/50%/10.5% | 11.8%/73.7%/14.5% | 0.003 |
| Disease behavior (B1/B2/B3) | 18.4%/39.5%/42.1% | 9.2%/48.7%/42.1% | 0.33 |
| Disease location (L1/L2/L3) | 23.7%/29%/47.4% | 27.6%/7.9%/64.5% | 0.01 |
| Medication exposure | |||
| 5-ASA | 29 (11) | 30.3 (23) | 0.88 |
| CS | 81.6 (31) | 72.4 (55) | 0.28 |
| IM | 79 (30) | 44.7 (34) | 0.001 |
| Surgical details | |||
| Time from diagnosis to surgery (mo) | 90 ± 63.1 | 48 ± 100.2 | 0.02 |
| Amount of small bowel resected (cm) | 23 ± 12.4 | 21.5 ± 19.3 | 0.92 |
| Length of hospital stay (d) | 9 ± 8.6 | 10 ± 7.7 | 0.76 |
Data are expressed as absolute numbers (percentage) or mean ± SD. 5-ASA: 5-aminosalysilic acid; CS: Corticosteroid; IM: Immunomodulator; M: Male; F: Female.
Patients who received anti-TNF agent had longer median time from disease diagnosis to first surgical resection (Figure 1; 90 mo vs 48 mo, P = 0.02). Combination therapy with anti-TNF and IM lead to much extended median time to surgery when compared to anti-TNF or IM alone (Figure 2; 96 mo vs 60 mo, 96 mo vs 54 mo, P = 0.03). There was no difference in smoking status (P = 0.82) or disease behavior (P = 0.33) in ones treated with and without anti-TNF agent (Table 2). The median time to surgery for patients not receiving anti-TNF therapy in the 2005-2010 cohort (cohort B) was shorter compared to those in the 1995-2000 cohort (cohort A) (Figure 1; 48 mo vs 72 mo).
Figure 1.
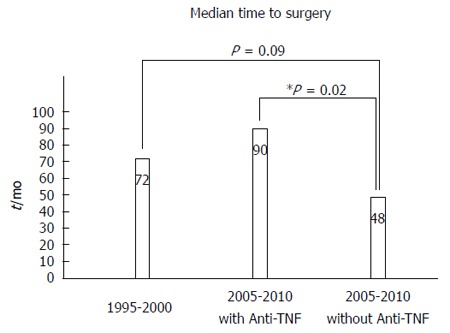
Patients treated with anti-tumor necrosis factor agents had significantly longer median time from Crohn’s disease diagnosis to surgery. TNF: Tumor necrosis factor.
Figure 2.
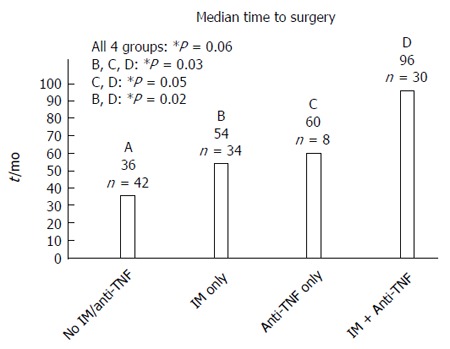
Combination therapy using immunomodulator and anti-tumor necrosis factor agent further extended the median time from Crohn’s disease diagnosis to surgery for patients requiring surgery in years 2005 to 2010 (cohort B). TNF: Tumor necrosis factor; IM: Immunomodulator.
Risk for surgical resection for patients in the 2005-2010 cohort (cohort B) was assessed using time-dependent multivariate Cox proportional hazard model controlling for gender, disease phenotype, smoking status, IM, and anti-TNF use. Patients who were treated with anti-TNF agent had a significantly higher time-dependent risk to surgery (adjusted hazard ratio (HR) = 3.57, 95%CI: 1.98-6.44, P < 0.0001) compared to those without. The median time from disease diagnosis to administration of anti-TNF agent was 66 mo and the median time from administration of anti-TNF agent to surgery was 14 mo. This suggests that anti-TNF agent was used too late in the treatment course.
DISCUSSION
The recent shift in medical management of Crohn’s disease has decreased the cumulative surgical rate of CD patients[15-20]. Our study echoed the observed trends that more IM but less 5-ASA products are currently used in CD management[18-22]. Immunomodulators are effective maintenance medications and azathioprine has been shown to modestly lower the risk of surgery[23-25]. A recent meta-analysis showed a combined pooled HR of 0.59 for first intestinal resection with thiopurine use[26]. Therefore, selecting patients who are most likely to benefit from IM may change the surgical rate of patients with CD.
Anti-TNF agents are very efficacious in treating refractory luminal and fistulising CD[8-13]. However, once CD is complicated with strictures and stenosis, surgical resection is typically the best therapeutic option. Our study confirmed that anti-TNF agents are the best for treating inflammatory CD[3,19,20,25,27]. We observed a significant shift in disease phenotypes from inflammatory to stricturing and penetrating phenotypes in patients requiring surgical resection. This shift in phenotypes suggests that the changes in medical management have lead to more success in managing patients with inflammatory CD, but the therapies are less effective in structuring and penetrating diseases. Additionally, our study infers that younger patients had more aggressive disease and they were more likely to require anti-TNF agents as rescue treatment.
The reported time from CD diagnosis to surgery varies widely in literature, ranging from one to 19 mo[17,27,28]. Our two cohorts had the same median time to surgery of 72 mo, much longer than reported in literature. Such discrepancies may be due to differences in regional surgical referral pattern. Interestingly, patients who did not receive anti-TNF therapy in our later cohort B (2005-2010) had shorter time to surgery than those in the earlier cohort A (1995-2000) (48 mo vs 72 mo). This suggests that either patients in the later cohort had more aggressive disease, or the phenotypes of the patients being seen in this tertiary centre were more prone to surgery. Stricturing and penetrating phenotypes were more common in the later cohort which is the likely cause of the shorter time to resection. Patients with disease status that may warrant surgical resection in the earlier decade were, instead, being placed on more aggressive medical therapy such as anti-TNF agent. Additionally, surgeons in the later time cohort may be more selective in operating on only the sicker patients although the overall surgical approach to CD management did no change throughout the study period.
A recent meta-analysis showed infliximab reduces hospitalization and major surgery[29]. Our study found that anti-TNF agents and combination therapy extend time from CD disease diagnosis to first surgical resection in CD patients. However, patients who received anti-TNF agents in the later cohort had a higher time-dependent risk to resective surgery. This suggests that anti-TNF agent was used too late in the patient’s treatment course at our center. With earlier introduction of anti-TNF agent, the patients may have a different time course to surgery. In Bouguen and Peyrin-Biroulet’s review, the surgical risk for adult CD within 5 year at a referral centre ranged from 17% to 35% in pre-anti-TNF era and 18% to 33% in anti-TNF era[4]. Our study reflects the changes described that, with wider and earlier use of IM and anti-TNF agents, we begin to see how these medications can alter the natural history of CD[4].
There are limitations to our study. As this is a single-centered, retrospective study with a relatively small sample size, we are unable to establish causal relationships. Although there were only 38 patients who received anti-TNF therapy in this study, it is proportionally higher when compared to the literature[18,20,21,30,31]. We found no significant difference in confounding variables to surgery such as smoking status and disease behavior in anti-TNF exposed and unexposed patients. A significantly higher proportion of patients were diagnosed at a young age in the anti-TNF exposed group. Younger patients with CD often have more aggressive disease that may lead to early surgery and requirement of anti-TNF agents[32-35]. Despite this, anti-TNF exposed still demonstrated longer median time to surgical resection. Studies conducted in tertiary centres may have skewed patient populations with more aggressive phenotypes as our study demonstrated.
Significant changes in patient phenotypes and medication exposure were observed between the two surgical cohorts separated by a decade. Patients received ant-TNF agents had prolonged time to surgery and those on combination therapy had the longest median time to their first surgical resection. However, the study result suggests that anti-TNF agent was often used too late in patient’s treatment course.
COMMENTS
Background
Since the past decade, new medical therapies are now available for treatment of Crohn’s disease (CD). Majority of CD patients require surgery within ten years of their disease diagnosis. To avoid or delay surgery while keeping patients in remission is a desirable goal in CD management.
Research frontiers
Immunomodulators are effective maintenance medications for CD. Anti-tumor necrosis factor (TNF) agents have demonstrated efficacy in inducing and maintaining CD remission in patients who have previously failed conventional therapy. However, the impact of medication on the natural progression of CD is unclear. This study evaluated changes in patient phenotypes, medication exposures, and time from disease diagnosis to surgery in CD patients requiring surgical resection.
Innovations and breakthroughs
Patients with CD requiring surgical resections shifted from more inflammatory to stricturing and penetrating phenotypes, and had more immunomodulators but less 5-aminosalysilic acid exposures. Patients treated with combination therapy had the longest time from disease diagnosis to their first surgical resection. Biologics were likely used too late in the patients’ treatment course.
Applications
Early and appropriate use of medical therapy may alter the natural progression of Crohn’s disease by preventing or delaying surgical resection. Combination therapy may lead to extended time from disease diagnosis to surgery.
Terminology
Immunomodulators include azathioprine, 6-mercaptopurine and methotrexate. Anti-TNF agents or biologics include infliximab and adalimumab. Combination therapy implies simultaneous use of an immunomodulator and an anti-TNF agent. Montreal classification denotes Crohn’s disease phenotypes based on age of diagnosis (A1 age < 16, A2 age 17-40, A3 age > 40), disease behavior (B1 non-stricturing/non-penetrating, B2 stricturing, B3 penetrating), and disease location (L1 ileal, L2 colonic, L3 ileocolonic). Modifiers for upper gastrointestinal (L4) and/or perianal (p) disease involvement can be applied.
Peer review
The paper focuses on a very interesting issue and reports data from a reasonably large series of patients. It would be nice to know about patients in the middle (years 2000-2005) in which a mixed population is present and a comparison between the beginning of anti-TNF use and a more mature utilization could be performed.
Footnotes
P- Reviewer: Freeman HJ, Vecchi M S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 4.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 5.Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, Harmsen WS, Zinsmeister AR. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. doi: 10.1016/s0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 6.Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–1837.e2. doi: 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 7.D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 8.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 9.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 10.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 13.Colombel JF, Schwartz DA, Sandborn WJ, Kamm MA, D’Haens G, Rutgeerts P, Enns R, Panaccione R, Schreiber S, Li J, et al. Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut. 2009;58:940–948. doi: 10.1136/gut.2008.159251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen GC, Nugent Z, Shaw S, Bernstein CN. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology. 2011;141:90–97. doi: 10.1053/j.gastro.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 17.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 18.Jess T, Riis L, Vind I, Winther KV, Borg S, Binder V, Langholz E, Thomsen OØ, Munkholm P. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a population-based study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481–489. doi: 10.1002/ibd.20036. [DOI] [PubMed] [Google Scholar]
- 19.Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–1206. doi: 10.1136/gut.2009.202101. [DOI] [PubMed] [Google Scholar]
- 20.Lakatos PL, Golovics PA, David G, Pandur T, Erdelyi Z, Horvath A, Mester G, Balogh M, Szipocs I, Molnar C, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol. 2012;107:579–588. doi: 10.1038/ajg.2011.448. [DOI] [PubMed] [Google Scholar]
- 21.Herrinton LJ, Liu L, Fireman B, Lewis JD, Allison JE, Flowers N, Hutfless S, Velayos FS, Abramson O, Altschuler A, et al. Time trends in therapies and outcomes for adult inflammatory bowel disease, Northern California, 1998-2005. Gastroenterology. 2009;137:502–511. doi: 10.1053/j.gastro.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 22.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2010;(6):CD000545. doi: 10.1002/14651858.CD000545.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630–642. doi: 10.1038/ajg.2011.64. [DOI] [PubMed] [Google Scholar]
- 25.Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930–936. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 26.Chatu S, Subramanian V, Saxena S, Pollok RC. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:23–34; quiz 35. doi: 10.1038/ajg.2013.402. [DOI] [PubMed] [Google Scholar]
- 27.Domènech E, Zabana Y, Garcia-Planella E, López San Román A, Nos P, Ginard D, Gordillo J, Martínez-Silva F, Beltrán B, Mañosa M, et al. Clinical outcome of newly diagnosed Crohn’s disease: a comparative, retrospective study before and after infliximab availability. Aliment Pharmacol Ther. 2010;31:233–239. doi: 10.1111/j.1365-2036.2009.04170.x. [DOI] [PubMed] [Google Scholar]
- 28.Sands BE, Arsenault JE, Rosen MJ, Alsahli M, Bailen L, Banks P, Bensen S, Bousvaros A, Cave D, Cooley JS, et al. Risk of early surgery for Crohn’s disease: implications for early treatment strategies. Am J Gastroenterol. 2003;98:2712–2718. doi: 10.1111/j.1572-0241.2003.08674.x. [DOI] [PubMed] [Google Scholar]
- 29.Costa J, Magro F, Caldeira D, Alarcão J, Sousa R, Vaz-Carneiro A. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19:2098–2110. doi: 10.1097/MIB.0b013e31829936c2. [DOI] [PubMed] [Google Scholar]
- 30.Slattery E, Keegan D, Hyland J, O’donoghue D, Mulcahy HE. Surgery, Crohn’s disease, and the biological era: has there been an impact? J Clin Gastroenterol. 2011;45:691–693. doi: 10.1097/MCG.0b013e318201ff96. [DOI] [PubMed] [Google Scholar]
- 31.Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830–835. doi: 10.1002/ibd.21118. [DOI] [PubMed] [Google Scholar]
- 32.Adamiak T, Walkiewicz-Jedrzejczak D, Fish D, Brown C, Tung J, Khan K, Faubion W, Park R, Heikenen J, Yaffee M, et al. Incidence, clinical characteristics, and natural history of pediatric IBD in Wisconsin: a population-based epidemiological study. Inflamm Bowel Dis. 2013;19:1218–1223. doi: 10.1097/MIB.0b013e318280b13e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piekkala M, Pakarinen M, Ashorn M, Rintala R, Kolho KL. Long-term outcomes after surgery on pediatric patients with Crohn disease. J Pediatr Gastroenterol Nutr. 2013;56:271–276. doi: 10.1097/MPG.0b013e318279871c. [DOI] [PubMed] [Google Scholar]
- 34.Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46:581–589. doi: 10.1097/MCG.0b013e318247c32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solberg IC, Cvancarova M, Vatn MH, Moum B. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn’s Disease (the IBSEN Study) Inflamm Bowel Dis. 2014;20:60–68. doi: 10.1097/01.MIB.0000436956.78220.67. [DOI] [PubMed] [Google Scholar]


