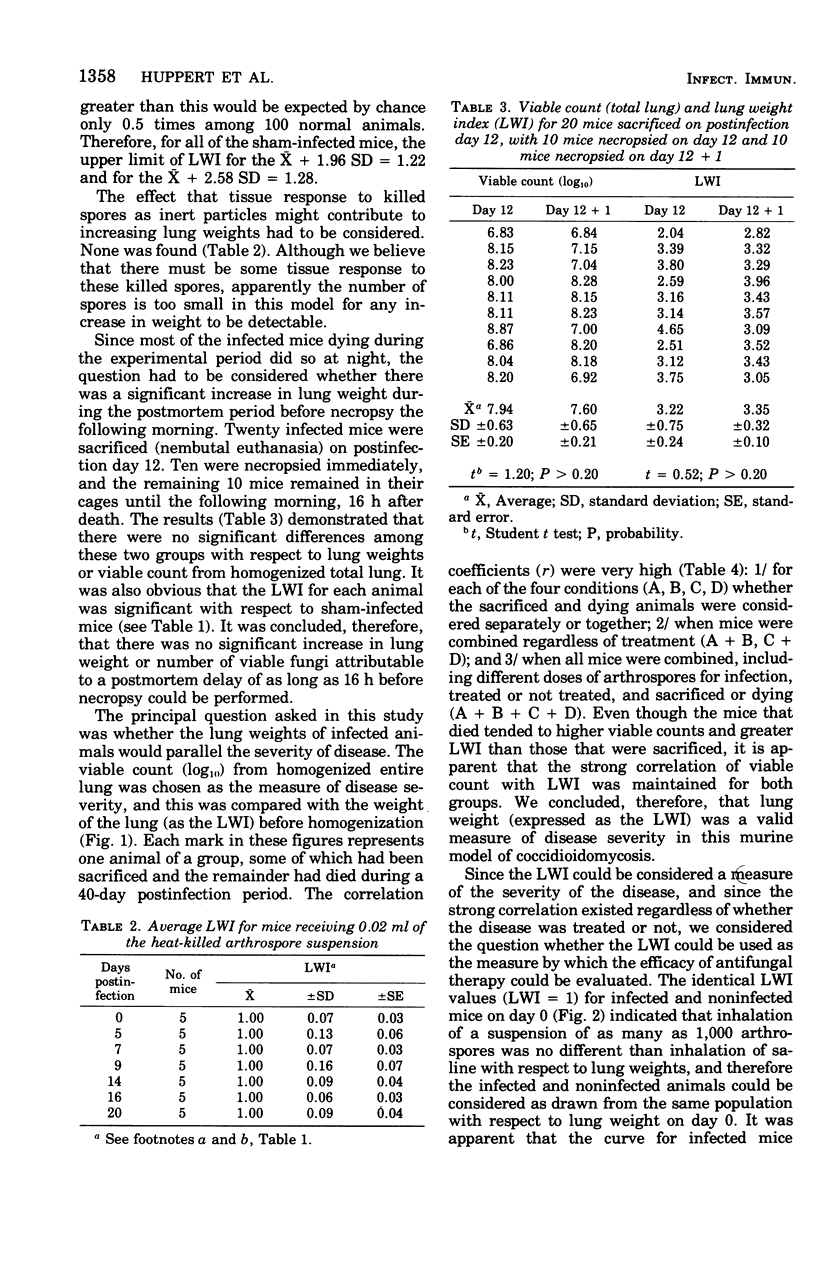
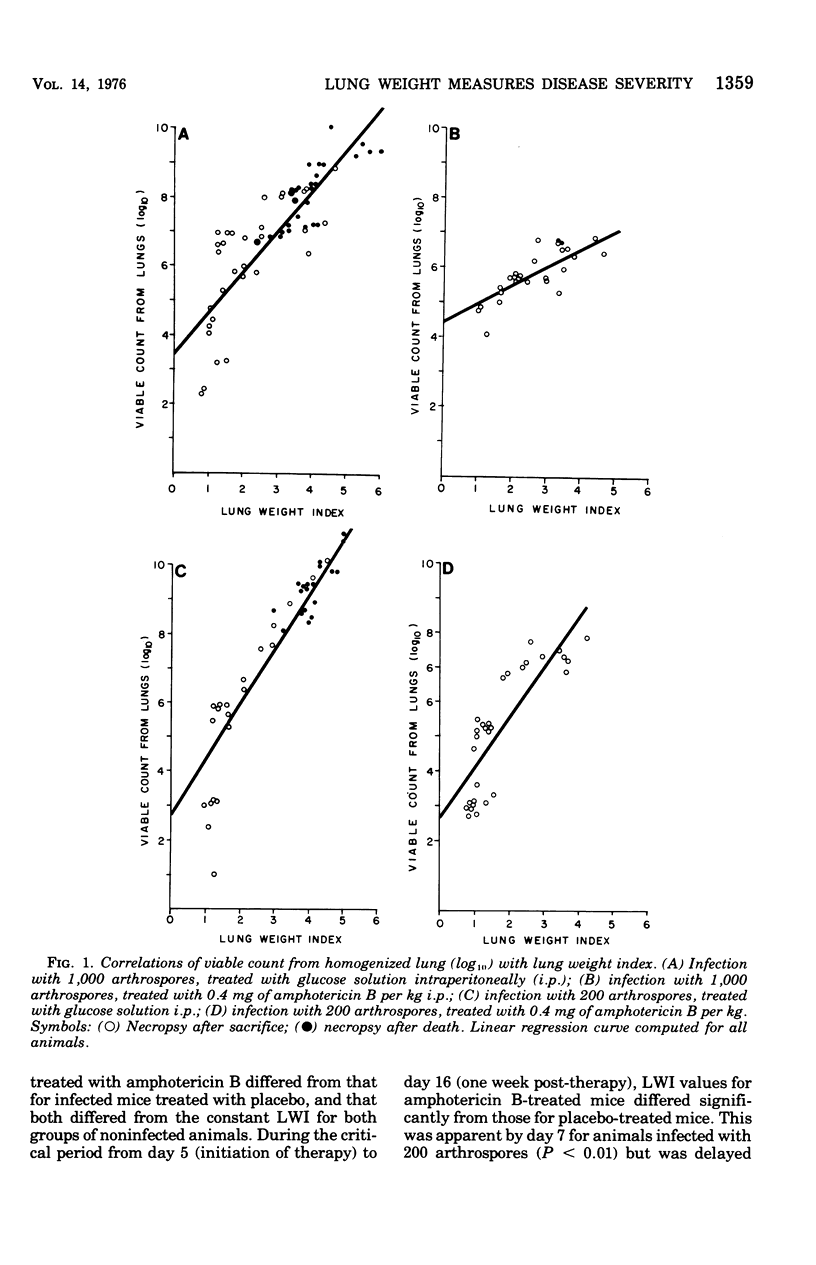
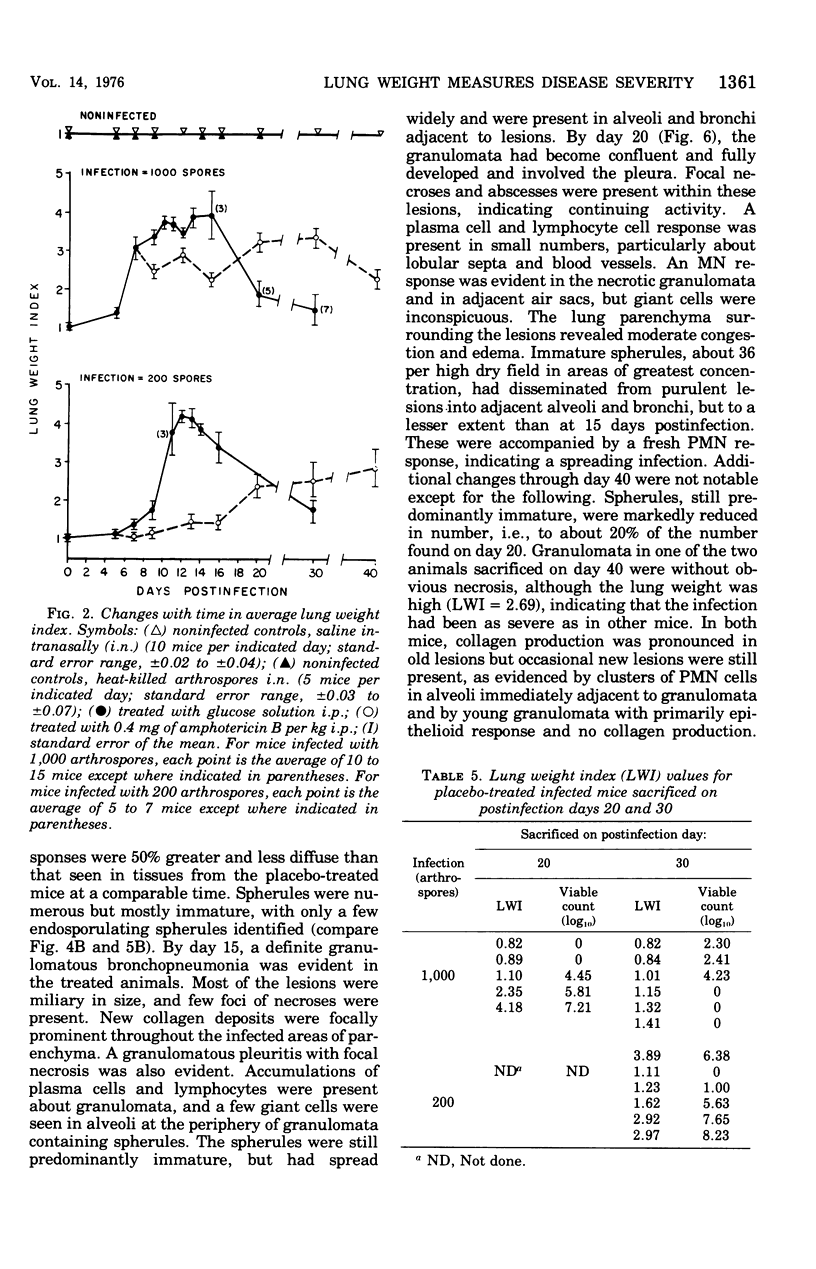
Abstract
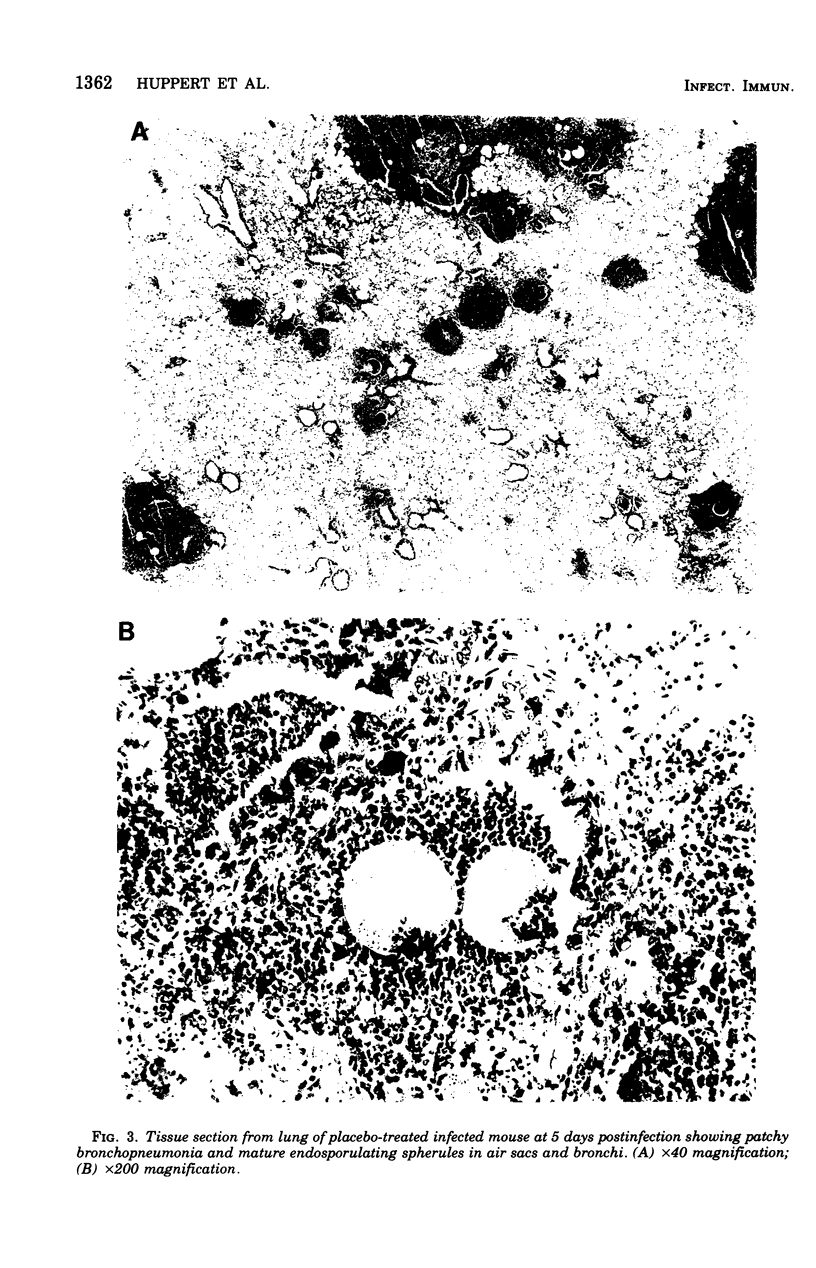
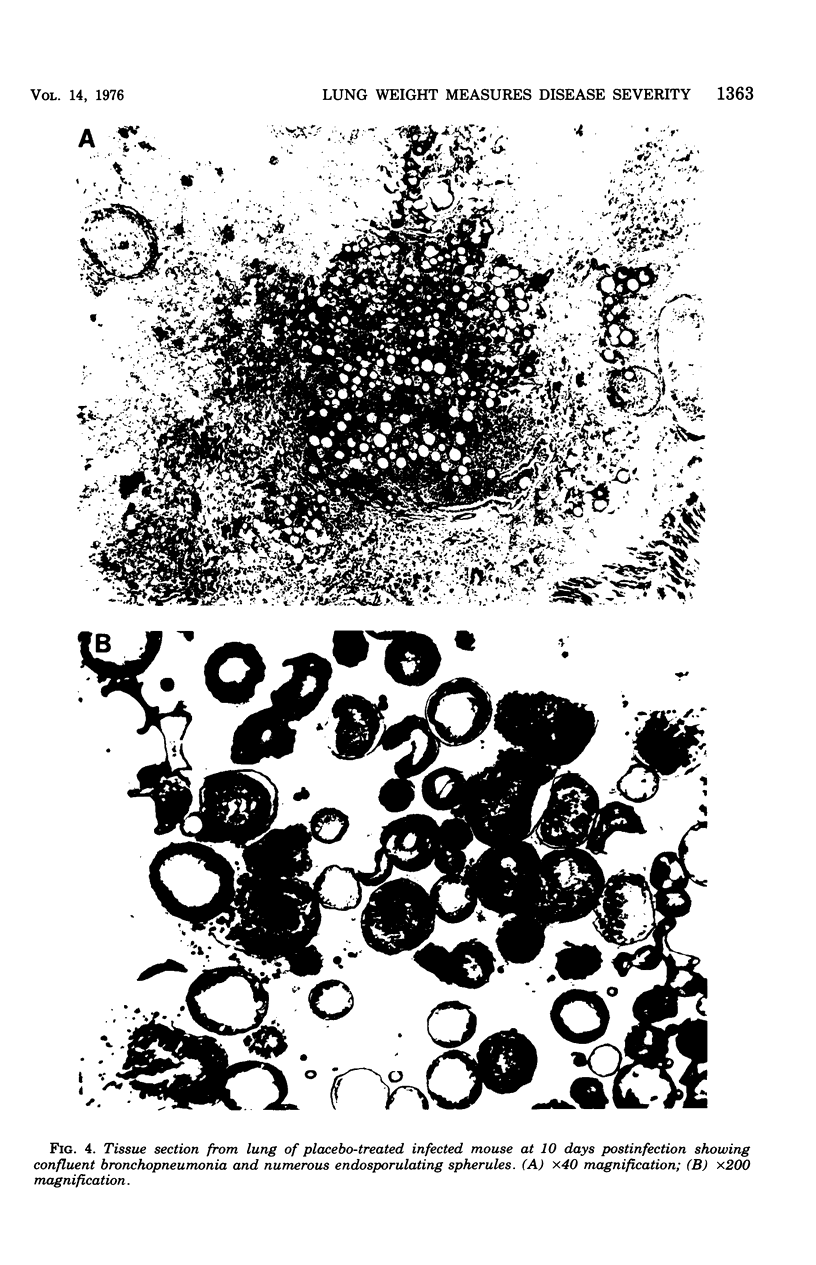
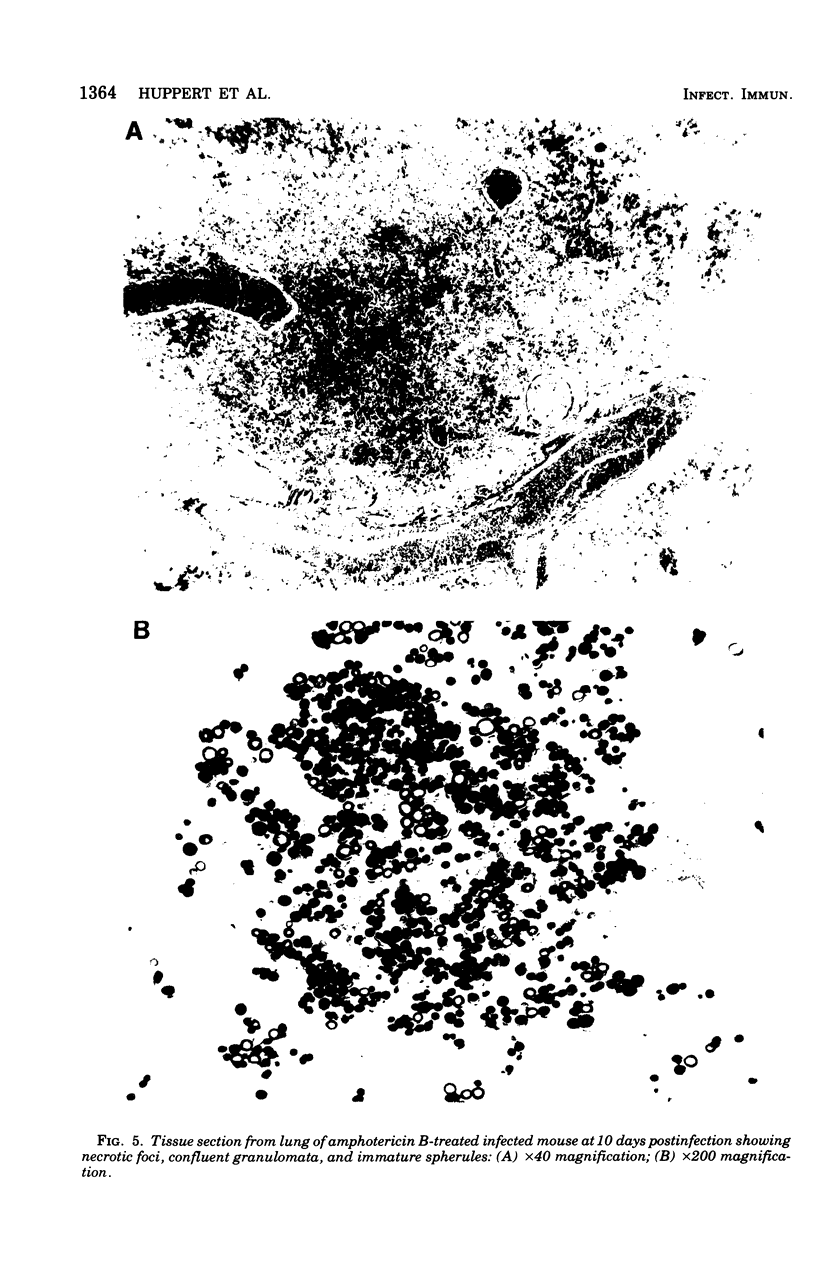
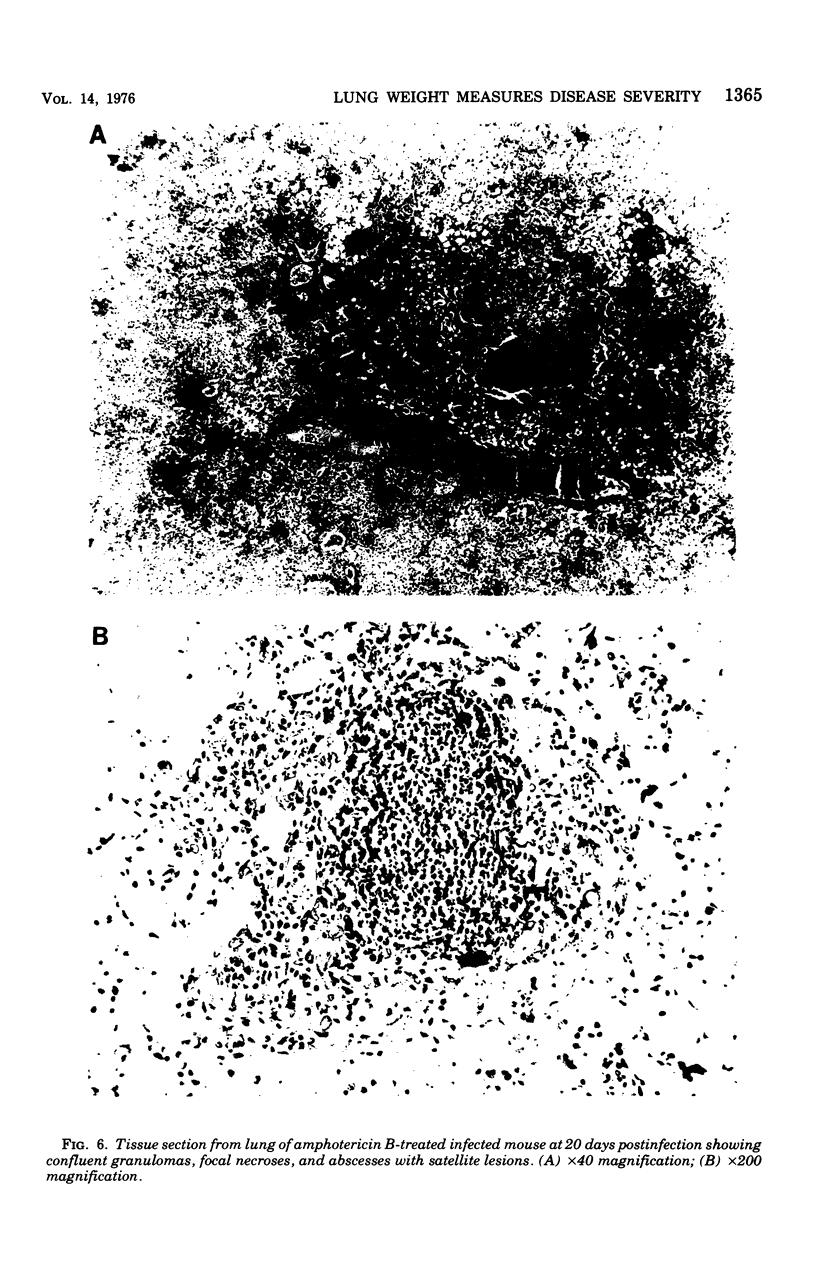
Evidence provided by histopathological study of lesions is a valuable adjunct for evaluating chemotherapeutic efficacy in experimental animal models, In addition, this should be correlated with a measure of disease severity in the same animal. The latter could be obtained by homogenization of infected organs and quantitative enumeration of viable cells of the etiological agent, but this would preclude histopathological studies in the same animal. Progression of disease in pulmonary infection is associated with replacement of air space by fluid, cells, and cellular debris. Therefore, an increase in lung weight should reflect severity of disease. Results with the murine model of coccidioidomycosis demonstrate that increasing lung weight parallels the increasing census of fungus cells in the lungs of both treated and nontreated infected mice. This was supported with evidence obtained from microscopic studies of lesions indicating that specific chemotherapy limited spread of the infection and inhibited multiplication of the fungus in the lung. Therefore, lung weight can be used as a measure of disease severity in the murine model of coccidioidomycosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTLEBERRY M. W., CONVERSE J. L., SINSKI J. T., LOWE E. P., PAKES S. P., DELFAVERO J. E. COCCIDIOIDOMYCOSIS: STUDIES OF CANINE VACCINATION AND THERAPY. J Infect Dis. 1965 Feb;115:41–48. doi: 10.1093/infdis/115.1.41. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN L., PAPAGIANIS D., BERMAN R. J., SMITH C. E. Studies on Coccidioides immitis: morphology and sporulation capacity of forty-seven strains. J Lab Clin Med. 1953 Sep;42(3):438–444. [PubMed] [Google Scholar]
- HUGENHOLTZ P. G., REED R. E., MADDY K. T., TRAUTMAN R. J., BARGER J. D. Experimental coccidioidomycosis in dogs. Am J Vet Res. 1958 Apr;19(71):433–437. [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Gross A. J. Evaluation of an experimental animal model for testing antifungal substances. Antimicrob Agents Chemother. 1972 May;1(5):367–372. doi: 10.1128/aac.1.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Vukovich K. R. Combined amphotericin B-tetracycline therapy for experimental coccidioidomycosis. Antimicrob Agents Chemother. 1974 May;5(5):473–478. doi: 10.1128/aac.5.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobbayashi G. S., Medoff G., Schlessinger D., Kwan C. N., Musser W. E. Amphotericin B potentiation of rifampicin as an antifungal agent against the yeast phase of Histoplasma capsulatum. Science. 1972 Aug 25;177(4050):709–710. doi: 10.1126/science.177.4050.709. [DOI] [PubMed] [Google Scholar]
- Kwan C. N., Medoff G., Kobayashi G. S., Schlessinger D., Raskas H. J. Potentiation of the antifungal effects of antibiotics by amphotericin B. Antimicrob Agents Chemother. 1972 Aug;2(2):61–65. doi: 10.1128/aac.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., MILLER R. L., SMITH C. E. Influence of vaccination on respiratory coccidiodial disease in cynomolgous monkeys. J Immunol. 1962 Aug;89:242–251. [PubMed] [Google Scholar]
- Levine H. B., Cobb J. M., Scalarone G. M. Spherule coccidioidin in delayed dermal sensitivity reactions of experimental animals. Sabouraudia. 1969 Feb;7(1):20–32. doi: 10.1080/00362177085190051. [DOI] [PubMed] [Google Scholar]
- Levine H. B., Kong Y. M. Immunity development in mice RECEIVING KILLED Coccidioides immitis spherules: effect of removing residual vaccine. Sabouraudia. 1965 Oct;4(3):164–170. [PubMed] [Google Scholar]
- Medoff G., Comfort M., Kobayashi G. S. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proc Soc Exp Biol Med. 1971 Nov;138(2):571–574. doi: 10.3181/00379727-138-35943. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Kwan C. N., Schlessinger D., Venkov P. Potentiation of rifampicin and 5-fluorocytosine as antifungal antibiotics by amphotericin B (yeast-membrane permeability-ribosomal RNA-eukaryotic cell-synergism). Proc Natl Acad Sci U S A. 1972 Jan;69(1):196–199. doi: 10.1073/pnas.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., MILLER R. L., SMITH C. E., KOBAYASHI G. S. Response of monkeys to respiratory challenge following subcutaneous inoculation with Coccidioides immitis. Am Rev Respir Dis. 1960 Aug;82:244–250. doi: 10.1164/arrd.1960.82.2.244. [DOI] [PubMed] [Google Scholar]
- Pulliam J. D., Converse J. L., Snyder E. M., Esterly J. R., Lowe E. P. Experimental irradiated arthrospore vaccine against coccidioidomycosis in mice. J Bacteriol. 1967 Nov;94(5):1394–1399. doi: 10.21236/ad0819931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. Z., Medoff G., Kobayashi G. S. Changes in murine resistance to Listeria monocytogenes infection induced by amphotericin B. J Infect Dis. 1973 Apr;127(4):373–377. doi: 10.1093/infdis/127.4.373. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. AN ACUTE PULMONARY GRANULOMATOUS RESPONSE IN MICE PRODUCED BY MYCOBACTERIAL CELLS AND ITS RELATION TO INCREASED RESISTANCE AND INCREASED SUSCEPTIBILITY TO EXPERIMENTAL TUBERCULOUS INFECTION. J Infect Dis. 1964 Apr;114:135–151. doi: 10.1093/infdis/114.2.135. [DOI] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Immunizing capacity of viable and killed attenuated mycobacterial cells against experimental tuberculous infection. J Bacteriol. 1969 Jan;97(1):107–113. doi: 10.1128/jb.97.1.107-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]