INTRODUCTION
Individuals infected with HIV have higher rates of atherosclerosis than those who are not HIV-infected, even after controlling for traditional risk factors [1, 2]. Previous studies have associated such higher rates with both the use of combination antiretroviral therapy [3, 4, 5] and with higher levels of T cell activation [1, 6]. The latter finding is particularly interesting because infiltrates of activated T cells also dominate early atherosclerotic lesions in the absence of HIV infection [7].
The presence of activated T cells within atheromatous lesions implies the presence of a specific antigenic stimulation. Although the cause of such stimulation remains unclear, it is intriguing that, in HIV-infected patients, CMV infection can not only induce a large fraction of antigen-specific T cells but is also associated with a higher frequency of subclinical atherosclerosis [8]. This juxtaposition suggests a potential role for CMV-specific immunity in the pathogenesis of HIV-associated atherogenesis.
Indeed, CMV is a “non-traditional” risk factor in atherogenesis and has been implicated in the pathogenesis of atherosclerosis mainly because of its ability to activate endothelial cells, the first and perhaps most important step in the development of the atherosclerosis [9]. In immunosuppressed patients, clinical studies suggest a direct relationship between CMV infection and atheroma. This seems particularly true in the setting of cardiac allograft recipients [10], where treatment with anti-CMV therapy is associated with a reduced risk of transplantation-associated arteriosclerosis.
The continuous immigration and infiltration of activated macrophages and T cells into and within atherosclerotic lesions are prominent features in both human and experimental atherosclerotic disease. Among chemokine receptors that mediate the tissue migration of T cells, polymorphisms in CX3CR1 (encoding the receptor for CX3CL1, or fractalkine) have been previously associated with coronary artery disease in human [11]. Interestingly, CMV-specific CD4+ T cells have themselves been shown to induce CX3CL1 in endothelial cells [12].
Given the above observations that CMV infection, CMV-specific T cells, and CX3CR1 have each been associated with atherosclerosis, we hypothesized that, in the setting of HIV infection, CMV co-infection might enhance the expansion of CMV-specific T cells expressing CX3CR1, induce endothelial cells to produce CX3CL1, and drive the migration of CX3CR1+ T cells into the arterial wall, promoting a chronic inflammatory process that culminates in atherosclerosis. The following studies provide compelling data in support of this hypothesis.
METHODS (See also Supplemental Methods for complete details)
Populations studied
HIV-infected subjects were recruited from an ongoing clinic-based cohort (SCOPE) based at the University of California, San Francisco. Carotid intima-media (IMT) measurements were available for 29 previously published individuals [8]. Control subjects were selected mainly from among subjects answering advertisements to participate in clinical studies who were of similar age and gender to the HIV-infected participants. The University of California, San Francisco Committee on Human Research approved the study and all subjects provided written informed consent.
Carotid Intima-Media T measurements
Carotid IMT was measured by high resolution ultrasound, as described previously [1]. Briefly, carotid IMT was measured in 12 predefined segments (six segments per side) using the standardized protocol of the Atherosclerosis Risk in Communities (ARIC) Study, which includes measurements of the near and far wall of the common carotid, the carotid bifurcation and the internal carotid [13]. Measurement reproducibility in our laboratory has been described previously and is greater than 0.9 [1]. Baseline carotid IMT measurements were obtained within one month of the immunophenotyping analysis. IMT progression was calculated as the mean IMT at follow up – mean IMT at baseline/duration of follow up (in years).
Flow cytometry
Panels of antibodies used for phenotypic detection and intracellular cytokine detection are described in Table S1. FACS analysis was performed on a four-laser BD LSR-II flow cytometers and data were analyzed with FlowJo software v8–6 (Treestar). The strategy used to gate the different subsets of peripheral blood mononuclear cells (PBMCs) is shown in Figure S1.
Enzyme-linked immunosorbent assay
Soluble fractalkine (CX3CL1) was detected in culture supernatants with a commercially available enzyme-linked immunosorbent assay (ELISA) detection kit (R&D Systems).
In vitro CMV infection
VR1814, an endothelial cell–tropic clinical strain of CMV isolated from the cervix, was adapted for growth in human artery endothelial cells (HAEC, Lonza) [14]. CMV infection was identified by production of its characteristic cytopathic effects (CPE) and immunostaining for CMV immediate early proteins (Millipore).
Co-cultures of PBMC or subset populations with HAEC monolayers
Co-cultures were set up with confluent primary human aortic endothelial cells (HAEC) monolayers overlaid with either 106 PBMC or CD4+ T cells overnight. CMV infection of HAEC was assessed when more than 50% of the cells showed CPE. The CD4+ T cells were depleted from PBMCs with the use of a positive selection MidiMACS system and LD immunomagnetic column (Miltenyi Biotech).
Neutralization assays
Blocking antibodies specific for TNFα (BD) and IFNγ (BD) were added at the time of co-cultivation of PBMC with CMV-infected HAEC. Purified mouse IgG1 and IgG2aK antibodies were used as isotype controls (BD). The manufacturer’s recommended concentrations were used for neutralization assays.
Transendothelial migration assay
HAEC were seeded on the membrane of transwell inserts (12 mm diameter, 3 μm pore size, Corning) to form polarized monolayers. The purity of HAEC was verified by immunofluorescence staining with rabbit anti-human Von Willebrand Factor (Abcam). The formation of polarized monolayers was monitored by staining with mouse anti-human VE-cadherin (R&D systems). CD4+ T cells were enriched from PBMC populations using a negative selection MidiMACS system and LS immunomagnetic column (Miltenyi Biotech). Recombinant human CX3CL1 (R&D Systems) or control media were loaded into lower well chambers. CD4+ T cells (105) were placed into the upper-chamber well inserts for a cell-migration time period of four hours. For each assay, three replicate wells were set up.
Immunohistochemistry assay
Specimens of coronary arteries were collected from autopsies (NCI AIDS and Cancer Specimen Resource, UCSF), fixed in buffered formalin, and embedded in paraffin. Sections were classified into minimal atherosclerotic and diffuse atherosclerotic coronary arteries. EDTA buffer was used for antigen retrieval. Primary antibodies used were anti-human CD4 mouse monoclonal (clone 1F6), anti-human CD3 mouse monoclonal (clone PS1), or anti-human CX3CR1 rabbit polyclonal (Thermoscientific). Detection of primary antibodies was performed with horseradish peroxidase polymer (DAKO Envision kit)–conjugated antibodies and developed with 3,3′-diaminobenzidine. Counterstains were done with Mayer’s hematoxylin.
Statistical analyses
Exact nonparametric two-tailed tests were used. The nonparametric Mann-Whitney test was used to compare continuous variables. The Fisher’s exact test was used to compare dichotomous variables. The Spearman rank correlation test was used to determine correlations between variables, with r being the Spearman correlation coefficient. Statistical analysis was performed with GraphPad Prism 5.01 software. Multiple linear regression was used to assess the strength of association between pairs of variables while adjusting for confounders (e.g., age). P values of <0.05 were considered statistically significant.
RESULTS
Characteristics of subjects studied
Table 1 outlines the characteristics of the 29 HIV-infected subjects and 48 HIV-uninfected controls. HIV-infected subjects were classified into two groups with “high” or “low” carotid artery intima-media thickness (IMT), i.e., above or below 1 mm in thickness (the mean value of carotid IMT in HIV+ subjects) [1]. The mean age of the HIV-infected subjects was 47.3 years and 27 (93.1%) were male. Sixteen (55.2%) were receiving antiretroviral therapy that, in each case, included a protease inhibitor. The median CD4+ T cell count was 514 cells/μl. Most (55.2%) had undetectable HIV RNA levels (<75 copies/ml). Among HIV+ subjects with detectable viral load, the mean (± SD) and median (range) value of HIV RNA levels were of 55,521 (± 133,341) and 8,871 (735–470,066) copies/ml. Compared to controls, HIV-infected subjects did not show any statistical differences in terms of age, gender, tobacco use, hypertension, cholesterol treatment, or body mass index (BMI).
Table 1.
Characteristics of subjects
| HIV-uninfected (n=48) | HIV-infected | |||
|---|---|---|---|---|
| All (n=29) | IMT <1mm (n=16) | IMT ≥1mm (n=13) | ||
| Age (years, SD) | 45 (12.1) | 47.3 (10) | 42.5 (7.9)1 | 53.8 (9.1)† |
| Male sex, n (%) | 42 (87.5) | 27 (93.1) | 14 (87.5) | 13 (100) |
| Tobacco use, n (%) | 20 (41.7) | 19 (65.5) | 12 (75) | 7 (53.8) |
| History of diabetes, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hypertension treatment, n (%) | 9 (18.7) | 4 (13.8) | 2 (12.5) | 2 (15.4) |
| Cholesterol treatment, n (%) | 8 (16.7) | 4 (13.8) | 4 (25) | 0 (0) |
| 10-year risk of coronary heart disease*, SD | NA | 10.9 (7.9) | 8.5 (7.2) | 12.5 (8.9) |
| Total cholesterol (mg/dl, SD) | NA | 198.6 (54.2) | 201.7 (45.9) | 194.5 (65.5) |
| HDL cholesterol (mg/dl, SD) | NA | 39.4 (9.8) | 39.6 (10.3) | 39.25 (9.7) |
| Triglycerides (mg/dl, SD) | NA | 300.8 (240.6) | 337.3 (230.4) | 252.2 (255.4) |
| BMI (kg/m2, SD) | 26.3 (3.7) | 26.1 (5.5) | 26.5 (6.3) | 25.5 (4.4) |
| Years since HIV diagnosis (SD) | 14.5 (5.2) | 13.7 (5.3) | 16 (5.1) | |
| Current CD4 count (cells/μl, SD) | 514 (267) | 573 (273) | 436 (247) | |
| CD4 nadir (cells/μl SD) | 239 (296) | 257 (330) | 215 (256) | |
| Viral load <75 copies/ml, n (%) | 16 (55.2) | 11 (68.7) | 5 (38.4) | |
| Total years on HAART (SD) | 10.7 (8) | 10.3 (7.8) | 11.2 (8.8) | |
| Protease inhibitor, n (%) | 16 (55.2) | 9 (56.2) | 7 (53.8) | |
HDL: high density lipoprotein, BMI: body mass index; HAART: highly active antiretroviral therapy; NA: not available;
calculated using the Framingham risk equation;
p=0.002
The mean IMT measurement at baseline in HIV+ high IMT and HIV+ low IMT subjects was 1.32 (±0.4) and 0.73 (±0.11) mm, respectively. The mean IMT progression in these two groups was 0.1 (±0.15) and 0.05 (±0.15) mm/year, respectively (p=0.53). As compared to the HIV+ low IMT group, members of the HIV+ high IMT group were older (p=0.002) but did not show any other statistically significant differences in term of classical risk factors for coronary disease. However, subjects with high IMT tended to have a higher 10-year risk of coronary heart disease (calculated using the Framingham risk equation).
The frequency of CD4+CX3CR1+ T cells correlates with IMT progression in HIV-infected subjects
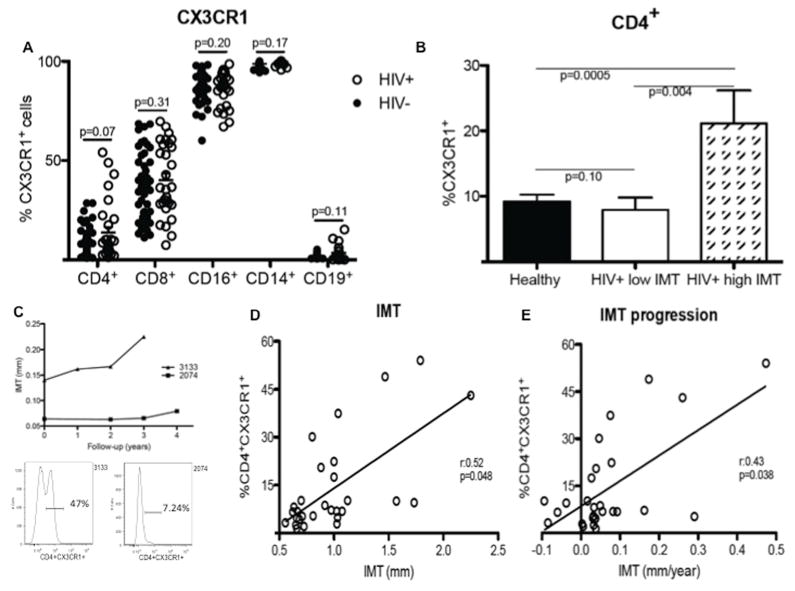
We first investigated the expression of CX3CR1 on PBMCs in HIV-infected and -uninfected subjects using multiparameter flow cytometry (Figure 1A). Among HIV-uninfected adults, CX3CR1 was found to be expressed on 9.2% (±7.2) of CD4+ T cells, 35.7% (±17.6) of CD8+ T cells, 86.2% (±9.5) of CD16+ cells (NK cells), 98.8% (±1.6) of CD14+ (monocytes), and 2% (±1) of CD19+ cells (B cells). Among HIV-infected subjects, 13.8% (±14.7) of CD4+ T cells, 40.2% (±18.1) of CD8+ T cells, 86.6% (±8.7) of NK cells, 99.3% (±1) of monocytes, and 3.4 % (±3) of B cells expressed CX3CR1. The percentage of cells expressing CX3CR1 did not differ significantly between HIV-infected and -uninfected subjects for any of these subsets of PBMCs. However, HIV-infected subjects tended to have a higher percentage of CD4+CX3CR1+ T cells than did those who were HIV-uninfected (p=0.07). The expression of CX3CR1 on CD4+ T cells was not associated with the HIV viral load (Figure S2A).
Figure 1. High percentage of CD4+CX3CR1+ T cells in HIV-infected subjects correlates with IMT progression.
(A) Flow cytometric analysis of CX3CR1 expression on CD4+, CD8+, CD16+, CD14+, and CD19+ cells found in PBMCs of HIV-uninfected (HIV−, n=48, blacks circles) and HIV-infected (HIV+, n=29, white circles) subjects. (B) Comparison of the frequency of CX3CR1+CD4+ T cells in HIV− and HIV+ subjects separated into two groups: one with low (<1mm) and another with high (≥1mm) IMT. (C) Carotid artery IMT progression over time in two HIV+ subjects, one (3133) showing a high frequency (47%; see flow cytogram on left) of CX3CR1+CD4+ T cells and the other (2074) showing a low frequency (7.24%; see flow cytogram on right) of CD4+CX3CR1+ T cells. (D) Positive statistical correlation between the frequency of CX3CR1+CD4+ T cells and IMT measurement at baseline. (E) IMT progression over time in HIV+ subjects. Significant correlations were found after adjustment for age.
Measurement of carotid intima-media thickness (IMT) assesses the presence of subclinical atherosclerosis and has been strongly correlated with coronary atherosclerosis [15]. Interestingly, the percentage of CD4+ T cells expressing CX3CR1 in HIV+ low IMT subjects was 7.9% (±7.4) and did not differ from that observed in HIV-uninfected subjects (p=0.10) (Figure 1B). By contrast, the percentage of CD4+ T cells expressing CX3CR1 in HIV+ high IMT subjects [21.1% (±18.2)] was higher than that found in each of the former groups (p=0.0005 relative to HIV-uninfected subjects and p=0.004 relative to HIV+ low IMT subjects) (Figure 1B). Thus, in HIV-infected subjects, a high frequency of CD4+ T cells expressing CX3CR1 was associated with a high IMT. The HIV viral load did not significantly differ between HIV+ low IMT and HIV+ high IMT subjects (Figure S2B). Baseline IMT measurements were also similar in subjects whether or not they were being treated with a protease inhibitor (Figure S2C).
To further characterize the link between the frequency of CD4+CX3CR1+ T cells in the peripheral blood and measurements of carotid artery IMT in HIV-infected subjects, we analyzed the relationship between these two parameters on a longitudinal basis. A subject with a high frequency of CD4+CX3CR1+ T cells (3133) was found to have a higher IMT measurement at baseline than did a subject with a low frequency of CD4+CX3CR1+ T cells (2074); subject 3133 also showed faster progression of IMT over time (Figure 1C). These observations were extended to a larger group of subjects to find that the frequency of CD4+CX3CR1+ T cells was correlated with IMT measurements at baseline (r=0.52; p=0.048 after adjustment for age) (Figure 1D) and with IMT progression over time (r=0.43; p=0.038 after adjustment for age) (Figure 1E) in HIV-infected subjects.
CD4+CX3CR1+ T cells are antigen-primed and activated T cells producing high levels of TNFα and IFNγ upon TCR stimulation
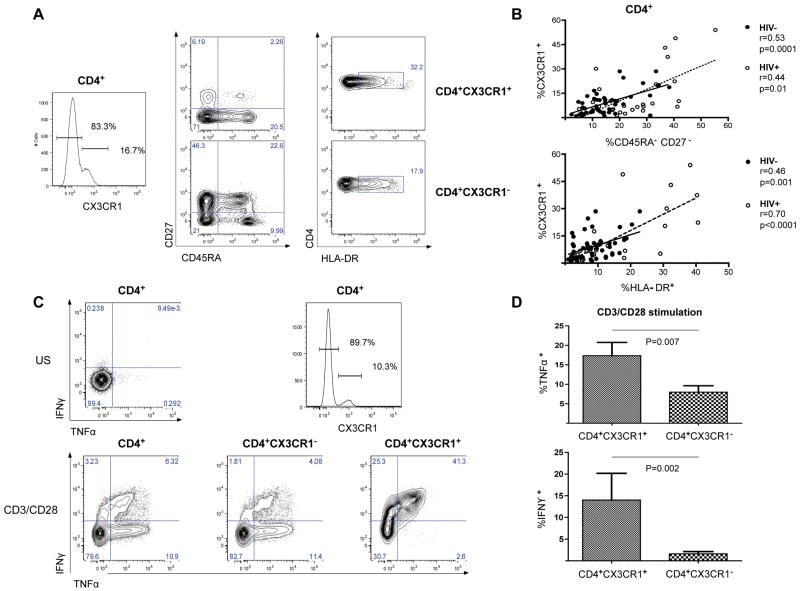
Multiparameter immunophenotyping was carried out on CD4+ T cells to define the subsets of cells expressing CX3CR1, focusing on CD27 (a co-stimulatory receptor involved in generation and long term maintenance of T cell immunity) [16], CD45RA (a marker associated with the definition of naïve and memory T cells) [17], and HLA-DR (a marker of T cell activation) [18]. CD4+ T cells that expressed CX3CR1 were predominantly CD45RA−CD27−, a phenotype of antigen-primed CD4+ T cells. These cells were also found to be CD57+, CCR7−, and PD-1+ (Figure S3). In contrast, CD4+ T cells that did not express CX3CR1 were mostly CD45RA+/−CD27+. Both of these subpopulations had high frequencies of cells that were HLA-DR+ (Figure 2A). In a larger group of subjects, the expression of CX3CR1 on CD4+ T cells was found to correlate with the lack of expression of CD45RA and CD27 in both HIV-uninfected (r=0.53, 95% CI: 0.28–0.71; p=0.0001) and in HIV-infected subjects (r=0.44. 95% CI: 0.08–0.70; p=0.01) (Figure 2B, upper panel). The expression of CX3CR1 on the surface of the CD4+ T cells was also correlated with the expression of HLA-DR in both groups (HIV-negative: r=0.46, 95% CI: 0.19–0.66; p=0.001) (HIV-positive: r=0.70, 95% CI: 0.44–0.85; p<0.0001) (Figure 2B, lower panel). Consistent with the phenotype of antigen-primed cells, CD4+ T cells that expressed CX3CR1 showed the functional properties of pro-inflammatory T cells: upon TCR activation by CD3/CD28 co-stimulation in vitro, CD4+CX3CR1+ T cells produced significantly more TNFα (17.3% ± 11.8 vs 8% ± 5.7; p=0.007) and IFNγ (14% ± 19 vs 1.6% ± 1.7; p=0.002) than did CD4+CX3CR1− T cells (Figure 2C and 2D).
Figure 2. Phenotype and functional characteristics of CD4+CX3CR1+ T cells.
(A) Flow cytometric analysis of CD27 and CD45RA (middle) and of HLA-DR (right) co-expression on resting CD4+CX3CR1+ (top) and CD4+CX3CR1− T cells (bottom) from a representative subject. (B) Positive statistical correlation in both groups (HIV− and HIV+ subjects) between the expression of CX3CR1 and the lack of expression of CD27 and CD45RA (top) and the expression of HLA-DR (bottom) on CD4+ T cells. (C) Flow cytometric analysis of TNFα and IFNγ production after TCR stimulation (using CD3/CD28 stimulation) of CD4+ T cells (left), CD4+CX3CR1− T cells (middle), and CD4+CX3CR1+ T cells (right) from a representative subject. CX3CR1 expression was defined as per the histogram on the upper right, with an unstimulated (US) control in the panel to the upper left. (D) Mean production of TNFα (top) and IFNγ (bottom) by CD4+CX3CR1+ T cells and CD4+CX3CR1− T cells upon polyclonal stimulation, as analyzed in 12 subjects.
CD4+CX3CR1+ T cells are mostly CMV-specific CD4+ T cells and CD4+ T cell response to CMV antigen induces endothelial cell production of CX3CL1, the cognate chemokine ligand of CX3CR1
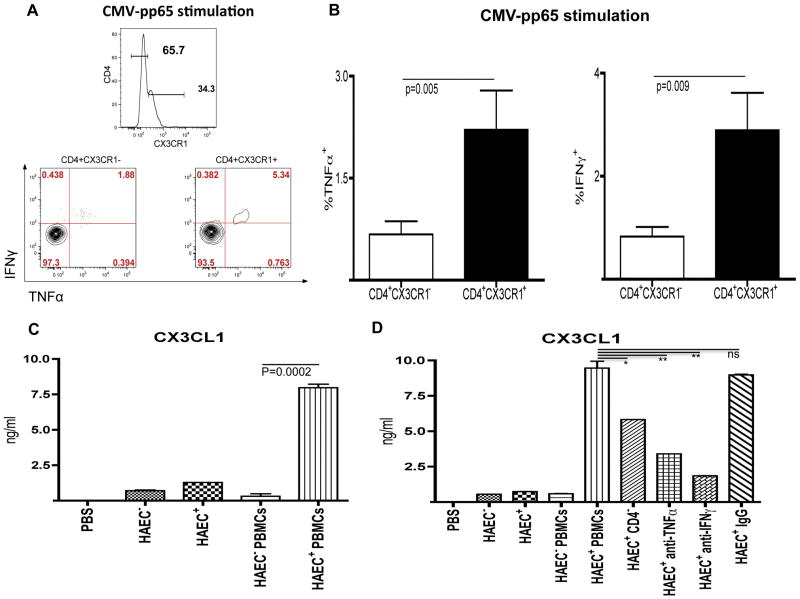
CMV is a non-traditional risk factor for atheroma that may be particularly important in the context of HIV infection wherein the prevalence of CMV infection is high [19], subclinical replication of CMV is frequent [20], and CMV-specific T cell responses are associated with increased IMT [8]. To determine whether the CD4+CX3CR1+ T cell population in HIV-infected subjects might harbor a high frequency of CMV-specific cells, these cells were tested for CMV specificity using a cytokine production assay. In HIV+ subjects, the percentage of CD4+ T cells producing TNFα and IFNγ upon stimulation with CMV pp65 peptide pools was 2.9% (±3) and 3.7% (±3.4), respectively (Figure S4). Interestingly, CD4+ T cells that expressed CX3CR1 produced significantly more TNFα (2.2% ±2.3 vs 0.7% ±0.8; p=0.005) and IFNγ (2.9 ±2.9 vs 0.8 ±0.7; p=0.009) than did CD4+CX3CR1− T cells. Thus, based on the TNFα and IFNγ production, more than 75% of the CMV-specific CD4+ T cells expressed CX3CR1 (Figures 3A and 3B).
Figure 3. CD4+CX3CR1+ T cells are CMV-specific and CX3CL1 production is induced in CMV-infected human artery endothelial cells.
(A) Flow cytometric analysis of TNFα and IFNγ production after CMV-pp65 stimulation of CD4+CX3CR1− T cells (left panel), and CD4+CX3CR1+ T cells (right panel) from a representative subject. CX3CR1 expression was defined as per the histogram above the two panels. (B) Mean production of TNFα (left) and IFNγ (right) by CD4+CX3CR1+ T cells and CD4+CX3CR1− T cells upon CMV-pp65 stimulation, as analyzed in 16 HIV-infected subjects. (C) Secretion of CX3CL1 (as detected by ELISA) into the supernatant of uninfected HAEC (HAECCMV−), CMV-infected HAEC (HAECCMV+), uninfected HAEC co-cultured with PBMCs from CMV-positive subjects (HAECCMV− PBMCs), and CMV-infected HAEC co-cultured with PBMCs from CMV-positive subjects (HAECCMV+ PBMCs). (D) Secretion of CX3CL1 (as detected by ELISA) into the supernatant of HAEC cultured in the same conditions as described above and in the supernatant of CMV-infected HAEC (HAECCMV+) co-cultured with either CD4-depleted PBMCs (CD4−) or with PBMCs and neutralizing antibodies against TNFα (anti-TNFα) or IFNγ (anti-IFNγ), or with isotype control antibodies (IgG). Neutralizations were carried out directly in co-cultivations of PBMCs with HAECCMV+ at a concentration of 30 μg/ml (for anti-TNFα) or 20 μg/ml (for anti-IFNγ). The data are representative of four separate experiments with different blood donors and CMV-infected HAEC monolayers.
*, means p<0.05; **, means p>0.005; ns, means not significant
We then tested the possibility that CMV infection of human arterial endothelial cells (HAEC) might induce the production of CX3CL1, the cognate chemokine ligand of CX3CR1, and thus facilitate the migration of CD4+CX3CR1+ T cells through the arterial wall. Limited CX3CL1 production was detected (by ELISA) in the supernatant of uninfected HAEC culture (HAECCMV−) and there was only a slight increase after infection of endothelial cells by CMV (HAECCMV+). When PBMCs from CMV-infected subjects were co-cultured with uninfected endothelial cells (HAECCMV− PBMCs), there was no increase in the level of CX3CL1 in the supernatant. However, when such PBMCs were co-cultured with CMV-infected endothelial cells (HAECCMV+ PBMCs), there was a dramatic increase in the level of CX3CL1 (Figure 3C). Interestingly, when CD4+ T cell-depleted PBMCs were co-cultured with CMV-infected HAEC, the production of CX3CL1 was significantly reduced (mean reduction of 33% ±24, p=0.04) (Figure 3D). These results, reproducible across all four donors tested, demonstrate that CMV-specific CD4+ T cells can induce CX3CL1 expression in CMV-infected endothelial cells.
The identification of the CD4+ T cell subset that induces CX3CL1 in endothelial cells and the ability of CD4+CX3CR1+ T cells to produce high levels of TNFα and IFNγ suggested that these cytokines may play a role in the induction of CX3CL1 in endothelial cells. Indeed, a marked reduction in CX3CL1 levels was observed when CMV-infected HAECs, co-cultured with PBMCs, were treated with neutralizing antibodies against either TNFα or IFNγ (Figure 3D). Thus, we conclude that CD4+ T cell-derived TNFα and IFNγ are likely to be the dominant soluble factors induce the production of CX3CL1 by endothelial cells.
CX3CL1 can drive the transendothelial migration of CD4+ T cells
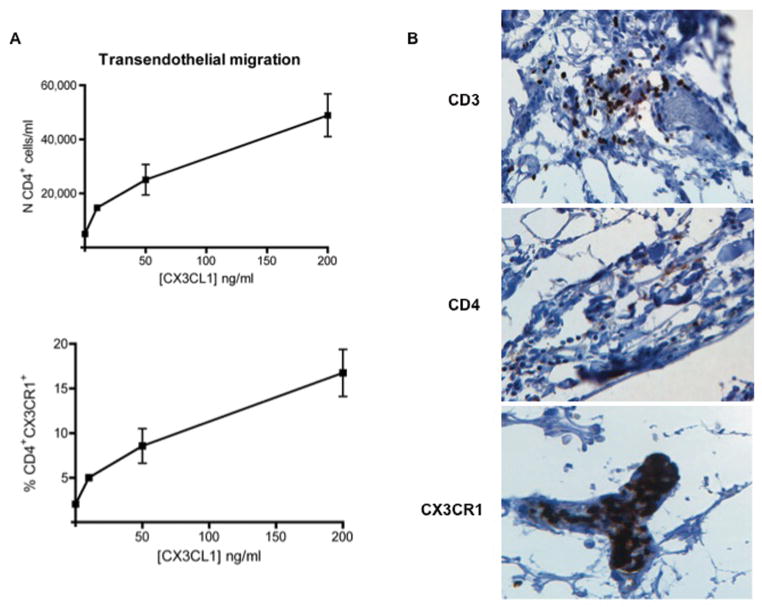
The ability of CX3CL1 to induce the directed migration of CD4+ T cells in vitro was assessed in a series of transendothelial migration assays. Enriched CD4+ T cells were loaded onto polarized HAEC in the upper compartment of the transwell filter insert, with the lower compartment containing varying concentrations of human recombinant CX3CL1. The number and percentage of input CD4+ T cells and of CD4+CX3CR1+ T cells deposited in the upper compartment were determined by flow cytometric analysis. After four hours, transmigrated CD4+ populations were collected from the lower chambers and replicate pooled samples were used to calculate the mean transmigrated cell number per milliliter. As shown in Figure 4A, CX3CL1 induced the transendothelial migration of CD4+ T cells in a concentration-dependent manner, with the highest numbers (48,900±7,900 cells/ml; 16.7±2.6 % of the CD4+CX3CR1+ T cells loaded) observed at a concentration of 200 ng/ml and the lowest (5,000±1,000 cells/ml; 1.6±0.4 % of the CD4+CX3CR1+ T cells loaded) with the chemokine negative control.
Figure 4. CX3CL1 supports chemoattraction of CD4+ T cells.
(A) Transmigrated CD4+ T cells from transendothelial migration assays incubated with varying concentration of CX3CL1 (10 ng/ml, 50 ng/ml, and 200 ng/ml) and with control media, expressed as the absolute number of CD4+ T cells/ml (top) or as the percentage of input CD4+ T cells expressing CX3CR1 (bottom). For each sample, three transmigration wells were set up and the transmigrated populations were counted from each well independently. Results are representative of three independent assays. (B) Immunohistochemical detection of CD3, CD4, and CX3CR1 in adjacent sections of the same coronary artery showing early atheromatous lesions from an HIV+ subject. An aggregate of CD3+ immunoreactive cells is observed in the perivascular space of a small blood vessel running in the adventitia of the artery (×20, top). CD4+ cells (x20, middle) and CX3CR1+ cells (x20, bottom) are scattered in the wall and present in the lumen of a small blood vessel running in the adventitia of the artery, respectively.
To extend these findings to conditions that might exist in vivo, we next asked whether CD4+CX3CR1+ T cells are localized within the wall of the coronary artery in HIV-positive subjects with atherosclerosis. Immunohistochemical analysis of the coronary arterial wall in different developmental stages of atherosclerotic plaques showed expression of CD3, CD4, and CX3CR1 within lesions at an early stage of the disease (Figure 4B). Immunoreactivity for all three markers was located in the external tunic of the artery in proximity to the atherosclerotic lesions and was mostly confined to the lumen (CX3CR1), in the wall (CD4), or around the vessels running in the adventitia (CD3). Except for the detection of CX3CR1, no immunoreactivity was observed in atherosclerotic lesions examined at late stage of disease (Figure S5).
DISCUSSION
In this study, we show that HIV-associated atherosclerosis is correlated with a high frequency of peripheral blood CD4+ T cells expressing CX3CR1, the chemokine receptor of CX3CL1 (fractalkine); many of these cells are CMV-specific and CMV can drive the migration of them through the arterial wall by enhancing the production of CX3CL1 by human arterial endothelial cells. Given prior data from our group that HIV infection is associated with expansion of CMV-specific T cells and that CMV-specific T cells are associated with atherosclerosis in HIV-infected adults [8, 21], these data support a conceptual model in which the immune dysfunction associated with HIV infection is associated with: (1) an increased frequency of circulating CD4+CX3CR1+ T cells, many of which are specific for CMV, (2) an increased presentation of CMV by endothelial cells to T cells, (3) an increased production of TNFα and IFNγ by antigen-stimulated CD4+CX3CR1+ T cells, driving the endothelial production of the ligand CX3CL1, and (4) the transendothelial migration of pro-inflammatory CD4+ T cells (see Figure S6 for a schematic model depicting these events).
Consistent with the potential proatherogenic role for the CD4+ T cells expressing CX3CR1, CD4+CX3CR1+ T cells shared the memory effector phenotype of T cells found in the atherosclerotic plaque [22]. In addition, by being mostly CCR7− (Figure S3), CD4+CX3CR1+ T cells also have the ability to accumulate in inflammatory tissue [23]. Finally, mice deficient in the inhibitory molecules programmed death–ligand 1 (PD-L1) and PD-L2 have been shown to have larger atheroma plaques with massive infiltration of CD4+ T cells [24]. These data indicate that these cells might be controlled by PD-1 in atherosclerosis and we here observed that CD4+CX3CR1+ T cells mostly expressed PD-1 (Figure S3).
Since CD4+ T cells expressing CX3CR1+ are antigen-primed and activated, the heightened expression of CX3CR1 in HIV disease may be a consequence of the immune activation that is the hallmark of HIV disease progression [18]. However, because CD4+CX3CR1+ T cells are mainly CMV-specific, the higher level of circulating CD4+CX3CR1+ T cells may also be the simple consequence of greater exposure to CMV antigen during the course of HIV disease. Accordingly, we observed that CD4+CX3CR1+ T cells predominantly expressed CD57+, an antigen-primed marker highly expressed on CMV-specific T cells [25] [26]. In sum, because they are both CX3CR1+ and pro-inflammatory, CMV-specific CD4+ T cells are ideally suited to migrate through the endothelium and to play a pro-atherogenic role in the arterial wall.
We determined how a high level of CD4+CX3CR1+ T cells in blood could be mechanistically linked to atherosclerosis by showing that CMV-induced T cell immunopathology can drive the migration of these cells through the wall artery. Here, we largely confirmed what was previously described by Bolovan-Fritts et al. [12]: that the production of TNFα and IFNγ by CMV-specific CD4+ T cells induces the production of CX3CL1 by endothelial cells. We have also extended this previous study to show that the CD4+CX3CR1+ T cells targeted by CX3CL1 are correlated with HIV-associated atherosclerosis in vivo, comprise the majority of the CMV-specific CD4+ T cells, produce high levels of TNFα and IFNγ (two key pro-inflammatory cytokines involved in atherosclerosis [7, 9, 27]) and can migrate through the artery endothelial cells. Our study accordingly offers a novel and comprehensive picture of how CMV-induced T cell immunopathology may be involved in the pathogenesis of atherosclerosis. However, it is important to note that, given the rarity of the material, we have only studied two sets of coronary arteries from one donor by immunohistochemistry: one set at an early stage of atherosclerosis and another at a later stage. More samples need to be studied in the future, especially at a pre-atherosclerotic stage, so that the current observations can be confirmed and extended.
Interestingly, we observed that almost all monocytes, the most important players of atherosclerosis pathogenesis, expressed CX3CR1. It is accordingly possible that endothelial production of CX3CL1 may also promote proatherogenic inflammatory events by affecting the trafficking of monocytes. These observations suggest that CX3CL1 and its cognate receptor, CX3CR1, are part of a chemokine-based positive feedback circuit involved in atherosclerosis, one that may represent an attractive therapeutic target. The ongoing inflammatory process in the arterial wall would presumably be most pronounced in those with the highest frequency of CMV-specific T cells, as has been observed in the context of HIV infection [21]. If so, then treatment of CMV infection (e.g., with valganciclovir) [28] would be an attractive therapeutic option.
In summary, our data suggest that CD4+ T cells expressing CX3CR1 play a key role in the pathogenesis of HIV-associated atherosclerosis, one that is proximally driven by CMV infection of endothelial cells. Elucidation of this mechanism provides support for treatment interventions (e.g., inhibition of CMV replication and/or of CX3CR1/CX3CL1 interactions) that might ultimately slow the pace of atherosclerosis in vivo. Given the prevalence of CMV infection and of T cell activation in the aging individuals who are not infected with HIV [29], such interventions may prove beneficial to a much larger patient population than just those with HIV alone.
Supplementary Material
Acknowledgments
We are thankful to Dr. R. Dunham for help with manuscript preparation, Dr. M. Revello for providing the CMV clinical strain (VR1814), and M. Weinstein for technical assistance with immunohistochemistry. The HCMV pp65 Peptide Pool was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Sources of Fundings. This research was supported in part by Assistance Publique Hôpitaux de Paris and Monahan Foundation (K.S.), R37 AI40312 (J.M.M.), R21 AI087035 (P.W.H.), R01 HL095130 (P.Y.S.), and by the Harvey V. Berneking Living Trust. The SCOPE cohort (J.N.M. and S.G.D.) was supported by grants from the NIAID (AI52745 and K24AI069994), the UCSF–GIVI Center for AIDS Research (P30 MH59037), and the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131). J.M.M. is a recipient of the NIH Director’s Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant number DPI OD00329.
Footnotes
AUTHOR CONTRIBUTIONS
K.S. and B.A. conceived the project. J.M.M. and K.S. designed and interpreted the experiments, and wrote the manuscript. K.S. conducted the experiments. E.M. designed experiments and provided virus. S.G.D, J.N.M., P.W.H., and P.Y.H. selected appropriate subjects for analysis, provided samples and IMT data, and helped to analyze data and to write the paper.
There are no financial conflicts of interest associated with this work.
References
- 1.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 2.Barbaro G, Fisher SD, Lipshultz SE. Pathogenesis of HIV-associated cardiovascular complications. Lancet Infect Dis. 2001;1:115–124. doi: 10.1016/S1473-3099(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. Journal of Acquired Immune Deficiency Syndromes. 2011;57:245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 4.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:826–835. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vonderen MG, Hassink EA, van Agtmael MA, Stehouwer CD, Danner SA, Reiss P, et al. Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. The Journal of Infectious Diseases. 2009;199:1186–1194. doi: 10.1086/597475. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Valantine HA, Gao SZ, Menon SG, Renlund DG, Hunt SA, Oyer P, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation. 1999;100:61–66. doi: 10.1161/01.cir.100.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 12.Bolovan-Fritts CA, Trout RN, Spector SA. Human cytomegalovirus-specific CD4+-T-cell cytokine response induces fractalkine in endothelial cells. J Virol. 2004;78:13173–13181. doi: 10.1128/JVI.78.23.13173-13181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 14.Grazia Revello M, Baldanti F, Percivalle E, Sarasini A, De-Giuli L, Genini E, et al. In vitro selection of human cytomegalovirus variants unable to transfer virus and virus products from infected cells to polymorphonuclear leukocytes and to grow in endothelial cells. J Gen Virol. 2001;82:1429–1438. doi: 10.1099/0022-1317-82-6-1429. [DOI] [PubMed] [Google Scholar]
- 15.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 16.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Jackson JB, Erice A, Englund JA, Edson JR, Balfour HH., Jr Prevalence of cytomegalovirus antibody in hemophiliacs and homosexuals infected with human immunodeficiency virus type 1. Transfusion. 1988;28:187–189. doi: 10.1046/j.1537-2995.1988.28288179029.x. [DOI] [PubMed] [Google Scholar]
- 20.Drew WL. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158:449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 21.Sacre K, Carcelain G, Cassoux N, Fillet AM, Costagliola D, Vittecoq D, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 23.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 24.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 26.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir Reduces T Cell Activation in HIV-infected Individuals With Incomplete CD4+ T Cell Recovery on Antiretroviral Therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, et al. Old age and anti-CMV immunity are associated with altered T cell reconstitution in HIV-1 infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.