Abstract
AIM: To investigate the association between serum alpha-fetoprotein (AFP) levels and fatty liver disease (FLD) in a Chinese population.
METHODS: A cross-sectional study was performed among subjects who presented for a health examination at the First Affiliated Hospital, College of Medicine, Zhejiang University in 2013. FLD was diagnosed based on an ultrasonography examination. Serum AFP levels were measured with a chemiluminescence immunoassay.
RESULTS: Of the 9800 subjects enrolled, 2601 were diagnosed with FLD. Subjects with FLD had higher serum AFP levels than those without the disease. Subjects with high serum AFP levels had a higher prevalence of FLD, metabolic syndrome, and its components. Univariate logistic analysis showed that elevated serum AFP levels were associated with an increased risk of FLD (OR = 1.057, 95%CI: 1.031-1.084). However, after adjusting for covariates, AFP no longer remained significantly associated with the risk factors for FLD.
CONCLUSION: Our results suggest that serum AFP levels are significantly associated with FLD and that AFP acts as a cofactor, but not as an independent factor, for FLD.
Keywords: Fatty liver, Alpha-fetoprotein, Association, Obesity, Risk factor
Core tip: Fatty liver disease (FLD) is a common liver disease that may progress to cirrhosis and hepatocellular carcinoma. In this study, we observed that serum alpha-fetoprotein (AFP) levels are significantly increased in subjects with FLD, and that AFP levels are significantly associated with metabolic parameters. Univariate logistic analysis showed that elevated serum AFP levels are associated with an increased risk of FLD. However, multivariate logistic regression analysis showed that AFP is not independently associated with the risk factors for FLD. Our results suggest a significant association between AFP and FLD, as well as suggesting that AFP acts as a cofactor, but not as an independent factor, for FLD.
INTRODUCTION
Fatty liver disease (FLD) is a common condition that is characterized by significant lipid deposition within the liver parenchyma. FLD could be induced through both alcoholic and non-alcoholic pathways. Non-alcoholic fatty liver disease (NAFLD) is also called metabolic fatty liver disease, and is closely associated with obesity and metabolic syndrome[1]. Alcoholic fatty liver disease, on the other hand, is caused by excessive alcohol consumption[2]. In parallel with the epidemic of obesity, NAFLD has become an epidemic around the world. More than 30% of adults are affected by NAFLD in the United States[3], with a prevalence as high as 20% in developing countries such as China[4]. With increasing consumption of alcohol, alcoholic fatty liver disease has also become a growing public concern worldwide[5].
FLD includes a wide clinical and histological spectrum, including simple steatosis, steatohepatitis, fibrosis, and cirrhosis. Simple steatosis is generally considered to be a benign condition, while steatohepatitis may progress to cirrhosis in up to 20% of patients[6]. FLD may progress to hepatocellular carcinoma (HCC) as well[7,8]. Non-alcoholic steatohepatitis has recently been reported to be the second leading etiology of HCC requiring liver transplantation, and is recognized as the most rapidly growing indication for liver transplantation in patients with HCC in the United States[9].
Alpha-fetoprotein (AFP) is a well-established tumor marker for HCC. The potential association between AFP and FLD has been examined in recent studies. Babali et al[10] observed that patients with fatty liver had a significantly increased serum AFP level, with said level being positively correlated with the grade of hepatic steatosis. Kara et al[11] compared serum AFP levels in 130 male NAFLD patients with those in 57 healthy male controls, but did not observe any significant association between AFP and histopathological findings in NAFLD patients. Polyzos et al[12] did not observe any positive association between AFP and NAFLD. The inconsistency of these studies may arise from differences in study population, sample size, and diagnostic methods for FLD.
In this study, we performed a large-sample, cross-sectional survey to analyze the association between AFP and FLD in a Chinese population.
MATERIALS AND METHODS
Study population
This study was performed among adults who presented for their annual health examinations at the First Affiliated Hospital, College of Medicine, Zhejiang University in 2013. The analyses were limited to participants who had full records of anthropometric and biochemical data, as well as results of hepatic ultrasonography examination. Pregnant women and subjects with a self-reported history of chronic viral hepatitis, cirrhosis, or malignant disease were excluded from the analysis. A total of 9800 participants (5880 men and 3920 women) with a median (interquartile range) age of 46.0 (39.0-53.0) years were included in the final analysis. All participants were informed verbally about the purpose and design of the study. Written informed consent was not required due to the observational nature of the study. The personal information of each participant was anonymized both at collection and prior to analysis. The study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Clinical examinations
Clinical examinations were performed following standard procedures as previously described[13,14]. All participates were instructed to complete an overnight fast. Body weight (kg) and standing height (cm) were recorded in light, indoor clothing without shoes. Waist circumference was measured using a non-stretchable standard tape at the minimum circumference between the iliac crest and the rib cage. Blood pressure was recorded using an automated sphygmomanometer with the subject in a sitting position.
Approximately 10-mL whole blood samples were collected from each subject, with serum samples being separated for further analysis without freezing. Serum biochemical values were measured using a Hitachi 7600 AutoAnalyzer (Hitachi, Tokyo, Japan) using standard methods. Serum AFP levels were determined with a chemiluminescence immunoassay using an Abbott-Architect Immunoanalyzer (Abbott Laboratories, Abbott Park, IL).
Diagnosis of FLD and metabolic syndrome
FLD was diagnosed based on ultrasonography criteria suggested by the Chinese Liver Disease Association[15]. The criteria are described as follows: FLD is considered to be present if the ultrasound examination shows a diffuse enhancement of near-field echo in the hepatic region and gradual attenuation of the far-field echo combined with any of the following: (1) unclear display of intrahepatic lacuna structure; (2) mild-to-moderate hepatomegaly with a round and blunt border; or (3) color Doppler ultrasonography showing a reduction in the blood flow signal in the liver or a blood flow signal that is difficult to display even when the distribution of the blood flow is normal.
Metabolic syndrome was defined according to the revised ATP III criteria of metabolic syndrome in an Asian Study[16]. Metabolic syndrome was considered to be present if subjects fulfilled any three of the following five factors: (1) central obesity: waist circumference ≥ 90 cm for males and ≥ 80 cm for females, and/or BMI ≥ 25 kg/m2 for both genders; (2) hypertriglyceridemia: triglycerides ≥ 1.7 mmol/L; (3) reduced high-density lipoprotein cholesterol (HDL-C): HDL-C < 1.03 mmol/L for males and < 1.29 mmol/L for females; (4) elevated blood pressure: systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg; and (5) elevated fasting blood glucose: fasting blood glucose ≥ 5.6 mmol/L or previously diagnosed type 2 diabetes.
Statistical analysis
Data were expressed as the mean and standard deviation or the median and interquartile range as appropriate. Student’s t-test, Mann-Whitney U test, and χ2 test were used for comparisons between the groups. Spearman’s analysis was used to determine the correlations between serum AFP levels and other metabolic parameters. Univariate and multivariate logistic regression analyses were applied to assess the factors associated with the presence of fatty liver. All of the analyses were performed using SPSS 13.0 software for Windows (SPSS Inc., Chicago, IL). P < 0.05 (2-tailed) was considered to be statistically significant.
RESULTS
Clinical characteristics of the subjects
Of the 9800 subjects enrolled in this study, 2601 (26.5%) and 3110 (31.7%) fulfilled the diagnostic criteria of FLD and metabolic syndrome, respectively. The prevalence of metabolic syndrome parameters including central obesity, elevated blood pressure, hypertriglyceridemia, reduced HDL-C, and elevated fasting blood glucose was 45.1%, 44.3%, 31.4%, 52.7%, and 11.3%, respectively.
Clinical characteristics were compared between subjects with and without FLD. As shown in Table 1, subjects with FLD are older and predominantly male. Significantly more unfavorable anthropometric and biochemical variables are observed among subjects with FLD compared with those without FLD. A notable finding is that serum AFP levels are significantly increased in subjects with FLD compared with those without FLD (Table 1), suggesting a potential association between AFP and FLD.
Table 1.
Comparison of clinical characteristics between the subjects with and without fatty liver disease
| Without FLD1 | With FLD2 | t value | P value | |
| Age (yr) | 45.0 (39.0–53.0) | 48.0 (41.0–55.0) | 8.248 | < 0.001 |
| Gender (male/female, n) | 3748/3451 | 2132/469 | 712.0053 | < 0.0013 |
| Body mass index (kg/m2) | 22.92 (2.71) | 26.72 (2.72) | 61.262 | < 0.001 |
| Waist circumference (cm) | 80.4 (8.9) | 92.7 (7.7) | 58.903 | < 0.001 |
| Systolic blood pressure (mmHg) | 123.8 (17.0) | 134.9 (16.1) | 29.053 | < 0.001 |
| Diastolic blood pressure (mmHg) | 75.4 (10.9) | 83.3 (10.5) | 31.739 | < 0.001 |
| Alanine aminotransferase (U/L) | 17.0 (13.0–24.0) | 29.0 (21.0–43.0) | 42.1944 | < 0.0014 |
| Aspartate aminotransferase (U/L) | 20.0 (17.0–24.0) | 23.0 (19.0–29.0) | 25.3394 | < 0.0014 |
| Gamma-glutamyltransferase (U/L) | 19.0 (13.0–31.0) | 39.0 (25.0–64.0) | 39.8464 | < 0.0014 |
| Direct bilirubin (μmol/L) | 3.0 (2.0–4.0) | 3.0 (2.0-4.0) | 2.113 | 0.053 |
| Indirect bilirubin (μmol/L) | 9.0 (6.0–11.0) | 9.0 (7.0–12.0) | 4.7064 | < 0.0014 |
| Triglyceride (mmol/L) | 1.10 (0.80–1.58) | 1.92 (1.40–2.68) | 41.7974 | < 0.001 |
| Total cholesterol (mmol/L) | 4.75 (0.90) | 5.08 (0.96) | 15.839 | < 0.001 |
| HDL cholesterol (mmol/L) | 1.19 (0.29) | 1.02 (0.23) | 26.529 | < 0.001 |
| LDL cholesterol (mmol/L) | 2.54 (0.62) | 2.63 (0.66) | 6.19 | < 0.001 |
| Fasting blood glucose (mmol/L) | 4.72 (4.44–5.02) | 5.04 (4.67–5.56) | 26.2504 | < 0.0014 |
| Serum uric acid (μmol/L) | 317.7 (85.3) | 390.8 (83.3) | 37.671 | < 0.001 |
| Alpha-fetoprotein (μg/L) | 2.90 (2.20–3.90) | 3.10 (2.40–4.10) | 7.6534 | < 0.0014 |
n = 7199;
n = 2601;
χ2 value;
Z value. The data are expressed as the mean ± SD or median (interquartile range) depending on the data distribution. FLD: Fatty liver disease; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
Correlations between AFP and metabolic syndrome
To better understand the association between AFP and FLD, we analyzed the association between AFP and the features of metabolic syndrome, which are closely associated with FLD. Spearman’s analysis showed that serum AFP levels are significantly and positively correlated with body mass index, waist circumference, systolic and diastolic blood pressure, triglyceride level, and fasting blood glucose, whereas they are negatively correlated with high density lipoprotein cholesterol (Table 2). These results suggest that subjects with higher serum AFP levels are associated with more unfavorable metabolic parameters.
Table 2.
Correlations between alpha-fetoprotein and features of metabolic syndrome
| BMI | WC | SBP | DBP | TG | HDL-C | FBG | |
| r value | 0.101 | 0.17 | 0.11 | 0.135 | 0.17 | -0.043 | 0.086 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
BMI: Body mass index; DBP: Diastolic blood pressure; FBG: Fasting blood glucose; HDL-C: High density lipoprotein cholesterol; SBP: Systolic blood pressure; TG: Triglyceride; WC: Waist circumference.
We also analyzed the association between serum AFP levels and the prevalence of metabolic syndrome and its components. We divided all of the subjects into two groups according to the median level of serum AFP (3.0 μg/L), and observed a significantly higher prevalence of FLD, metabolic syndrome, and its components including central obesity, elevated blood pressure, hypertriglyceridemia, and elevated fasting blood glucose in subjects with serum AFP ≥ 3.0 μg/L compared with those with serum AFP < 3.0 μg/L (Figure 1). These results suggest a significant association between AFP and metabolic syndrome, while also indirectly supporting an association between serum AFP levels and FLD.
Figure 1.
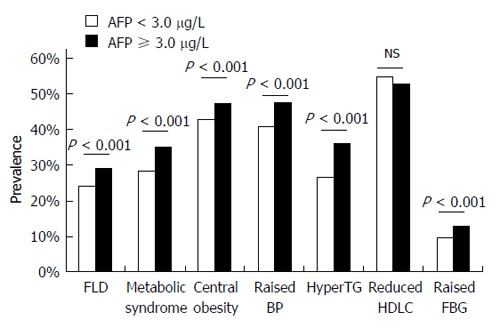
Prevalence of fatty liver disease, metabolic syndrome, and its components in subjects with different serum alpha-fetoprotein levels. Subjects were classified into two groups according to the median level of serum alpha-fetoprotein (AFP) (3.0 μg/L). Significantly higher prevalence of fatty liver disease, metabolic syndrome, and its components including central obesity, elevated blood pressure (BP), hypertriglyceridemia (hyperTG), and elevated fasting blood glucose (FBG) were observed in subjects with serum AFP ≥ 3.0 μg/L compared with those with serum AFP < 3.0 μg/L. FLD: Fatty liver disease; HDLC: High-density lipoprotein cholesterol; FBG: Fasting blood glucose.
Risk factors for the presence of FLD
We further applied both univariate and multivariate logistic regression analyses to assess the risk factors for FLD. In the univariate model, elevated serum AFP levels were observed to be associated with an increased risk of FLD (OR = 1.057, 95%CI: 1.031-1.084; Table 3). However, after adjusting for the 17 variables listed in Table 4 using a multivariate stepwise logistic analysis (Backward: Wald; Entry: 0.05, Removal: 0.10), AFP no longer remained significantly associated with the risk factors for FLD. This result indicates that the relation between AFP and FLD is somehow influenced by other variables.
Table 3.
Univariable analysis for factors associated with fatty liver disease
| Variables | OR | 95%CI | P value |
| Age (yr) | 1.017 | 1.013-1.021 | < 0.001 |
| Male gender | 4.185 | 3.748-4.672 | < 0.001 |
| Body mass index (kg/m2) | 1.667 | 1.629-1.706 | < 0.001 |
| Waist circumference (cm) | 1.187 | 1.177-1.197 | < 0.001 |
| Systolic blood pressure (mmHg) | 1.038 | 1.035-1.041 | < 0.001 |
| Diastolic blood pressure (mmHg) | 1.068 | 1.063-1.073 | < 0.001 |
| Alanine aminotransferase (U/L) | 1.050 | 1.047-1.053 | < 0.001 |
| Aspartate aminotransferase (U/L) | 1.039 | 1.033-1.045 | < 0.001 |
| Gamma-glutamyltransferase (U/L) | 1.013 | 1.012-1.015 | < 0.001 |
| Direct bilirubin (μmol/L) | 1.006 | 0.982-1.031 | 0.631 |
| Indirect bilirubin (μmol/L) | 1.020 | 1.010-1.030 | < 0.001 |
| Triglyceride (mmol/L) | 1.992 | 1.898-2.091 | < 0.001 |
| Total cholesterol (mmol/L) | 1.462 | 1.392-1.534 | < 0.001 |
| HDL cholesterol (mmol/L) | 0.079 | 0.065-0.097 | < 0.001 |
| LDL cholesterol (mmol/L) | 1.248 | 1.163-1.340 | < 0.001 |
| Fasting blood glucose (mmol/L) | 1.828 | 1.724-1.938 | < 0.001 |
| Serum uric acid (μmol/L) | 1.010 | 1.009-1.010 | < 0.001 |
| Alpha-fetoprotein (μg/L) | 1.057 | 1.031-1.084 | < 0.001 |
HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
Table 4.
Multivariable analysis for factors associated with fatty liver disease
| Variables | OR | 95%CI | P value |
| Age (yr) | 1.011 | 1.004-1.018 | 0.003 |
| Male gender | 1.408 | 1.160-1.709 | 0.001 |
| Body mass index (kg/m2) | 1.268 | 1.218-1.321 | < 0.001 |
| Waist circumference (cm) | 1.064 | 1.049-1.080 | < 0.001 |
| Diastolic blood pressure (mmHg) | 1.020 | 1.014-1.027 | < 0.001 |
| Alanine aminotransferase (U/L) | 1.043 | 1.036-1.051 | < 0.001 |
| Aspartate aminotransferase (U/L) | 0.953 | 0.940-0.966 | < 0.001 |
| Triglyceride (mmol/L) | 1.293 | 1.226-1.362 | < 0.001 |
| HDL cholesterol (mmol/L) | 0.411 | 0.307-0.550 | < 0.001 |
| LDL cholesterol (mmol/L) | 1.318 | 1.189-1.461 | < 0.001 |
| Fasting blood glucose (mmol/L) | 1.338 | 1.251-1.430 | < 0.001 |
| Serum uric acid (μmol/L) | 1.004 | 1.003-1.005 | < 0.001 |
HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
DISCUSSION
In this study, we aimed to investigate the association between serum AFP and FLD in a Chinese population. Of the 9800 subjects enrolled, the prevalence of FLD was 26.5%. Subjects with FLD exhibited higher serum AFP levels than those without FLD. A significant association between serum AFP levels and metabolic syndrome indirectly supports the link between AFP and FLD. Univariate logistic analysis showed that elevated serum AFP levels are associated with an increased risk of FLD. However, multivariate logistic regression analysis showed that AFP is not independently associated with the risk factors for FLD. These results suggest a significant association between AFP and FLD, as well as suggesting that AFP may act as a cofactor, but not as an independent factor, for FLD.
FLD is a common liver disease that affects approximately 27% of the urban adult population in China[17] and an even higher proportion of adults in developed countries[18]. FLD may progress to end-stage liver diseases such as cirrhosis and HCC. A recent study reported that FLD, both alcoholic and non-alcoholic, accounted for 9.2% of all HCC cases diagnosed in Japan between 2006 and 2009[19]. Another recent study also reported that non-alcoholic steatohepatitis was ranked as the second most common etiology of HCC requiring liver transplantation in the United States[9], and non-alcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with HCC in the United States[9]. Postoperative morbidity and 30-d mortality rates were significantly higher in the FLD-related HCC patients than viral hepatitis-associated HCC patients[20]. Based on these findings, screening for HCC in FLD patients would be beneficial, and this may be particularly applicable for steatohepatitis patients.
AFP is a major serum protein produced by the liver of a fetus. Serum AFP levels peak around the 12th week of gestation and then decline to negligible levels during the first year of life. AFP is observed to be elevated in several non-neoplastic hepatic disorders[21]. A statistically significant increase in serum AFP levels was also observed in patients with fatty liver compared with healthy controls (4.09 ± 1.68 ng/mL vs 2.95 ± 0.41 ng/mL, P = 0.008) by Babali et al[10], and their correlation analysis showed that serum AFP levels positively correlate with the grade of hepatic steatosis[10]. However, there have been other studies which not observe any difference in serum AFP levels between subjects with or without fatty liver[11,12]. The inconsistency among these studies may be explained by the differences in the study population, sample size, and diagnostic methods for FLD.
By analyzing 9800 subjects who presented for their health examination at a large medical center, we observed that subjects with ultrasonography diagnosed FLD have higher serum AFP levels than those without FLD. We also observed that serum AFP levels are significantly and positively correlated with metabolic syndrome and its components. Our univariate logistic regression analyses showed that AFP is significantly associated with the risk factors for FLD. However, AFP no longer remains significantly associated with the risk factors for FLD in the multivariate model. Our results suggest a significant association between serum AFP levels and FLD.
It is interesting to consider why serum AFP levels are elevated in subjects with FLD. Hepatocyte necrosis and subsequent hepatic regeneration is hypothesized to be responsible for the increases in serum AFP levels[21]. Hepatocyte proliferation during liver regeneration is also observed to be associated with dedifferentiation of mature hepatocytes and temporarily increased expression of AFP in the liver[22]. Serum AFP levels may also increase as a result of altered hepatocyte-hepatocyte interaction and the loss of normal architectural arrangements[23]. Although the precise mechanisms remain unclear, our results still have significant clinical implications. Based on the fact that FLD may progress to HCC and that serum AFP levels are elevated in subjects with FLD, monitoring serum AFP levels to screen for HCC in FLD patients has significant clinical importance. Indeed, a study reported that AFP combined with prothrombin induced by a lack of vitamin K or a vitamin K antagonist-II (PIVKA-II) may be considerably valuable for surveillance of HCC in FLD[24].
Several limitations are acknowledged in this study. First, FLD was diagnosed based on hepatic ultrasonography, which is not sensitive for mild hepatic steatosis; neither is it sensitive for diagnosing steatohepatitis or fibrosis. Therefore, the association of serum AFP levels with histopathological findings in FLD could not be analyzed in this study. Liver biopsy is the gold standard for the diagnosis of FLD, but such a procedure is invasive and may cause complications[25]. Ultrasonography is widely used in large sample studies of FLD because it is widely available, safe, and has acceptable diagnostic value for detecting hepatic steatosis[26]. Second, FLD can be caused through both alcoholic and non-alcoholic etiologies. In this study, we did not record alcohol consumption information in all subjects. Whether AFP is differentially associated with alcoholic and non-alcoholic fatty liver disease remains to be determined. Third, serum insulin levels have not been analyzed in this study. It remains unclear whether serum AFP levels are associated with insulin resistance. Nevertheless, our correlation analysis showed that serum insulin levels were significantly associated with metabolic variables, and subjects with higher serum AFP levels had a higher prevalence of metabolic syndrome and its components. Our results indirectly suggested a potential positive association between serum AFP levels and insulin resistance. Moreover, the degree of apoptosis in hepatocytes is known to be elevated in steatotic livers[27,28]. Thus, it would be valuable to correlate AFP levels with markers of hepatocyte cell death, such as cytokeratin 18 fragments. Further studies are needed to clarify these issues.
In conclusion, our large cross-sectional study shows that serum AFP levels are significantly association with FLD and may act as a cofactor, but not as an independent factor, for FLD. Our results indirectly suggest that it may be worthwhile to monitor serum AFP levels to screen for HCC in FLD patients.
COMMENTS
Background
Fatty liver disease (FLD) is a common chronic liver disease that may progress to cirrhosis and hepatocellular carcinoma. The association between serum alpha-fetoprotein (AFP) levels and FLD remains controversial in the literature.
Research frontiers
The factors associated with the development and progression of FLD have been extensively investigated during recent years.
Innovations and breakthroughs
The authors observed that serum AFP levels are significantly increased in subjects with FLD, and AFP levels are significantly associated with metabolic parameters. Univariate logistic analysis showed that elevated serum AFP levels are associated with an increased risk of FLD. However, multivariate logistic regression analysis showed that AFP is not independently associated with the risk factors for FLD.
Applications
This results show that serum AFP levels are significantly associated with FLD and may act as a cofactor, but not as an independent factor, for FLD. This results indirectly suggest that it may be worthwhile to monitor serum AFP levels for screening of hepatocellular carcinoma in FLD patients.
Terminology
AFP is a major serum protein produced by the liver of a fetus. Serum AFP level may be elevated in patients with hepatocellular carcinoma and several non-neoplastic hepatic disorders.
Peer review
This large cross-sectional study was well-designed, and the methods are valid. The results suggested that serum AFP levels are significantly associated with FLD and that AFP acts as a cofactor, but not as an independent factor, for FLD. Although there were several limitations in this study, as the authors have mentioned in the discussion, the observations are useful and, based on the findings, it is recommended that physicians monitor serum AFP levels to screen for hepatocellular carcinoma in FLD patients.
Footnotes
Supported by National Key Basic Research Development Program, No. 2012CB524905; National Science and Technology Support Plan Project, No. 2012BAI06B04; National Natural Science Foundation of China, No. 81100278, No. 81170378, No. 81230012, and No. 81270487; International Science and Technology Cooperation Projects of Zhejiang Province, No. 2013C24010; and Science Foundation of Health Bureau of Zhejiang Province, No. 2012RCA026
P- Reviewer: Kucera O, Ma H, Ruan XZ S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Ma S
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Szabo G, Mandrekar P. Focus on: alcohol and the liver. Alcohol Res Health. 2010;33:87–96. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Wong VW. Nonalcoholic fatty liver disease in Asia: a story of growth. J Gastroenterol Hepatol. 2013;28:18–23. doi: 10.1111/jgh.12011. [DOI] [PubMed] [Google Scholar]
- 5.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 8.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 9.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 10.Babalı A, Cakal E, Purnak T, Bıyıkoğlu I, Cakal B, Yüksel O, Köklü S. Serum α-fetoprotein levels in liver steatosis. Hepatol Int. 2009;3:551–555. doi: 10.1007/s12072-009-9156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kara M, Genc H, Tapan S, Meral C, Ercin CN, Erdal M, Dogru T. Alpha fetoprotein levels and its relationship with histopathological findings in patients with non-alcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2013;17:1536–1541. [PubMed] [Google Scholar]
- 12.Polyzos SA, Kountouras J. Serum alpha-fetoprotein in patients with nonalcoholic fatty liver disease. Eur Rev Med Pharmacol Sci. 2013;17:2411–2412. [PubMed] [Google Scholar]
- 13.Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56:241–247. doi: 10.1016/j.jhep.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299–1304. doi: 10.1038/ajg.2013.104. [DOI] [PubMed] [Google Scholar]
- 15.Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 17.Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28 Suppl 1:11–17. doi: 10.1111/jgh.12036. [DOI] [PubMed] [Google Scholar]
- 18.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 19.Tokushige K, Hashimoto E, Kodama K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J Gastroenterol Hepatol. 2013;28 Suppl 4:88–92. doi: 10.1111/jgh.12239. [DOI] [PubMed] [Google Scholar]
- 20.Wakai T, Shirai Y, Sakata J, Korita PV, Ajioka Y, Hatakeyama K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J Gastrointest Surg. 2011;15:1450–1458. doi: 10.1007/s11605-011-1540-8. [DOI] [PubMed] [Google Scholar]
- 21.Bloomer JR, Waldmann TA, McIntire KR, Klatskin G. alpha-fetoprotein in noneoplastic hepatic disorders. JAMA. 1975;233:38–41. doi: 10.1001/jama.233.1.38. [DOI] [PubMed] [Google Scholar]
- 22.Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825–5834. [PubMed] [Google Scholar]
- 23.Goldstein NS, Blue DE, Hankin R, Hunter S, Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels in patients with chronic hepatitis C. Relationships with serum alanine aminotransferase values, histologic activity index, and hepatocyte MIB-1 scores. Am J Clin Pathol. 1999;111:811–816. doi: 10.1093/ajcp/111.6.811. [DOI] [PubMed] [Google Scholar]
- 24.Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D, Reeves H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol. 2003;15:539–543. doi: 10.1097/01.meg.0000059112.41030.2e. [DOI] [PubMed] [Google Scholar]
- 26.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 27.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]


